-
PDF
- Split View
-
Views
-
Cite
Cite
Zunyang Song, Hangcong Chen, Xiuhua Lai, Lihua Wang, Yulin Yao, Jiajia Qin, Xuequn Pang, Hong Zhu, Weixin Chen, Xueping Li, Xiaoyang Zhu, The Zinc Finger Protein MaCCCH33-Like2 Positively Regulates Banana Fruit Ripening by Modulating Genes in Starch and Cell Wall Degradation, Plant and Cell Physiology, Volume 65, Issue 1, January 2024, Pages 49–67, https://doi.org/10.1093/pcp/pcad115
Close - Share Icon Share
Abstract
As zinc finger protein transcription factors (TFs), the molecular mechanism of Cys-Cys-Cys-His (CCCH) TFs in regulating plant development, growth and stress response has been well studied. However, the roles of CCCH TFs in fruit ripening are still obscure. Herein, we report that MaCCCH33-like2 TF and its associated proteins modulate the fruit softening of ‘Fenjiao’ bananas. MaCCCH33-like2 interacts directly with the promoters of three genes: isoamylase2 (MaISA2), sugar transporter14-like (MaSUR14-like) and β-d-xylosidase23 (MaXYL23), all of which are responsible for encoding proteins involved in the degradation of starch and cell wall components. Additionally, MaCCCH33-like2 forms interactions with abscisic acid–insensitive 5 (ABI5)-like and ethylene F-box protein 1 (MaEBF1), resulting in enhanced binding and activation of promoters of genes related to starch and cell wall degradation. When MaCCCH33-like2 is transiently and ectopically overexpressed in ‘Fenjiao’ banana and tomato fruit, it facilitates softening and ripening processes by promoting the degradation of cell wall components and starch and the production of ethylene. Conversely, the temporary silencing of MaCCCH33-like2 using virus-induced gene silencing (VIGS) inhibits softening and ripening in the ‘Fenjiao’ banana by suppressing ethylene synthesis, as well as starch and cell wall degradation. Furthermore, the promoter activity of MaCCCH33-like2 is regulated by MaABI5-like. Taken together, we have uncovered a novel MaCCCH33-like2/MaEBF1/MaABI5-like module that participates in fruit softening regulation in bananas.
Introduction
Banana is an internationally traded fruit crop widely cultivated and consumed in subtropical and tropical countries (Li et al. 2016, Paul et al. 2016). Banana fruit ripens and softens rapidly after being harvested due to its climacteric character (Han et al. 2016). Fruit softening is a key indicator determining the edibility of bananas (Song et al. 2023). Therefore, it is essential to study the regulatory mechanisms of fruit softening in this commercially important fruit and identify the key functional genes to improve fruit quality.
Zinc finger protein genes constitute a huge family and are involved in various molecular functions such as transcriptional regulation, protein–protein interaction, DNA and lipid binding and RNA binding and degradation (Laity et al. 2001, Qu et al. 2014, Noman et al. 2019). Tandem C3H zinc finger (TZF) is one subfamily of the zinc-type finger protein family, which could interact with the A(Adenine)U(Uracil)-rich elements (AREs) of the 3ʹ untranslated region (UTR) of mRNAs of some key regulators and regulate RNA metabolism and stability, involved in different cellular functions (Qu et al. 2014). The CCCH family proteins contain a signature motif, which consists of one histidine residue and three cysteines (Bogamuwa and Jang, 2014). AtTZF1, one CCCH-type zinc finger protein, could bind to RNA molecules and cause the ARE-containing mRNAs to decay and regulate RNA turnover (Qu et al. 2014). In rice, OsTZF1 (a CCCH-type zinc finger protein) could bind to ARE-like, AREs and U-rich motif regions in the 3ʹ UTR of mRNA and is involved in the regulation of genes related to stress response (Jan et al. 2013). Except for the RNA-binding regulation, CCCH-type zinc finger protein could bind to DNA and serve as transcription factors (TFs). In plants, many CCCH TFs have been identified to regulate downstream genes by binding different motifs. For example, AtC3H14 regulates NIMIN1 and NIMIN2 activity by binding the promoter sequence of the CHRE motif (GGGACA/GGGAGA) in Arabidopsis (Kim et al. 2014). Zc3h10 could bind to GC-rich sequences with repeated CC doublets (Yi et al. 2019). PuC3H35 binds to the CT-rich motif (CTCACCTTC/CTTTTTTCT/CTCTCTTCT) of PuANR and PuEARLI1 (Li et al. 2022). IbC3H18 can bind to several consensus motifs, such as TGAATCGA, TGGGCGAGAA, CGATAGCA and TTTACCGAATAA (Zhang et al. 2019). In Arabidopsis, 68 CCCH proteins have been isolated, among which somnus (SOM) affects seed germination by regulating phytochrome-interacting factor3-like 5 (PIL5) (Kim et al. 2008). Two other CCCH-type zinc finger proteins, AtKHZ1 and AtKHZ2, play redundant roles in regulating senescence and flowering (Yan et al. 2017). BnZFP1 positively regulates oleic acid levels in Brassica napus (Zhang et al. 2018a). Moreover, CCCH-type zinc finger protein in Populus functions in abiotic stress response, where overexpressing PdC3H17 improves drought tolerance (Zhuang et al. 2020). Although the CCCH TFs play important regulatory roles in plants, their functions in fruit ripening and softening are still uncovered.
‘Fenjiao’ (Musa ABB Pisang Awak) is widely planted in South China for its better resistance to environmental stresses and high nutrition (Hu et al. 2015). ‘Fenjiao’ (ABB genotype) is found to be quite different from the Cavendish banana (AAA genotype) in relation to the breakdown of starch and the production of ethylene (Wang et al. 2019). ‘Fenjiao’ fruit ripens, softens and deteriorates more rapidly than the Cavendish banana fruit due to more active ethylene biosynthesis and starch metabolism pathways. The ‘Fenjiao’ banana tends to suffer from ripening disorder compared to the Cavendish banana fruit, i.e. an incomplete softening disorder induced by low temperature, which is mainly due to the failure of complete starch and cell wall degradation (Song et al. 2022). The ripening regulation network in the ‘Fenjiao’ banana is not clear, which could serve as a good example of fruit softening regulation. In our previous investigation, we discovered a correlation between fruit softening and a set of 12 genes related to the cell wall degradation as well as 11 genes associated with starch breakdown (Song et al. 2022). However, the CCCH TF regulates starch, and cell degradation is rarely found in bananas.
Overall, 89 CCCH-type zinc finger genes have been identified from genome-wide analysis in bananas (Xu 2014, Sun et al. 2015), which could potentially serve as a significant factor in controlling the process of fruit ripening (Kuang et al. 2021). Nevertheless, their molecular functions are not yet characterized in fruits. In this study, we find that the expression of MaCCCH33-like2 is closely associated with fruit ripening. The introduction of MaCCCH33-like2 through ectopic overexpression and transient expression in tomatoes and ‘Fenjiao’ bananas expedites the ripening process of the fruits. This acceleration is attributed to the upregulation of genes associated with the cell wall and starch degradation, facilitated by the interaction between MaCCCH33-like2 and important regulatory proteins, such as abscisic acid–insensitive 5 (ABI5)-like protein and ethylene F-box protein 1 (MaEBF1). Additionally, we found that MaABI5-like regulates the promoter activity of MaCCCH33-like2. In summary, a new regulatory module of MaCCCH33-like2/MaEBF1/MaABI5-like was identified in the present work, which mediates fruit softening and ripening of the ‘Fenjiao’ banana.
Results
Change in physiological indexes of fruit ripening
The ‘Fenjiao’ banana ripens quickly when stored at 25℃, the color index reaches >5 and the pulp firmness reaches the lowest level of about 4 N after 5 d of harvest (Fig. 1A–C). Ethylene treatment promotes ‘Fenjiao’ banana ripening and softening by accelerating the color change and the decline of firmness and inducing ethylene production. However, 1-methyl-cyclopropene (1-MCP) treatment severely represses ‘Fenjiao’ banana ripening and softening by inhibiting color change, the decline of firmness and ethylene production during the storage (Fig. 1A–D). After 5 d of storage, 1-MCP-treated fruits were accelerated ripen by ethephon (ETH) treatment. ETH treatment greatly induced the ripening of fruit, with gradually increasing in color index and ethylene production, and decreasing in fruit firmness (Fig. 1A–D). 1-MCP-treated fruits ripen completely after 5 d of treatment. The 1-MCP400-treated fruits could also ripen completely without ethephon treatment as reported in our previous work (Zhu et al. 2020).
