-
PDF
- Split View
-
Views
-
Cite
Cite
Wenxin Xu, Sai V Vemula, Samuel M Niman, Xiaowen Liu, Ayumi Takakura, Zimo Huang, Toni K Choueiri, Matthew L Freedman, Paul J Catalano, Joseph V Bonventre, Saurabh Gupta, David F McDermott, Rupal S Bhatt, Circulating KIM-1 is a minimally invasive biomarker correlated with treatment response to nivolumab in patients with metastatic renal cell carcinoma, The Oncologist, Volume 28, Issue Supplement_1, September 2023, Page S2, https://doi.org/10.1093/oncolo/oyad216.004
Close - Share Icon Share
Abstract
There are currently no circulating biomarkers used for clinical monitoring of clear cell renal cell carcinoma (ccRCC). Such a biomarker could facilitate individualized treatment decisions and minimize exposure to ineffective therapies. Prior studies have suggested that circulating KIM-1 is a potential minimally invasive biomarker for ccRCC, but the utility of KIM-1 for identifying early response to nivolumab therapy is not known.
CheckMate-009 was a prospective trial investigating nivolumab (every 3 weeks at 0.3, 2, or 10 mg/kg) in patients with metastatic clear cell RCC. We measured serum KIM-1 at baseline and after 3 weeks of treatment (prior to cycle 2) using a custom sandwich immunoassay using the R-PLEX platform. Human KIM-1 antibody (R&D systems, #AF1750) was used to prepare biotin conjugated antibodies and detection antibodies. The assay lowest limit of detection for KIM-1 was 4.88 pg/mL. We assessed the association between early changes in serum KIM-1 and treatment related clinical outcomes.
Clinical data and serum KIM-1 was analyzed in 54 patients. KIM-1 was high in all patients at baseline (median serum KIM-1 5913 pg/mL, IQR 2137-25101 pg/mL). 25 patients (48%) had a decrease in KIM-1 at 3 weeks after a single dose of nivolumab. Decrease in KIM-1 at 3 weeks was associated with improved PFS (univariable HR 0.26, 95% CI 0.13-0.52; multivariable HR 0.22, 95% CI 0.097-0.50 after adjustment for sex, prior nephrectomy, nivolumab dose, and IMDC risk factors).
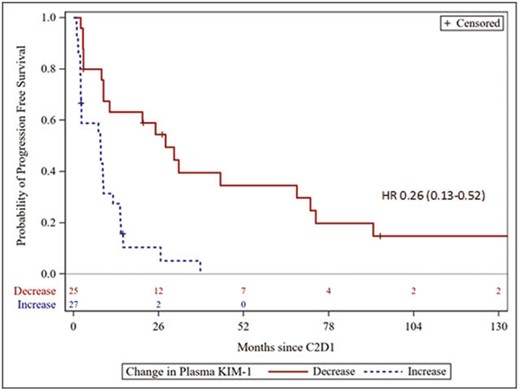
Serum KIM-1 is elevated in patients with metastatic ccRCC and is associated with clinical outcomes. Among patients treated with nivolumab in the CheckMate-009 trial, early decrease in KIM-1 from baseline to 3 weeks was predictive for PFS.
CDMRP DOD Funding: yes



