-
PDF
- Split View
-
Views
-
Cite
Cite
Melika Shirdarreh, Orly Aziza, Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner, Patients’ and Oncologists’ Knowledge and Expectations Regarding Tumor Multigene Next-Generation Sequencing: A Narrative Review, The Oncologist, Volume 26, Issue 8, August 2021, Pages e1359–e1371, https://doi.org/10.1002/onco.13783
Close - Share Icon Share
Abstract
Tumor multigene next-generation sequencing (NGS) is increasingly being offered to cancer patients to guide clinical management and determine eligibility for clinical trials. We undertook a review of studies examining the knowledge and attitudes of patients and oncologists regarding the primary results and potential secondary findings of such testing.
A search was conducted through the MEDLINE database using the following keywords: “neoplasms” and “molecular sequencing / genome sequencing / tumor profiling / NGS / whole exome sequencing” and “patient / oncologist” and “knowledge / attitudes / satisfaction / experience / evaluation / perspective / practice / preference.” Articles meeting the inclusion criteria and additional relevant articles from their references were selected.
From 1,142 publications identified by the search and 9 from references, 21 publications were included in the final review. Patients generally had positive attitudes toward tumor NGS despite relatively little knowledge of test-related genetics concepts, but their expectations often exceeded the reality of low clinical utility. Patients with higher education and greater genetics knowledge had more realistic expectations and a more altruistic view of the role of NGS. Attitudes toward disclosure of secondary findings were highly variable. Oncologists had poor to moderate genomic literacy; they communicated challenges with tempering patient expectations and deciding what information to disclose.
Patients considering undergoing tumor NGS should be provided with easily understandable resources explaining the procedure, goals, and probable outcomes, whenever possible based on evidence-based guidelines. Continuing medical education programs on this topic for oncology health care professionals should strive to improve their genomic literacy and instruct them on how to optimally present this information to their patients.
Oncologists are increasingly offering tumor multigene testing to patients with advanced cancers to guide more “personalized” treatment and/or determine eligibility for clinical trials. However, patients often have inadequate understanding and unrealistic expectations. Oncologists must ensure that they themselves have sufficient knowledge of the benefits and limitations of testing and must provide their patients with appropriate educational resources. Prior to testing, patients should be told the likelihood of finding a mutation in their specific tumor type for which a targeted treatment or clinical trial is available. Patients also need clear information about the possibility and implications of secondary findings.
Introduction
In the past few years, technological advances have allowed for broad uptake of tumor genetic profiling for patients with various malignancies. Whole exome and whole genome next-generation sequencing (NGS) can now be performed rapidly and at relatively low cost. In oncology, this has provided more opportunities for patients to receive anticancer therapies targeted to particular genomic alterations but has also led to the discovery of incidental potentially germline mutations requiring further investigation.
Germline mutations are inherited from a parental gamete, are present in every cell of the body, and will be passed on to the future generation. Somatic mutations, on the other hand, occur in a single somatic cell and are neither inherited nor transmitted. Clinical testing for specific germline mutations that predispose to late-onset diseases, such as Huntington's chorea or cancer, has been ongoing for several decades with the primary aim of determining disease risk to enable prevention and screening (if possible) and life planning (if relevant). In the past, germline testing was generally performed in the context of a family history of a specific disease. However, more recently, NGS to screen multiple genes for pathogenic germline mutations has frequently been offered to subgroups of patients with a personal history of cancer, even in the absence of a family history. This has made the interpretation of findings more difficult. Disclosure of test results has been further complicated by the frequent discovery of genetic variants of uncertain significance with respect to their pathogenicity.