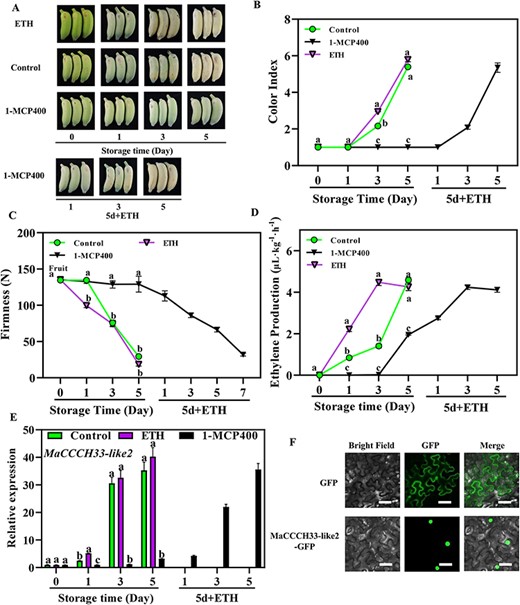
Fruit ripening and expression profile of MaCCCH33-like2. (A) Phenotype of ‘Fenjiao’ banana treated by ethylene and 1-MCP. Modifications in physiological indicators throughout the storage period: color index (B), firmness of the fruit (C) and ethylene production (D). (E) The expression level of MaCCCH33-like2 transcript in the pulp during the storage and ripening stages of banana fruit. The transcription of MaCCCH33-like2 at various time points is compared relative to day 0, which is set as 1. Different letters represent statistically significant differences (P < 0.05) in different temperature storage conditions. ETH: ethephon treatment; 1-MCP400: 400 nl L−1 of 1-MCP treatment for 1 h. 1-MCP-treated fruit was stored for 5 d and then treated by ethephon for ripening (5 d + ETH). (F) The subcellular localization of MaCCCH33-like2 in tobacco leaves. The empty-GFP-positive control and MaCCCH33-like2-GFP were temporarily introduced into tobacco leaves, resulting in transient expression. The fluorescence microscope was used to capture images, specifically detecting green fluorescence. The scale bars in the images represent a length of 50 μm.
Characterization of MaCCCH33-like2 properties and expression pattern
Herein, 116 CCCH-type zinc finger genes were identified based on RNA-seq data, and the expression of these genes exhibited a strong correlation with the ripening and softening of the fruits (Supplementary Fig. S1). MaCCCH33-like2 stood out among these genes for further investigation due to its higher levels of abundance and significantly greater expression difference compared to the others. The expression of MaCCCH33-like2 was subsequently validated using real-time quantitative PCR (RT-qPCR) (Fig. 1E). The expression of MaCCCH33-like2 was markedly increased during fruit ripening under control conditions, which is closely associated with fruit ripening (Fig. 1E). Ethylene treatment significantly induced the expression of MaCCCH33-like2 as compared with control after 1 d of treatment (Fig. 1E). However, the application of 1-MCP400 significantly suppressed the expression of MaCCCH33-like2 throughout the storage period, resulting in a consistently low level of expression. Ethylene induced the expression of MaCCCH33-like2, which gradually increased with fruit ripening after being treated with ethephon (Fig. 1E). This information suggests that MaCCCH33-like2 likely plays a pivotal role in the process of fruit ripening.
MaCCCH33-like2 contains 1,950 bases encoding 650 amino acids with a CCCH domain (Supplementary Fig. S2). The phylogenetic analysis found that MaCCCH33-like2 is closely related to MaCCCH33-like (Supplementary Fig. S3). However, there is no further information about the function of these closely related CCCH family genes. Typical for a TF, MaCCCH33-like2 is a nuclear protein (Fig. 1F).
MaCCCH33-like2 acts upon genes associated with the degradation of starch and cell wall components
In our previous study, a total of 12 genes involved in cell wall degradation and 11 genes involved in starch degradation, which have strong relevance to fruit softening, were extensively examined (Song et al. 2019, 2022). In order to explore the influence of MaCCCH33-like2 on the expression of these genes, dual-luciferase transient expression (DLR) experiments were carried out using relevant vectors (Supplementary Fig. S4A). Among the genes analyzed, the promoter activities of β-d-xylosidase23 (MaXYL23), sugar transporter14-like (MaSUR14-like) and isoamylase2 (MaISA2) were significantly enhanced by MaCCCH33-like2 compared to the control (Fig. 2A, B). Then, we analyzed the promoter sequences of MaXYL23, MaSUR14-like and MaISA2 by PlantCARE database, which found that the promoter sequences of all these genes contained ARE (TGGTTT/AAACCA), CAAT-box, MYB, MYC, TATA-box and Unnamed-4 motifs (Supplementary Fig. S5). Then, MaCCCH33-like2 recombinant protein was produced and assayed with probes containing ARE (TGGTTT/AAACCA), CAAT-box, MYB, MYC, TATA-box and Unnamed-4 motif, respectively, of MaISA2 in the electrophoretic mobility shift assay (EMSA), which found that MaCCCH33-like2 directly binds to the ARE (TGGTTT/AAACCA) motif but not to others (Supplementary Fig. S6). It indicated that MaCCCH33-like2 might regulate downstream genes by directly binding to the ARE (TGGTTT/AAACCA) motif in the promoters.
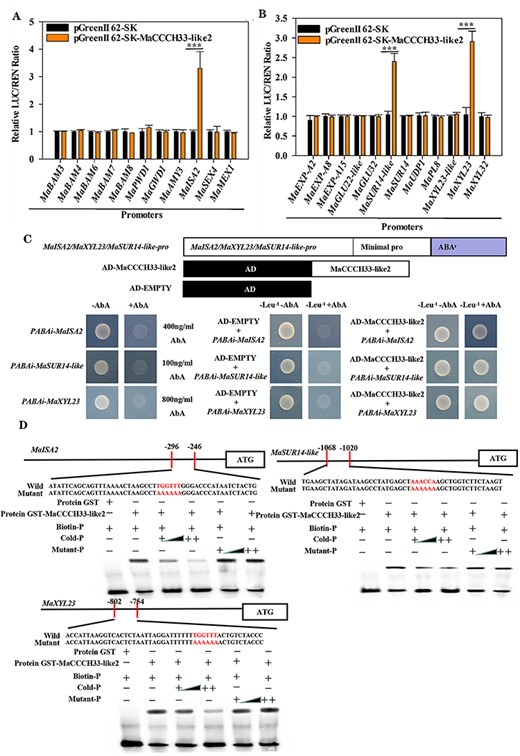
MaCCCH33-like2 interacts with the promoters of genes in cell wall and starch degradation pathways both in vivo and in vitro (A, B). In vivo, MaCCCH33-like2 exhibits transcriptional activity on the starch promoter regions (A) and cell wall (B) degradation genes. An empty vector was set as a reference (set as 1). The notation ‘***’ indicates significantly different values at a statistical significance level of P < 0.001. (C) Yeast growth assays demonstrate the interaction between MaCCCH33-like2 and the promoters of MaISA2, MaSUR14-like and MaXYL23. The yeast cells transformed with these constructs were cultivated on a selective SD medium containing ABA but lacking Leu-. (D) The binding of MaCCCH33-like2 with the promoters of MaISA2, MaSUR14-like and MaXYL23 in EMSAs. The fusion protein GST-MaCCCH33-like2 was combined with the promoters of MaISA2, MaSUR14-like and MaXYL23, which included the ARE-binding site and mutant probes labeled with red letters (AAAAAA). The symbols ‘+’ and ‘−’ represent the presence or absence of the probes, respectively. ‘++’ indicates increasing concentrations of the probes. Triangles indicate increasing concentrations of unlabeled or mutated probes used for competition. Biotin-P signifies that the probe was labeled with biotin, Cold-P indicates that the probe was not labeled with biotin and Mutant-P indicates that the probe was mutated to contain AAAAAA.
To further study whether MaCCCH33-like2 directly interacts with the promoter of MaXYL23, MaSUR14-like and MaISA2 in vivo, a yeast one-hybrid (Y1H) experiment was performed. The results showed that MaCCCH33-like2 could bind to the promoters of MaXYL23, MaSUR14-like and MaISA2 in yeast (Fig. 2C). The purified recombinant protein of GST-MaCCCH33-like2 was used for EMSAs (Supplementary Fig. S7B). As shown in Fig. 2D, the recombinant MaCCCH33-like2 protein directly interacts with the specific cis-elements (ARE) of the MaXYL23, MaSUR14-like and MaISA2 promoters.
MaCCCH33-like2 forms an interaction with MaEBF1, leading to the enhancement of promoter activities for genes related to the cell wall and starch degradation
In the previous study, it was demonstrated that ethylene triggers the upregulation of MaEBF1 expression during the ripening phase and governs the ripening process in ‘Fenjiao’ bananas (Song et al. 2019). Here, we found that MaCCCH33-like2 physically interacts with MaEBF1 in yeast (Fig. 3A). This interaction was also verified by bimolecular fluorescence complementation (BiFC) assays, which showed a strong yellow fluorescent protein (YFP) fluorescent signal of MaEBF1 co-expressed with MaCCCH33-like2 in the nuclei. However, when MaCCCH33-like2-YFPN, MaCCCH33-like2-YFPC, MaEBF1-YFPN and MaEBF1-YFPC were co-expressed with an unfolded YFP fragment, no fluorescent signal was detected (Fig. 3B).
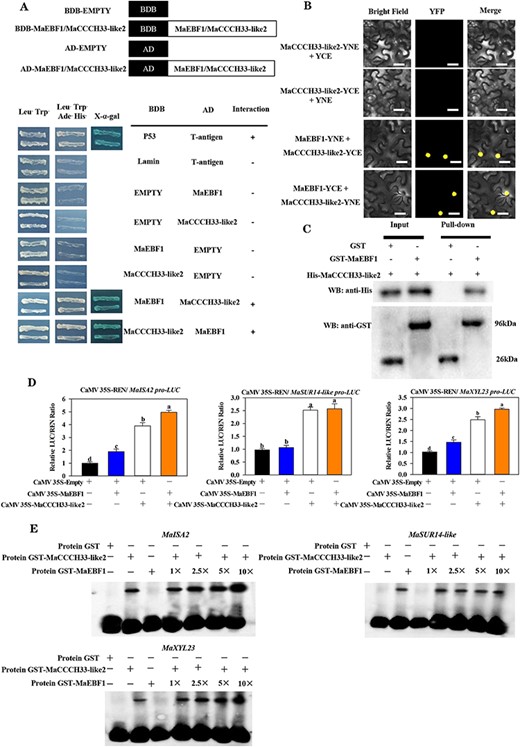
The interplay between MaCCCH33-like2 and MaEBF1 proteins and their influence on genes associated with fruit softening. (A) The Y2H analysis demonstrated an interaction between MaCCCH33-like2 and MaEBF1. The yeast cells carrying the transformed constructs were cultivated on an SD/-4 medium. (B) The utilization of BiFC in tobacco leaf epidermal cells revealed the interactions between MaCCCH33-like2 and MaEBF1. MaCCCH33-like2 and MaEBF1 were cloned, with the N-terminus and C-terminus of YFP, respectively. The fusion protein (MaCCCH33-like2) was used as the experimental sample, while the N-terminus and C-terminus alone served as the negative control. YFP signals were observed under a fluorescence microscope, with the scale bars indicating a size of 50 μm. (C) The interaction between MaCCCH33-like2 and MaEBF1 was analyzed by GST pull-down assay in vitro. The His-MaCCCH33-like2 protein was subjected to incubation with either GST-MaEBF1 or GST alone. The immunoprecipitated fractions obtained were then analyzed using the anti-GST antibody to detect the interaction with GST-MaEBF1 and the anti-His antibody to identify the presence of His-MaCCCH33-like2. (D) Transcriptional activity of MaCCCH33-like2, MaEBF1 and MaEBF1+MaCCCH33-like2 on the activities of the promoter of MaISA2, MaSUR14-like and MaXYL23. An empty vector was used as a reference control (set as 1). Letters a, b, c and d were used to denote significant differences at a significance level of P < 0.05. In experiment (E), it was observed that MaEBF1 increased the binding activity of MaCCCH33-like2 to the promoters of MaISA2, MaSUR14-like and MaXYL23. The values 1×, 2.5×, 5× and 10× corresponded to 50, 125, 250 and 500 ng of MaEBF1 protein, respectively. The symbol ‘−’ indicated the absence, while ‘+’ indicated the presence of the mentioned factors.
Moreover, GST pull-down experiments were conducted using purified recombinant proteins of His-MaCCCH33-like2 (Supplementary Fig. S7a) and GST-MaEBF1 obtained in the previous work (Song et al. 2022). The results show that His-MaCCCH33-like2 could pull down GST-MaEBF1 (Fig. 3C), indicating that MaEBF1 interacts with MaCCCH33-like2 in vitro.
To verify the impact of MaEBF1 interaction on the promoter activity of genes in the cell wall and starch degradation pathway, we introduced co-expression of MaCCCH33-like2, MaEBF1 and MaCCCH33-like2 regulations into tobacco leaves. The promoter activity of MaISA2 and MaXYL23 were significantly upregulated compared to transformations with only MaCCCH33-like2 (Fig. 3D). In contrast, the activity of the promoter of MaSUR14-like was not affected by the interaction between MaCCCH33-like2 and MaEBF1 (Fig. 3D).
Subsequently, the EMSA was performed by incubating GST-MaCCCH33-like2 and GST-MaEBF1 with MaISA2, MaSUR14-like and MaXYL23 probes. The findings revealed that GST-MaEBF1 alone did not exhibit DNA binding, whereas the binding affinity of GST-MaCCCH33-like2 with MaISA2 and MaXYL23 increased significantly with higher quantities of GST-MaEBF1. However, there was no observed enhancement in the binding activity between GST-MaCCCH33-like2 and MaSUR14-like (Fig. 3E).
MaCCCH33-like2 cooperates with MaABI5-like to increase the activation of promoters of genes related to the cell wall and starch degradation
In our prior investigation, we demonstrated that MaABI5-like plays a role in governing the ripening and softening of ‘Fenjiao’ bananas (Song et al. 2022). Here, the data indicated that MaCCCH33-like2 physically cooperates with MaABI5-like in yeast (Fig. 4A). The interaction between MaCCCH33-like2 and MaABI5-like was also verified by GST pull-down and BiFC experiments. The BiFC results showed that a strong YFP fluorescent signal was detected in the nuclei when MaCCCH33-like2 was co-expressed with MaABI5-like (Fig. 4B). However, the fluorescent signal was not detected when MaCCCH33-like2-YFPN and MaCCCH33-like2-YFPC were co-expressed with an unfolded YFP fragment, and MaABI5-like-YFPN and MaABI5-like-YFPC were co-expressed with an unfolded YFP fragment, which was reported in the previous work (Song et al. 2022). Moreover, the GST pull-down experiment was conducted using purified recombinant GST-MaCCCH33-like2 (Supplementary Fig. S7B) and His-MaABI5-like obtained in the previous work (Song et al. 2022). The results show that His-MaABI5-like could pull down GST-MaCCCH33-like2 (Fig. 4C).
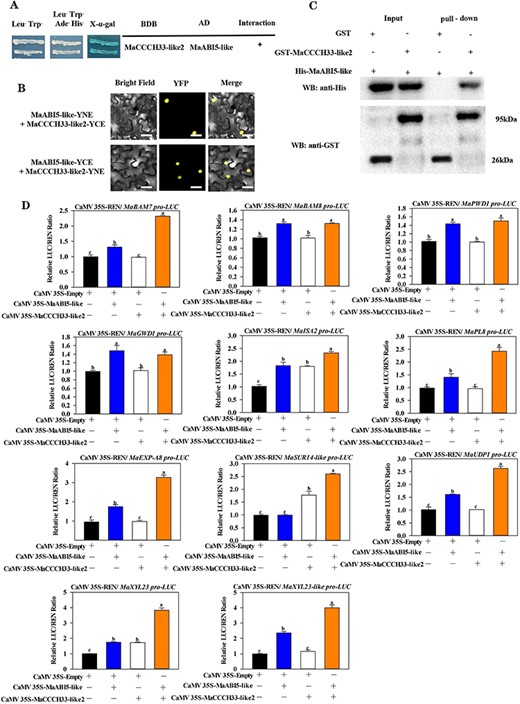
The interaction between MaCCCH33-like2 and MaABI5-like proteins and regulation on fruit softening–related genes. (A) The interaction between MaCCCH33-like2 and MaABI5-like was detected using the Y2H assay. The yeast cells that were genetically modified were cultivated on a medium called SD/-4 to facilitate the growth and observation of the interaction. (B) The BiFC technique demonstrated the interactions between MaCCCH33-like2 and MaABI5-like in tobacco leaves. MaCCCH33-like2 and MaABI5-like were genetically engineered to fuse with the N- and C-terminus of the YFP, respectively. As a negative control, the fusion protein (MaCCCH33-like2) was combined with the N- and C-terminus alone. YFP signals were detected using a fluorescence microscope, with a scale bar indicating a length of 50 μm. (C) The interaction between MaCCCH33-like2 and MaABI5-like was investigated through the GST pull-down assay performed in vitro. His-MaABI5-like protein was co-incubated with either GST-MaCCCH33-like2 or GST alone. Immunoprecipitated fractions were subsequently detected using specific antibodies targeting GST and His proteins. (D) The transcriptional activity of MaCCCH33-like2, MaABI5-like and the combination of MaABI5-like and MaCCCH33-like2 on the promoters of five starch-related genes and six cell wall degradation genes was evaluated. An empty vector served as a reference control, with its activity normalized and set as 1.