Somatic testing of tumor tissue to evaluate overexpression of certain nonmutated genes, specific single mutations, or a specific cluster of mutations has also been performed for several decades for cancer diagnosis, classification, prognostication, and tumor site–specific targeted treatment [1]. Well-known examples include Oncotype DX for prognosis and prediction of chemosensitivity for breast cancer [2] and HER2 (breast cancer) [3], EGFR (lung cancer) [4], and BRAF (melanoma) [4] for targeted treatment. More recently, tumor multigene NGS is being performed to look for mutations that are not necessarily tumor site specific, with the goal of finding one or more “actionable mutations” for which there might be a targeted drug. At present, such testing may be performed in a research setting or requested by the patient and performed by a private company at the patient's expense. Although tumor multigene NGS holds great promise, the clinical benefits may be overestimated by patients and oncologists alike, leading to unrealistic expectations. Moreover, neither patients nor their health care providers may be aware that tumor NGS can reveal incidental findings such as tumor prognostic information, germline mutations predisposing to cancer or to noncancerous conditions, or germline variants of uncertain significance. These secondary findings may have unexpected implications, not only for the patient but for his or her family. The European Society for Medical Oncology has recently released guidelines for the use of NGS for patients with metastatic cancer in order to help reduce some of the uncertainty surrounding such testing [5].
Compared with germline testing for cancer risk prediction, the relatively new phenomenon of tumor NGS has been much less studied with respect to patient and physician knowledge and attitudes. To the best of our knowledge, this is the first review to date of studies focusing on patient and/or physicians’ knowledge and attitudes regarding tumor multigene NGS with potential therapeutic intent.
Materials and Methods
Data Sources
A narrative review was conducted based on the guidelines outlined in the PRISMA Statement [6] using the MEDLINE database (January 1946 to June 2020) (Fig. 1). The search was limited to English language. The following search terms were used: neoplasms, molecular sequencing, genome sequencing, tumor profiling, next-generation sequencing, whole genome sequencing, whole exome sequencing, patient knowledge, attitudes, satisfaction, experience, evaluation, perspective, practice, preference. Reference lists of retrieved articles were also screened for additional relevant studies.
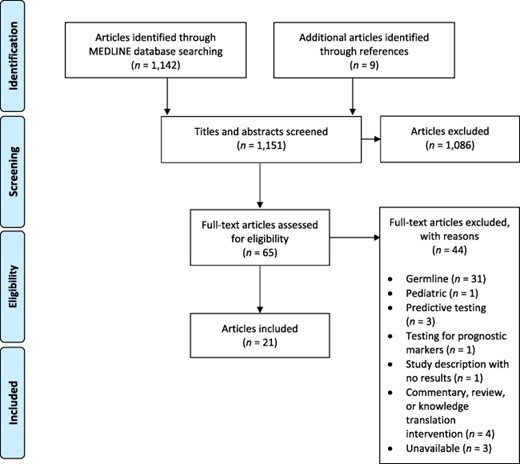
Flowchart for the search strategy conducted as per PRISMA guidelines.
Study Selection and Data Extraction
All studies selected surveyed adult cancer patients and/or physicians regarding their knowledge, attitudes, expectations, and/or concerns regarding whole genome or whole exon tumor NGS with potential therapeutic implications or adult cancer patients to whom testing results had been disclosed regarding their satisfaction with the testing process. Studies were excluded if they focused on germline rather than somatic NGS, mutations associated with hereditary risk, specific somatic mutations with known treatments, or molecular testing for prognostication. Commentaries and reviews were also excluded.
Study titles and abstracts were screened independently by all five authors, and potentially eligible studies were selected for further assessment. Full texts of the selected studies were retrieved and independently assessed for eligibility. Additional relevant articles were selected from the references of the final list of eligible studies.
Data from eligible studies were independently extracted by two authors (O.A. and M.S.) and reviewed by the other three authors. The data extracted included characteristics of the study population, study questions, survey methodology, and the major findings.
Results
Our MEDLINE search yielded a total of 1,142 publications, and an additional nine potentially relevant publications were identified from reference lists. After reviewing abstracts and titles, 1,086 studies were excluded. Of the remaining 65 studies, 44 were excluded after review of the full manuscript. Twenty- one articles met the inclusion criteria (Fig. 1) including a total of 3,883 patients and 275 physicians.
Patients
Tables 1–4 summarize, respectively, surveys and/or interviews of patients who had not been offered tumor NGS, those who were contemplating or about to undergo it, those who were awaiting test results, and those who had already received test results. Some of the publications were subsets of a single study as outlined below.
Studies evaluating cancer patients not currently being considered for somatic testing
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Rogith 2016 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated | 16-item self-administered questionnaire designed by investigators | Knowledge (objectively tested) | Knowledge relatively low (mean 8.7/16) 13% knew difference between somatic and germline testing 35% knew a target might not be found 75% aware of genetic privacy laws Higher knowledge scores associated with higher education and income |
| Rogith 2014 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated Patients offered opportunity for tissue banking for research | Nonvalidated 18-item self-administered questionnaire | Attitudes regarding privacy | 13% concerned about privacy of genetic data 60% willing to share identified data, and 76% deidentified data with researchers other than their primary physician even if no benefit to them 85% would consent to DNA banking if data deidentified No association between education and privacy concerns |
| Yusuf 2015 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% Researchers were there to answer any questions Participants had available a standard list of definitions | 20-item self-administered questionnaire | Attitude toward primary objectives Attitudes toward secondary findings | 75% willing to undergo a biopsy to guide treatment and 64% for research 81% would be willing to undergo testing for the selection of approved drugs and 59% for experimental therapy if testing covered by insurance (vs. 64% and 37% if not covered by insurance) Non-White patients less willing to undergo testing and biopsies even if costs covered 90% wanted to know findings related to new cancer risk and 87% about other preventable/treatable diseases |
| Yushak 2016 | U.S. | 413 | Multiple tumors Early-stage: 35% Advanced: 41% Unsure: 15% Patients were provided with brief background information on technical terms before survey | 45-item self-administered questionnaire | Attitudes, concerns, and expectations toward primary objectives of testing Attitudes toward secondary findings | 61% wanted to tumor profiling performed for only 1% chance of impact on treatment; willingness linked to White race and prior knowledge of tumor profiling 72% willing to have results used for research 45% concerned about costs Only 21% concerned about privacy 72% wanted to know information relevant to noncancerous conditions, 56% if disease serious but not preventable 49% wanted to know about variants of uncertain significance 48% concerned about impact on ability to obtain health insurance and 41% about impact on life insurance |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Rogith 2016 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated | 16-item self-administered questionnaire designed by investigators | Knowledge (objectively tested) | Knowledge relatively low (mean 8.7/16) 13% knew difference between somatic and germline testing 35% knew a target might not be found 75% aware of genetic privacy laws Higher knowledge scores associated with higher education and income |
| Rogith 2014 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated Patients offered opportunity for tissue banking for research | Nonvalidated 18-item self-administered questionnaire | Attitudes regarding privacy | 13% concerned about privacy of genetic data 60% willing to share identified data, and 76% deidentified data with researchers other than their primary physician even if no benefit to them 85% would consent to DNA banking if data deidentified No association between education and privacy concerns |
| Yusuf 2015 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% Researchers were there to answer any questions Participants had available a standard list of definitions | 20-item self-administered questionnaire | Attitude toward primary objectives Attitudes toward secondary findings | 75% willing to undergo a biopsy to guide treatment and 64% for research 81% would be willing to undergo testing for the selection of approved drugs and 59% for experimental therapy if testing covered by insurance (vs. 64% and 37% if not covered by insurance) Non-White patients less willing to undergo testing and biopsies even if costs covered 90% wanted to know findings related to new cancer risk and 87% about other preventable/treatable diseases |
| Yushak 2016 | U.S. | 413 | Multiple tumors Early-stage: 35% Advanced: 41% Unsure: 15% Patients were provided with brief background information on technical terms before survey | 45-item self-administered questionnaire | Attitudes, concerns, and expectations toward primary objectives of testing Attitudes toward secondary findings | 61% wanted to tumor profiling performed for only 1% chance of impact on treatment; willingness linked to White race and prior knowledge of tumor profiling 72% willing to have results used for research 45% concerned about costs Only 21% concerned about privacy 72% wanted to know information relevant to noncancerous conditions, 56% if disease serious but not preventable 49% wanted to know about variants of uncertain significance 48% concerned about impact on ability to obtain health insurance and 41% about impact on life insurance |