To test the effect of MaCCCH33-like2 interaction with MaABI5-like on the target genes, transient assays were constructed. As shown in Fig. 4D, when MaCCCH33-like2 and MaABI5-like were co-expressed, the promoter activities of MaBAM7, MaISA2, MaXYL23, MaPL8, MaSUR14-like, MaEXP-A8, MaUDP1 and MaXYL23-like was dramatically upregulated compared to transformations with MaABI5-like or MaCCCH33-like2 alone.
Next, GST-MaABI5-like and GST-MaCCCH33-like2 were hatched with MaBAM7, MaBAM8, MaPWD1, MaGWD1, MaPL8, MaEXP-A8, MaUDP1 and MaXYL23-like probes for the EMSA. The results demonstrated that GST-MaABI5-like alone binds to DNA, and the affinity of GST-MaABI5-like toward MaEXP-A8, MaBAM7, MaPL8, MaUDP1 and MaXYL23-like, but not MaBAM8, MaPWD1 and MaGWD1, was markedly strengthened with an increasing amount of GST-MaCCCH33-like2 (Fig. 5A). The binding signal of GST-MaABI5-like and GST-MaCCCH33-like2 to promoters of MaISA2 and MaXYL23 was markedly strengthened with an increasing amount of each protein (Fig. 5B). Moreover, GST-MaCCCH33-like2 alone binds to the promoter of MaSUR14-like, and the binding activity of GST-MaCCCH33-like2 was markedly improved with the increasing amount of GST-MaABI5-like (Fig. 5C). These data suggested that the interaction between MaABI5-like and MaCCCH33-like2 increases their binding to promoters of the corresponding target genes.
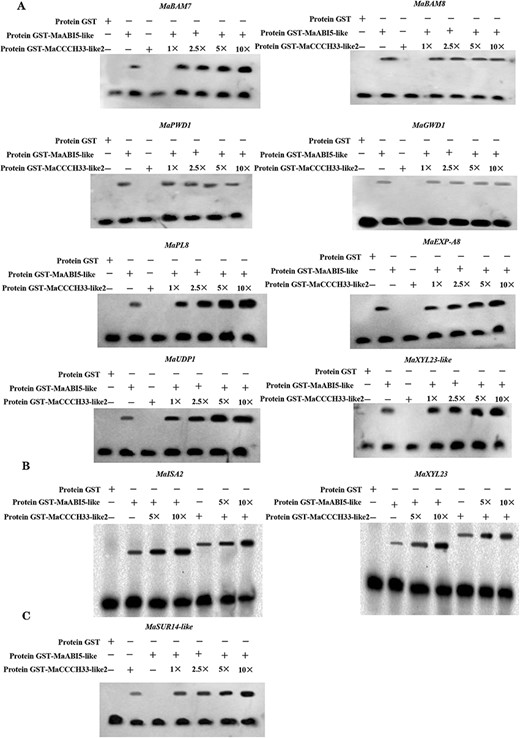
MaCCCH33-like2 and MaABI5-like increased the binding activity of MaABI5-like and MaCCCH33-like2 to the regulatory regions of genes involved in starch degradation and cell wall breakdown. (A) MaCCCH33-like2 enhanced the binding activity of MaABI5-like to the promoters of the corresponding target genes. (B) MaCCCH33-like2 and MaABI5-like mutually augmented their binding affinity to the MaISA2 and MaXYL23 promoters. (C) The presence of MaABI5-like protein increased the binding capacity of MaCCCH33-like2 to the MaSUR14-like promoters. The numerical values (1×, 2.5×, 5× and 10×) indicate the protein amounts (50, 125, 250 and 500 ng) of both MaCCCH33-like2 and MaABI5-like, respectively. The symbol ‘−’ represents absence, while ‘+’ represents presence.
Transient overexpression of MaCCCH33-like2 initiates the ripening process in ‘Fenjiao’ bananas
A transient overexpression experiment was utilized to investigate the role of MaCCCH33-like2 in controlling the softening and ripening of fruits. Transient overexpression of MaCCCH33-like2 in fruit showed high gene expression of MaCCCH33-like2 (Fig. 6A). ‘Fenjiao’ bananas overexpressing MaCCCH33-like2 showed accelerating fruit ripening, as the peel turned yellow rapidly compared to transformations with the empty vector (Fig. 6B). Ethylene production, fruit firmness decrease and color index were induced in MaCCCH33-like2–overexpressing fruit (Fig. 6C–F). Furthermore, the expression levels of MaABI5-like, MaEBF1, MaPWD1, MaBAM7/8, MaGWD1, MaISA2, MaEXP-A8, MaUDP1, MaSUR14-like, MaPL8, MaXYL23-like and MaXYL23 transcripts were found to be increased in fruit overexpressing MaCCCH33-like2 compared to the control group which had an empty vector (Fig. 6G–I).
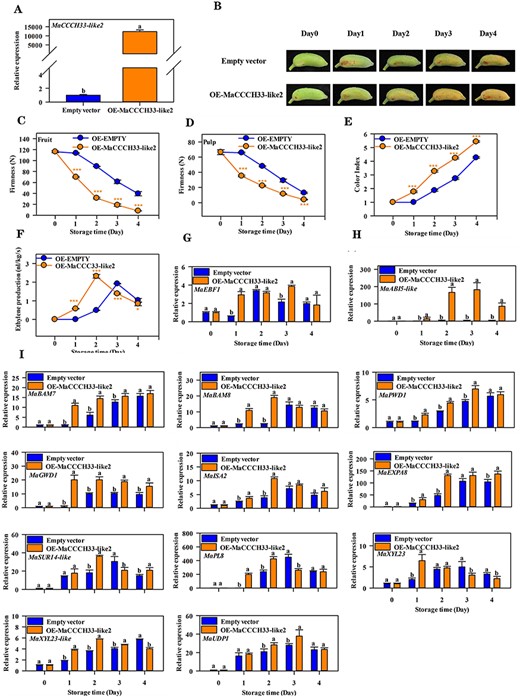
The impact of transiently overexpressing MaCCCH33-like2 in ‘Fenjiao’ banana fruit ripening. (A) The transcript levels of MaCCCH33-like2 were determined in both empty vector and MaCCCH33-like2-overexpressing fruit using RT-qPCR. The expression data for each gene at different time points are presented as a ratio relative to day 0 (freshly harvested fruit), which is set as 1. (B) The ripening phenotype of fruit from the empty vector and MaCCCH33-like2-overexpressing lines is depicted. Fruit firmness (C), pulp firmness (D), color index (E) and ethylene production (F) were monitored during the ripening process. For the transient overexpression experiment, the pretreated fruits were haphazardly divided into two groups of 100 fingers each, and the data and photos were collected from these treated fruits. The expression profiles of MaEBF1 (G), MaABI5-like (H) and genes associated with starch and cell wall degradation (I) were determined using RT-qPCR during fruit ripening. The expression data for each gene at different time points are presented as a ratio relative to day 0 (freshly harvested fruit), which is set as 1. Asterisks (***) indicate significant differences at the P < 0.001 level.