Studies evaluating cancer patients not currently being considered for somatic testing
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Rogith 2016 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated | 16-item self-administered questionnaire designed by investigators | Knowledge (objectively tested) | Knowledge relatively low (mean 8.7/16) 13% knew difference between somatic and germline testing 35% knew a target might not be found 75% aware of genetic privacy laws Higher knowledge scores associated with higher education and income |
| Rogith 2014 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated Patients offered opportunity for tissue banking for research | Nonvalidated 18-item self-administered questionnaire | Attitudes regarding privacy | 13% concerned about privacy of genetic data 60% willing to share identified data, and 76% deidentified data with researchers other than their primary physician even if no benefit to them 85% would consent to DNA banking if data deidentified No association between education and privacy concerns |
| Yusuf 2015 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% Researchers were there to answer any questions Participants had available a standard list of definitions | 20-item self-administered questionnaire | Attitude toward primary objectives Attitudes toward secondary findings | 75% willing to undergo a biopsy to guide treatment and 64% for research 81% would be willing to undergo testing for the selection of approved drugs and 59% for experimental therapy if testing covered by insurance (vs. 64% and 37% if not covered by insurance) Non-White patients less willing to undergo testing and biopsies even if costs covered 90% wanted to know findings related to new cancer risk and 87% about other preventable/treatable diseases |
| Yushak 2016 | U.S. | 413 | Multiple tumors Early-stage: 35% Advanced: 41% Unsure: 15% Patients were provided with brief background information on technical terms before survey | 45-item self-administered questionnaire | Attitudes, concerns, and expectations toward primary objectives of testing Attitudes toward secondary findings | 61% wanted to tumor profiling performed for only 1% chance of impact on treatment; willingness linked to White race and prior knowledge of tumor profiling 72% willing to have results used for research 45% concerned about costs Only 21% concerned about privacy 72% wanted to know information relevant to noncancerous conditions, 56% if disease serious but not preventable 49% wanted to know about variants of uncertain significance 48% concerned about impact on ability to obtain health insurance and 41% about impact on life insurance |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Rogith 2016 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated | 16-item self-administered questionnaire designed by investigators | Knowledge (objectively tested) | Knowledge relatively low (mean 8.7/16) 13% knew difference between somatic and germline testing 35% knew a target might not be found 75% aware of genetic privacy laws Higher knowledge scores associated with higher education and income |
| Rogith 2014 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% 79% highly educated Patients offered opportunity for tissue banking for research | Nonvalidated 18-item self-administered questionnaire | Attitudes regarding privacy | 13% concerned about privacy of genetic data 60% willing to share identified data, and 76% deidentified data with researchers other than their primary physician even if no benefit to them 85% would consent to DNA banking if data deidentified No association between education and privacy concerns |
| Yusuf 2015 | U.S. | 100 | Breast cancer Stage 0–3: 76% Stage 4: 24% Researchers were there to answer any questions Participants had available a standard list of definitions | 20-item self-administered questionnaire | Attitude toward primary objectives Attitudes toward secondary findings | 75% willing to undergo a biopsy to guide treatment and 64% for research 81% would be willing to undergo testing for the selection of approved drugs and 59% for experimental therapy if testing covered by insurance (vs. 64% and 37% if not covered by insurance) Non-White patients less willing to undergo testing and biopsies even if costs covered 90% wanted to know findings related to new cancer risk and 87% about other preventable/treatable diseases |
| Yushak 2016 | U.S. | 413 | Multiple tumors Early-stage: 35% Advanced: 41% Unsure: 15% Patients were provided with brief background information on technical terms before survey | 45-item self-administered questionnaire | Attitudes, concerns, and expectations toward primary objectives of testing Attitudes toward secondary findings | 61% wanted to tumor profiling performed for only 1% chance of impact on treatment; willingness linked to White race and prior knowledge of tumor profiling 72% willing to have results used for research 45% concerned about costs Only 21% concerned about privacy 72% wanted to know information relevant to noncancerous conditions, 56% if disease serious but not preventable 49% wanted to know about variants of uncertain significance 48% concerned about impact on ability to obtain health insurance and 41% about impact on life insurance |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Best 2019 | Australia | 569 | Advanced stage, any tumor, no further treatment options, eight patients previously had germline genetic sequencing | Semistructured interviews (n = 20) Open ended questions on survey | Knowledge (self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary finding | Participants cognizant of lack of knowledge, but this did not deter them from consenting to testing Confusion regarding germline vs. somatic testing Strong readiness to participate, primarily because of desire for new treatments (42%) and altruism (32%) Expectations unrealistically high Receiving treatment to prolong life was the priority for all participants Greatest concern that results would be negative or reveal negative information Some saw discovery of germline mutations as a benefit, others as a burden given overall prognosis Noncancer information seen as irrelevant |
| Butow 2020 | Australia | 777 | Advanced stage, no further treatment options Multiple tumor types Either completing or had completed last line of effective treatment | Self-administered questionnaire | Attitudes and expectations regarding primary objectives | 89% would have testing for as little as a 1% actionable return rate, but fewer were willing to pay for it (24% unwilling to pay even A$1,000) Immigrants less willing to undergo testing and to pay for it |
| Davies 2020 | Australia | 1,074 | Advanced stage Multiple tumor types Provided with education material about possibility of incidental germline findings and low possibility of finding actionable somatic mutation | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary objectives | Poor to moderate knowledge of somatic testing, with an average correct response score of 43% Perceived somatic testing to have high importance 76% significantly overestimated the utility of testing for making decisions about future cancer risks Greater satisfaction with the decision to undergo somatic testing associated with higher knowledge scores |
| Bijlsma 2018a | The Netherlands | 24 | Early stage 29% Advanced stage, NGS inexperienced: 38% Advanced stage, NGS experienced: 33% (experienced means already participating in a tumor and germline NGS study in which patients had been informed about possibility of secondary findings) Multiple tumor types 66% highly educated | Semistructured interviews done before and after a video outlining four specific types of secondary findings | Attitudes toward secondary findings | Initially, almost all wanted to receive all secondary findings After video, including four categories of genetic results, <50% preferred to only receive subsets of information, primarily for own interest but some to prevent a noncancerous disease, for research, or to benefit family members Main concern was their own and family members’ ability to cope with results Request for support and information to help communicate secondary findings to family members |
| Bijlsma 2018b | The Netherlands | 24 | Same patients as in Bijlsma 2018a | Semistructured interviews done after the two interviews described in Bijlsma 2018a | Attitudes toward secondary findings | Desire of patients to know findings in order to control their lives (preventive measures, screening, foregoing childbearing to avoid a hereditary disease, preparing financially), but some did not want to know about something that would negatively affect their life Desire to know information that might help family members but also concerned about the emotional impact of this information on family Concepts and information difficult to understand and remember Emotional conflict between desire for knowledge and desire to avoid further stress |
| Blanchette 2014 | Canada | 98 | Advanced stage Multiple tumor types Referred for phase I trial or genomic testing | 42-item self-administered questionnaire | Knowledge (objectively tested and self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary findings | Median knowledge score 8/12 (67%) true/false items correct; scores significantly associated with education level and income 48% reported having sufficient knowledge, and 34% indicated a need for formal genetic counseling to decide regarding testing 76% of patients were interested in learning more about testing 70% reported impact on treatment selection as most important reason to pursue testing 66% would consent to a needle biopsy and 39% to biopsy requiring general anesthetic if needed for testing 90% agreed to biobanking and sharing results for research purposes, 74% outside local hospitals 87% would want information about familial cancer risk 79% would want information about their own risk of developing noncancerous disease |
| Gray 2016 | U.S. | 167 | Advanced stage Lung: 53% Colorectal cancer: 47% | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary results | Moderately low genetic knowledge (mean score 4/7 correct) Interest in somatic testing correlated with interest in germline testing Almost all would wish to earn most cancer-related results including negative prognostic results Overall positive attitudes |