Suppressed expression of MaCCCH33-like2 inhibits the process of fruit ripening
To validate the function of MaCCCH33-like2, transient silencing of the gene was performed in ‘Fenjiao’ banana fruit using the virus-induced gene silencing (VIGS) assay. The expression of MaCCCH33-like2 was significantly reduced in the silencing fruit compared to the control group with an empty vector (Fig. 7A). The suppression of MaCCCH33-like2 led to delayed color development in ‘Fenjiao’ banana fruit when compared to the control group with an empty vector (Fig. 7B, C). In addition, the silencing of MaCCCH33-like2 resulted in the inhibition of ethylene production and a decrease in fruit firmness (Fig. 7D–F). Furthermore, the transcript levels of MaEBF1, MaABI5-like, MaPWD1, MaBAM7/8, MaGWD1, MaISA2, MaEXP-A8, MaSUR14-like, MaPL8, MaUDP1, MaXYL23 and MaXYL23-like were downregulated in the MaCCCH33-like2–silenced fruit compared to the control group with pTRV2-empty vector (Fig. 7G–I).
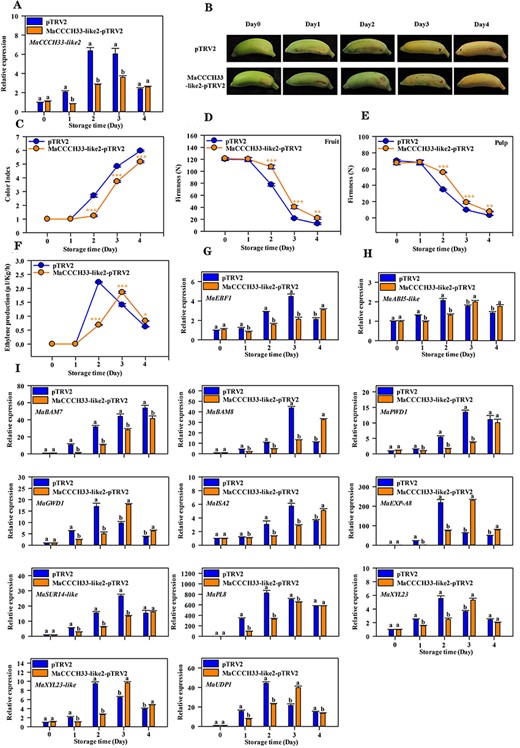
The repression of MaCCCH33-like2 hinders the ripening process of ‘Fenjiao’ banana fruit. (A) The transcript levels of MaCCCH33-like2 were determined in the pulp of ‘Fenjiao’ banana fruit with pTRV2 and MaCCCH33-like2-pTRV2 using RT-qPCR. (B) The ripening phenotype of fruit from the empty vector and MaCCCH33-like2-pTRV2 lines is shown. Changes in fruit color index (C), fruit firmness (D), pulp firmness (E) and ethylene production (F) during fruit ripening are illustrated. Asterisks (*, **, ***) indicate significant differences at the P < 0.05, P < 0.01 and P < 0.001 levels, respectively. Expression profiles of MaEBF1 (G), MaABI5-like (H) and genes associated with starch and cell wall degradation (I) during fruit ripening are presented. The transcription data for each gene at different time points are presented as a ratio relative to day 0, which is set as 1. Different letters represent significant differences(P < 0.05).
Overexpression or reduced expression of MaCCCH33-like2 in banana alters the interaction of MaABI5-like with the target genes
As we proved previously by the EMSA, the interaction between MaCCCH33-like2 and MaABI5-like enhanced their binding to promoters of the corresponding target genes (Fig. 5). In order to further confirm the collaborative role of MaABI5-like and MaCCCH33-like2 in regulating the expression of target genes, chromatin immunoprecipitation followed by qPCR (ChIP-qPCR) assays were performed using samples from fruit with transient overexpression and VIGS silencing. As depicted in Fig. 8A, the overexpression of MaCCCH33-like2 markedly strengthens the binding activity of MaABI5-like to the promoters of MaBAM7/8, MaPWD1, MaISA2, MaGWD1, MaSUR14-like, MaEXP-A8, MaPL8, MaUDP1 and MaXYL23-like. Conversely, the silencing of MaCCCH33-like2 substantially attenuated the binding capacity of MaABI5-like to the promoters of the corresponding target genes (Fig. 8B).
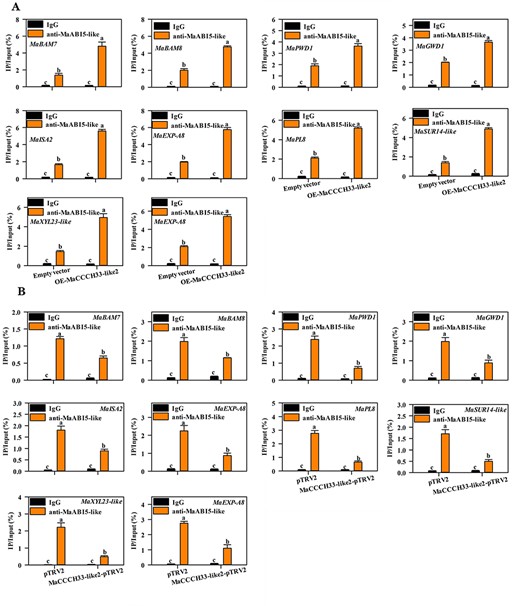
The effect of MaCCCH33-like2 overexpression or reduced expression on the binding of MaABI5-like to the promoters of MaBAM7, MaBAM8, MaPWD1, MaGWD1, MaISA2, MaPL8, MaEXP-A8, MaSUR14-like, MaUDP1, MaXYL23 and MaXYL23-like by ChIP-qPCR. Different letters represent significant differences (P < 0.05).
Overexpression of MaCCCH33-like2 in tomatoes accelerates fruit ripening
The function of MaCCCH33-like2 in fruit ripening was further demonstrated by ectopic overexpression in Micro-Tom tomatoes. Different independent transgenic lines (>10) showed a similar phenotype. Of these, three different transgenic lines (OC1, OC2 and OC3) with a relatively high expression of MaCCCH33-like2 were used for further studies (Fig. 9A, B). All transgenic lines showed accelerated fruit ripening (Fig. 9C, D). The ripening differences were observed between MaCCCH33-like2-OE lines and wild-type (WT) fruit at 32 d post-anthesis (dpa) when the MaCCCH33-like2-OE lines turned to the breaker (BR), but the WT fruit stayed at the green stage (Fig. 9D). The ripening period from the BR to the full ripening stage was promoted about 3 d as compared with WT lines (Fig. 9C). In addition, MaCCCH33-like2-OE lines induced the decrease in firmness (Fig. 9E), ethylene production and color index as compared to WT lines (Fig. 9F, G).
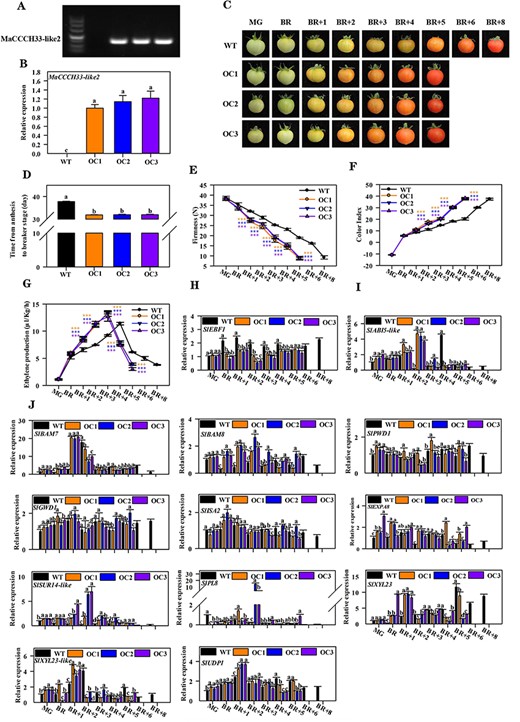
Overexpression of MaCCCH33-like2 in tomatoes accelerates fruit ripening and softening. The transcription of MaCCCH33-like2 in the fruit of WT and MaCCCH33-like2-overexpressing lines (OC1, OC2 and OC3) was detected by RT-PCR (A) and RT-qPCR (B). Experiments were conducted using pericarp tissues from MaCCCH33-like2-overexpressing and WT fruit at 30 dpas. The gene expression profiles were normalized using the reference gene SlUBI and further normalized against OC1 expression, which was set as 1. (C) The fruit ripening phenotype of WT and MaCCCH33-like2-overexpressing lines is shown. (D) The days from anthesis to the BR stage in WT and MaCCCH33-like2-overexpressing lines are presented. Changes in fruit firmness (E), color index (F) and ethylene release rate (G) during fruit ripening in WT and MaCCCH33-like2-overexpressing lines are illustrated. Asterisks (***) indicate significant differences at the P < 0.001 level. The expression profiles of SlEBF1 (H), SlABI5-like (I) and genes associated with cell wall and starch degradation (J) during fruit ripening in tomatoes are shown. Different letters represent significant differences (P < 0.05).
The expression of SlEBF1 was repressed in MaCCCH33-like2 overexpression lines compared with the WT (Fig. 9I). In contrast, the expression of SlABI5-like and genes related to the cell wall and starch degradation, including SlPWD1, SlBAM7/8, SlGWD1, SlISA2, SlEXP-A8, SlPL8, SlSUR14-like, SlUDP1, SlXYL23 and SlXYL23-like, were upregulated in MaCCCH33-like2-overexpressing lines compared with WT (Fig. 9I, J).
MaABI5-like controls the expression of MaCCCH33-like2
The overexpression of MaCCCH33-like2 and MaABI5-like resulted in a significant increase in the transcript level of MaABI5-like (Fig. 9H) and MaCCCH33-like2 (Fig. 10A) in ‘Fenjiao’ fruit and SlABI5-like (Fig. 9I) and SlCCCH33-like2 (Fig. 10B) in tomato fruit, respectively, indicating that MaABI5-like and MaCCCH33-like2 may regulate each other’s expression. To validate this hypothesis, the promoter regions of MaCCCH33-like2 and MaABI5-like were constructed into the pGreenII 0800-LUC vector. As shown in Fig. 10C and D, MaABI5-like and MaCCCH33-like2 can directly bind to each other’s promoters. Moreover, MaABI5-like increases the promoter activity of MaCCCH33-like2 (Fig. 10E), but MaABI5-like is not regulated by MaCCCH33-like2 (Fig. 10F).
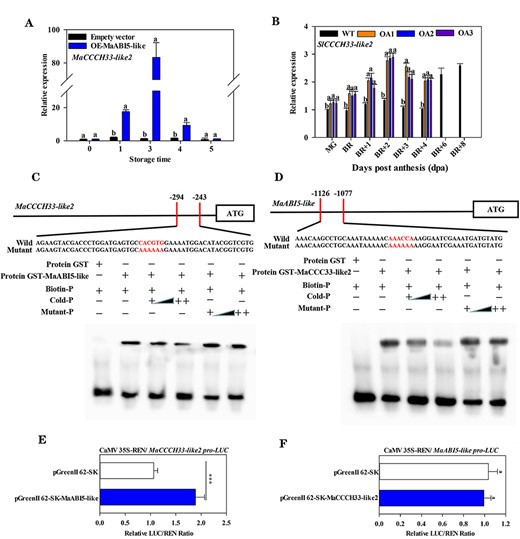
The regulatory relationship between MaCCCH33-like2 and MaABI5-like. The expression of MaCCCH33-like2 in the MaABI5-like transient overexpression banana fruit (A) and the expression of SlCCCH33-like2 in MaABI5-like ectopic overexpression tomato fruit (B) are shown. The expression data for each gene at different time points are presented as a ratio relative to day 0 (freshly harvested fruit) or the mature green (MG) stage of WT fruit, set as 1. (C) The binding activity of MaABI5-like to the promoters of MaCCCH33-like2 was examined using EMSA. The GST-MaABI5-like protein was mixed with the MaCCCH33-like2 promoter probes containing the ABRE/G-box-binding site, along with mutant probes (AAAAAA) indicated in red letters. (D) The GST-MaCCCH33-like2 protein was mixed with the MaABI5-like promoter probes containing the ARE-binding site, along with mutant probes (AAAAAA) indicated in red letters. ‘+’ represents the presence, and ‘−’ represents the absence of binding. ‘++’ indicates increasing amounts of probes. (E) The in vivo interaction between MaABI5-like and the promoters of MaCCCH33-like2 was observed through transient assays in tobacco leaves. (F) The in vivo interaction between MaCCCH33-like2 and the promoters of MaABI5-like was detected through transient assays in tobacco leaves. An empty vector was set as a reference (set as 1). Asterisks (***) represent significant differences (P < 0.001).
Discussion
Fruit softening is a key index for the assessment of the ripeness and edible quality of fruits such as bananas (Luo et al. 2015, Song et al. 2022). The coordinated degradation regulation of starch and cell wall is an important issue during banana fruit softening (Shiga et al. 2011). Our previous work found that the genes involved in starch degradation and cell wall metabolism pathways were significantly enriched during the fruit ripening process and significantly affected by 1-MCP treatment (Song et al. 2020, Zhu et al. 2020). The expression of a group of key genes in starch and cell wall metabolism was repressed by 1-MCP treatment. Our previous study also found that the total content of starch, amylose and amylopectin; the activity of amylase and 11 starch degradation genes were markedly induced by ethylene in the ‘Fenjiao’ banana (Song et al. 2019). Moreover, the activities of pectate lyase, pectin methyl-esterase, polygalacturonase and the transcript level of 12 cell wall degradation genes were inhibited by chilling stress, preventing the decrease in banana fruit firmness (Song et al. 2022). In general, the fruit softening indicated the starch and cell wall degradation, resulting from the regulation of these key genes in starch and cell wall degradation pathways.
CCCH-type zinc finger proteins are widely identified in eukaryotes, which are critical in modulating plant development, growth and abiotic and biotic stress responses (Bogamuwa and Jang 2014). Many of them bind to RNA sequences and play crucial roles in the RNA metabolism process (Lai and Blackshear 2001, Qu et al. 2014, Zhang et al. 2018b), and a group of CCCH zinc finger proteins act as TFs to bind to DNA and modulate the transcript level of target genes (Zhang et al. 2019, Li et al. 2022). In Arabidopsis , AtC3H17 (a CCCH TF) affects seed development by modulating the transcript level of Oleo1, Oleo2 and CRU3 (Seok et al. 2016). IbC3H18 (a CCCH TF) enhances drought and salt tolerance by cooperating with IbPR5 in tomatoes (Zhang et al. 2019). In rice, overexpressing OsDOS represses the leaf senescence (Kong et al. 2006). Moreover, overexpression of PvC3H72, a zinc finger-CCCH gene in switchgrass, enhances its cold tolerance by regulating ABA-responsive genes (Xie et al. 2019). In this study, it was observed that the expression of MaCCCH33-like2 was significantly stimulated by ethylene and suppressed by treatment with 1-MCP. Furthermore, MaCCCH33-like2 was shown to directly bind and activate the promoters’ activity of starch and cell wall degradation–related genes, thereby influencing the expression levels of these target genes (Fig. 1 and 2). Consistently, MaCCCH33-like2 overexpression in ‘Fenjiao’ banana fruit significantly induced the expression of genes associated with cell wall and starch degradation (Fig. 6). MaCCCH33-like2 silencing in ‘Fenjiao’ banana fruit significantly inhibited the expression of genes related to cell wall and starch degradation (Fig. 7). Furthermore, in tomato plants, the overexpression of MaCCCH33-like2 led to a significant increase in the color index, firmness decline, ethylene production and the expression levels of genes in the cell wall and starch degradation pathways when compared to the WT line (Fig. 9). Taken together, these data indicate that MaCCCH33-like2 functions as a vital TF and a positive factor in fruit ripening and promotes fruit ripening and softening.
EIN3 binding F-Box (EBF) proteins are essential regulators in the ethylene signal pathway. Several previous studies showed that EBFs are involved in regulating fruit ripening. For instance, the ripening and softening of tomatoes were repressed/induced in EBF overexpression/knockout mutants (Yang et al. 2010, Deng et al. 2018, Guo et al. 2018). Nevertheless, despite not being a TF protein, EBF1 was found to have the ability to regulate the transcript levels of certain genes involved in cell wall and starch degradation (Ding et al. 2019, Song et al. 2022). EBF1 might work with other TFs as a transcriptional cooperator to modulate the transcript level by interacting with proteins affecting fruit ripening, as reported in apple, where MdEBF1/2 interacts with MdEIL1-3 to repress the expression of MdPG1 and thereby delays fruit softening (Tacken et al. 2012). Moreover, in papaya fruit, it has been shown that CpEBF1 cooperates with CpMADS1 and CpEIL1 in modulating the transcript level of genes related to cell wall degradation (Ding et al. 2019, Zhang et al. 2020). Our previous research findings suggested a close association between MaEBF1 and fruit softening in ‘Fenjiao’ bananas. We found that MaEBF1 regulates genes involved in cell wall and starch degradation through interactions with MaABI5-like and MaNAC67-like (Song et al. 2019, 2022). In this study, our data demonstrated that MaEBF1 collaborates with MaCCCH33-like2 both in vivo and in vitro, as illustrated in Fig. 3A–C. Furthermore, we observed that the interaction between MaCCCH33-like2 and MaEBF1 leads to the activation of the transcript levels of genes involved in cell wall and starch degradation, as depicted in Fig. 3D, E.
Our previous research findings also revealed a collaborative relationship between MaABI5-like and MaEBF1. Additionally, the overexpression of MaEBF1 and MaABI5-like in both tomato and ‘Fenjiao’ banana fruit was shown to expedite the process of fruit ripening (Song et al. 2022). In banana, MaABI5 cooperates with MaC3HC4-1 (a RING E3 ubiquitin ligase) and participates in ABA-independent cold tolerance (Chen et al. 2018). In this study, we discovered that MaCCCH33-like2 interacts with MaABI5-like and effectively stimulates the expression of genes involved in cell wall and starch degradation (Figs. 4, 5). Moreover, MaCCCH33-like2 overexpression in the ‘Fenjiao’ banana and tomato upregulated the transcript of MaABI5-like and SlABI5-like (Fig. 6H, 9I), and overexpression of MaABI5-like and MaEBF1 in ‘Fenjiao’ banana and tomato fruit upregulated the transcription level of MaCCCH33-like2 and SlCCCH33-like2 (Fig. 10A, B, and Supplementary Fig. S8). Additionally, we were able to show that MaABI5-like activates the expression of MaCCCH33-like2 by directly binding and regulating its promoter (Fig. 10C, E). These data indicate the cooperation of MaCCCH33-like2, MaABI5-like and MaEBF1, in coregulating ‘Fenjiao’ banana fruit softening and ripening. As shown in Supplementary Fig. S5, the promoter of MaCCCH33-like2 contained 35 motifs, such as ABRE, G-box, MYB binding site (MBS) and W-box motif. Some previous studies found that TFs could bind to the ABRE, G-box, MBS and W-box motif, participating in fruit softening. For example, MaABI5-like bound to the ABRE and G-box motif of cell wall and starch degradation genes promoted fruit ripening (Song et al. 2022). MaWRKY49 upregulated the expression of two pectate lyase (PL) genes MaPL3 and MaPL11 by interacting with their promoters, participating in banana ripening (Liu et al. 2023). CpMYB1 and CpMYB2 directly bound to the promoter of CpPME1, CpPME2 and CpPG5 by binding to the MBS motif in their promoters, participating in papaya fruit ripening (Fu et al. 2020). This information indicates that WRKYs, MYBs and other TFs may modulate the transcript level of MaCCCH33-like2 by interacting with MBS and W-box, participating in fruit ripening.
We found that ethylene is a key player in inducing the expression of MaCCCH33-like2 during the ripening process (Fig. 1E). Ethylene production was markedly induced by ethephon treatment but significantly repressed in 1-MCP treatment fruit. The overexpression of MaCCCH33-like2 in tomato and ‘Fenjiao’ banana fruit increased the ethylene production, and the reduced expression of MaCCCH33-like2 by VIGS in ‘Fenjiao’ banana inhibited the ethylene production (Figs. 6, 7, 9). We suppose that the expression of MaCCCH33-like2 could respond to ethylene and regulate the ripening process. However, how ethylene regulates the expression of MaCCCH33-like2 needs further investigation.
The transcriptional capacity of TFs is accurately regulated by many factors, such as posttranslational modification, redox-based regulation, protein–protein interaction and homo-/heterodimerization (Shaikhali 2015). For instance, in rice, although OsWRKY51 alone cannot bind to the Amy32b promoter, the cooperation of OsWRKY71 with OsWRKY51 enables the binding (Zhen et al. 2010). Similar results have also been found by Fu et al. (2017) and Wu et al. (2019). EBF1 is not a TF, so it is uncertain if it can bind the promoters of the corresponding target genes. However, it may interact with other TFs to regulate their activity. Multiple studies have demonstrated that the transient overexpression of non-TFs, such as CpEBF1 in papaya, MaMPK2 in banana fruit and OsMFT2 in rice, can independently regulate the expression of target genes (Ding et al. 2019, Wu et al. 2019, Song et al. 2022). In our current investigation, we have identified that MaEBF1 and MaCCCH33-like2 possess the ability to function as coactivator proteins, thereby augmenting the DNA-binding capacity of their interacting partner, such as MaABI5-like. This cooperative action influences the transcriptional regulation of target genes involved in cell wall and starch degradation. Furthermore, EMSAs conducted in this study have demonstrated that coactivator proteins, such as MaEBF1 and MaCCCH33-like2, can enhance the DNA-binding capacity of MaABI5-like although no super-shift band was observed, indicating an unstable binding to the protein complex (refer to Figs. 3, 5). Similar findings have been reported in previous studies, suggesting that coactivator proteins can enhance the DNA-binding capacity of target DNA without establishing stable binding to the protein complex (Song et al. 2020, 2023, An et al. 2021, Bai et al. 2022).
Based on our data, we propose a novel module of MaCCCH33-like2/MaEBF1/MaABI5-like in modulating ‘Fenjiao’ banana ripening and softening (Fig. 11). In Fig. 11, it is evident that the expression of MaCCCH33-like2, MaEBF1 and MaABI5-like undergoes a significant increase during fruit ripening and is induced by ethylene treatment. Both MaCCCH33-like2 and MaABI5-like play crucial roles in initiating and upregulating the expression of genes involved in starch and cell wall degradation by directly regulating the activities of the promoters of these genes. Moreover, the interaction of MaEBF1 with MaCCCH33-like2 and MaABI5-like enhances their activation of genes involved in starch and cell wall degradation pathways, thereby facilitating fruit softening and ripening. Furthermore, MaABI5-like also regulates the expression of MaCCCH33-like2, thereby contributing to the regulation of the fruit softening process.
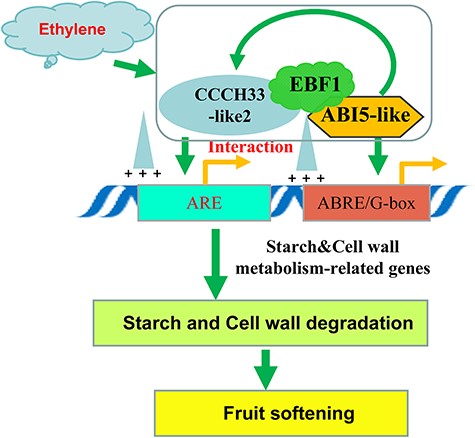
The proposed working model of MaCCCH33-like2/MaEBF1/MaABI5-like in modulating ‘Fenjiao’ banana ripening and softening. During fruit ripening, the expression levels of MaCCCH33-like2, MaEBF1 and MaABI5-like exhibit a significant increase, which is further induced by ethylene treatment. MaCCCH33-like2 and MaABI5-like play a crucial role in initiating and upregulating the transcription of genes involved in cell wall and starch degradation by directly binding to the promoters of these genes. Additionally, the interaction of MaEBF1 with MaCCCH33-like2 and MaABI5-like enhances their ability to activate genes involved in cell wall and starch degradation, thereby promoting fruit softening and ripening. Moreover, MaABI5-like also has the capability to regulate the transcription of MaCCCH33-like2, which further contributes to the regulation of fruit ripening.
Materials and Methods
Plant materials, growth conditions and treatments
Bananas of the ‘Fenjiao’ cultivar (Musa ABB Pisang Awak cv. ‘Guangfen NO.1’) were obtained from a local farm in Guangzhou city, China, when they reached commercial maturation, indicated by approximately 85–90% plumpness. The fruit treatment procedure followed the protocol described by Song et al. (2019). The pretreated banana fruits were then randomly divided into three groups for different treatments. Group 1 bananas, serving as the control group, were placed at a temperature of 25°C for natural ripening. Group 2 bananas were immersed in a solution of ethephon (1,000 μl · L−1) for 1 min and then stored at 25°C for ripening. Group 3 bananas were subjected to fumigation with 400 nl · L−1 (1-MCP400) for 1 h in a sealed box and subsequently stored at 25°C for ripening. After 5 d of storage, the 1-MCP-treated fruit were treated by ethephon as group 2 and then put at 25°C for ripening.
Tobacco (Nicotiana benthamiana) plant growth and Agrobacterium tumefaciens–mediated transient expression assays were performed according to Song et al. (2022). Tomato cv. Micro-Tom plant growth and genetic transformation were performed according to Song et al. (2022).
Measurements of physiological parameters
The fruit firmness and ethylene production of ‘Fenjiao’ bananas and tomatoes were assessed following the methods established by Hu et al. (2015) and Deng et al. (2018). The color index of tomatoes was evaluated according to López and Gómez (2004). The color index of ‘Fenjiao’ bananas was measured using the procedures by Hu et al. (2015). The duration between anthesis and the onset of the BR ripening stage in tomatoes was determined using the method described by Deng et al. (2018).
Gene expression analysis
The gene expression analysis was performed according to Song et al. (2019). Tomato SlUBI and SlACTIN (Huang et al. 2016) and banana MaACTIN (Chen et al. 2011) and MaRPS2 (ribosomal protein 2) were used as reference genes.
BiFC test and subcellular localization analysis
The complete coding sequence (CDS) of MaCCCH33-like2, MaABI5-like and MaEBF1, excluding the stop codon, was inserted into the appropriate vectors, such as the PEAQ vector and pBIFC vectors. These constructs were introduced into A. tumefaciens and then infiltrated into the leaves of N. benthamiana plants for transient expression. The Confocal Spectral Microscope Imaging System (Leica TCS SP5, Leica Microsystems, Wetzlar, Germany) was used to detect the fluorescence signals of GFP and YFP according to Ding et al. (2019).
Yeast two-hybrid assays
The yeast two-hybrid (Y2H) assay was performed according to Song et al. (2019). The CDS of MaCCCH33-like2, MaEBF1 and MaABI5-like was cloned into pGBKT7 and pGADT7 vectors, respectively. MaCCCH33-like2+MaEBF1 and MaCCCH33-like2+MaABI5-like were cotransformed into yeast strains to confirm their interaction. Positive and negative controls were established, where P53+T-antigen served as the positive control, and Lamin+T-antigen served as the negative control.
GST pull-down assays
In our previous study, we successfully obtained GST-MaEBF1 and His-MaABI5-like proteins (Song et al. 2019, 2022). The construction of GST-MaCCCH33-like2 and His-MaCCCH33-like2 was introduced into BM Rosetta (DE3) cells. The expression of recombinant proteins GST-MaCCCH33-like2 and His-MaCCCH33-like2 was induced using 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG, catalog no. 9030, TAKARA, Tokyo, Japan) at 28℃ and 20℃ for 6 h, respectively. Subsequently, GST-MaCCCH33-like2 and His-MaCCCH33-like2 were purified using the GST Purification Kit (Clontech, California, USA, catalog no. 635619) and Ni-NTA His Purification Kit (Clontech, catalog no. 635658), respectively. The GST pull-down assays were performed following the protocol by Song et al. (2019).
DLR assays
The promoter sequences of 12 cell wall degradation genes and 11 starch genes were incorporated into the pGreenII 0800-LUC vector as reporter constructs in the previous work. Additionally, the full-length sequences of MaEBF1 and MaABI5-like were inserted into the pGreenII 62-SK vector as effector constructs (Song et al. 2019, 2022). In this study, we inserted the full CDS of MaCCCH33-like2 into the pGreenII 62-SK vector. The resulting construct was then coinfiltrated with the effector and reporter constructs into N. benthamiana leaves. The DLR assay was performed following the protocol described by Song et al. (2019). The resulting Luciferase/Renilla (LUC/REN) ratio was determined from at least six biological replicates for each experiment.
Y1H library assays
The Y1H assay was performed according to Song et al. (2019). The promoters of MaISA2, MaSUR14-like and MaXYL23 were inserted into the pAbAi vector as bait. Subsequently, the resulting vectors were linearized and transferred into the Y1H Gold strain. AD-MaCCCH33-like2 (integrated into the pGADT7 vector as mentioned earlier) was then introduced into the yeast strain containing the bait-reporter system.
Electrophoretic mobility shift assay
The GST-MaCCCH33-like2 protein was acquired using the method described earlier. Probes containing the specific cis-elements (AREs) of MaCCCH33-like2 from the promoters of genes involved in cell wall and starch degradation were synthesized and labeled with the PierceTM Biotin 3ʹ End DNA Labeling Kit (Thermo Scientific, Massachusetts, USA, catalog no. 89818). The EMSA was performed following the protocol described by Song et al. (2022).
Transient overexpression assays in ‘Fenjiao’ banana
The construction of MaCCCH33-like2-pMDC32 was introduced into the A. tumefaciens strain GV3101 and infiltrated into ‘Fenjiao’ banana fruit for transient overexpression analysis, as performed by Song et al. (2022). The fruits were stored at a temperature of 20℃ and a relative humidity of 90% for a duration of 4 d. Ethylene production, fruit firmness, color index and transcript levels were assessed on days 0, 1, 2, 3 and 4 of the storage periods.
VIGS in ‘Fenjiao’ banana fruit
A 300 bp specific fragment of MaCCCH33-like2 was cloned and incorporated into the pTRV2 vector. The resulting constructs, MaCCCH33-like2-pTRV2 and pTRV2-empty, were introduced into an A. tumefaciens strain. ‘Fenjiao’ banana fruits were prepared following the methods mentioned earlier and randomly assigned to two groups. The pTRV2-empty and MaCCCH33-like2-pTRV2 A. tumefaciens strains, in combination with pTRV1 A. tumefaciens at a ratio of 1:3, were separately introduced into the fruits of the respective groups. Subsequently, all fruits were stored at a temperature of 22℃ for a duration of 4 d. Ethylene production, color index and transcript levels were measured on days 0, 1, 2, 3 and 4.
ChIP-qPCR assay
The ChIP-qPCR experiment was performed according to Song et al. (2022). The MaCCCH33-like2-overexpressing, MaCCCH33-like2-silencing and corresponding empty vector ‘Fenjiao’ banana fruit pulp were used for crosslink genomic DNA and proteins. MaABI5-like antibody was used to perform immunoprecipitation, which resulted in the cross-linking of DNA fragments with an average length of 500 bp. This process was carried out for 12 h at 4℃ with rotation. Negative controls were established by performing the procedure without the antibody or using IgG instead. The DNA–protein–antibody complex was then bound to protein A/agarose beads. Finally, the precipitated DNA fragment was detected and quantified using RT-qPCR. The primers used were according to Song et al. (2022).
Tomato transformation
The A. tumefaciens strain GV3101 carrying the MaCCCH33-like2-pMDC32 construct, which was obtained previously, was used for transformation. Tomato (Solanum lycopersicum L. cv. Micro-Tom) seedlings were used as the transformation material, according to Song et al. (2022). Transgenic plants with positive expression of MaCCCH33-like2 were identified by assessing the transcript levels. From these, three separate homozygous lines (T2) were chosen for further analysis of phenotypic characteristics. The ethylene production, color index and firmness of both WT tomato fruit and the MaCCCH33-like2 overexpression lines were measured at different mature stages, including mature green (MG) stage, BR stage, BR + 1 d, BR + 2 d, BR + 3 d, BR + 4 d and BR + 5 d.
Statistical analysis
The experimental data are displayed as the mean ± standard deviation (SD). All experiments were conducted using three or six independent biological replicates. Statistical significance between treatments and the control was analyzed using ANOVA, followed by Duncan’s multiple range test. Primer sequences used in all experiments are presented in Supplementary Table S1.
Supplementary Data
Supplementary Data are available at PCP online.
Data Availability
All data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
National Key Research and Development Program of China (2022YFD2100102); National Natural Science Foundation of China (32202554, 31372112); earmarked fund for CARS (CARS-31); Guangdong Banana and Pineapple Industry Technology System Innovation Team (2022KJ109); Natural Science Foundation of Guangdong Province (2023A1515010335); Pearl River Talent Program for Young Talent (2017GC010321).
Author Contributions
X.Z., W.C. and X.L. conceived and designed the experiment; Z.S., X.L., Y.Y., H.C. and J.Q. performed the experiments; X.Z. and Z.S. carried out the analysis; Z.S. and X.Z. wrote the manuscript; X.L., W.C., X.P. and H.Z. revised the manuscript and all the authors read and approved the final manuscript.
Disclosures
The authors have no conflicts of interest to declare.