| Gray 2012 | U.S. | 69 | Stage not specified Multiple tumor types Tested after survey Patients were provided with baseline knowledge about test types (somatic and germline) | Semistructured interviews | Knowledge (objective) Attitudes and expectations regarding primary results Attitudes toward secondary findings | 74% had never heard of cancer genetic testing of whom 60% thought it was only to determine risk 96% expressed willingness to undergo selective somatic testing if predictive and 93% if prognostic 71% had concerns, particularly disclosure of unwanted information about poor prognosis and other psychological harms Only 62% would consent to whole genome sequencing; those willing hoped to help children avoid illness; those against feared information overload, concern about noncancerous disease |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire Knowledge assessed by a six-item previously validated true/false questionnaire | Knowledge (objective and self-perceived) Attitudes, concerns, and expectations regarding primary results Attitudes toward secondary findings | Average knowledge score of 88% 55%–60% indicated that they understood the study purpose, procedures, and risks and benefits 40% expected to receive direct benefits from testing including participation in clinical trials 84% expected notifications for relevant clinical trials 74% expected to learn more about the causes of their cancer Low levels of concern Despite explanations from study personnel to the contrary, most participants (67%–76%) presumed that incidental germline sequencing findings relevant to noncancerous health conditions would automatically be disclosed to them |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Best 2019 | Australia | 569 | Advanced stage, any tumor, no further treatment options, eight patients previously had germline genetic sequencing | Semistructured interviews (n = 20) Open ended questions on survey | Knowledge (self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary finding | Participants cognizant of lack of knowledge, but this did not deter them from consenting to testing Confusion regarding germline vs. somatic testing Strong readiness to participate, primarily because of desire for new treatments (42%) and altruism (32%) Expectations unrealistically high Receiving treatment to prolong life was the priority for all participants Greatest concern that results would be negative or reveal negative information Some saw discovery of germline mutations as a benefit, others as a burden given overall prognosis Noncancer information seen as irrelevant |
| Butow 2020 | Australia | 777 | Advanced stage, no further treatment options Multiple tumor types Either completing or had completed last line of effective treatment | Self-administered questionnaire | Attitudes and expectations regarding primary objectives | 89% would have testing for as little as a 1% actionable return rate, but fewer were willing to pay for it (24% unwilling to pay even A$1,000) Immigrants less willing to undergo testing and to pay for it |
| Davies 2020 | Australia | 1,074 | Advanced stage Multiple tumor types Provided with education material about possibility of incidental germline findings and low possibility of finding actionable somatic mutation | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary objectives | Poor to moderate knowledge of somatic testing, with an average correct response score of 43% Perceived somatic testing to have high importance 76% significantly overestimated the utility of testing for making decisions about future cancer risks Greater satisfaction with the decision to undergo somatic testing associated with higher knowledge scores |
| Bijlsma 2018a | The Netherlands | 24 | Early stage 29% Advanced stage, NGS inexperienced: 38% Advanced stage, NGS experienced: 33% (experienced means already participating in a tumor and germline NGS study in which patients had been informed about possibility of secondary findings) Multiple tumor types 66% highly educated | Semistructured interviews done before and after a video outlining four specific types of secondary findings | Attitudes toward secondary findings | Initially, almost all wanted to receive all secondary findings After video, including four categories of genetic results, <50% preferred to only receive subsets of information, primarily for own interest but some to prevent a noncancerous disease, for research, or to benefit family members Main concern was their own and family members’ ability to cope with results Request for support and information to help communicate secondary findings to family members |
| Bijlsma 2018b | The Netherlands | 24 | Same patients as in Bijlsma 2018a | Semistructured interviews done after the two interviews described in Bijlsma 2018a | Attitudes toward secondary findings | Desire of patients to know findings in order to control their lives (preventive measures, screening, foregoing childbearing to avoid a hereditary disease, preparing financially), but some did not want to know about something that would negatively affect their life Desire to know information that might help family members but also concerned about the emotional impact of this information on family Concepts and information difficult to understand and remember Emotional conflict between desire for knowledge and desire to avoid further stress |
| Blanchette 2014 | Canada | 98 | Advanced stage Multiple tumor types Referred for phase I trial or genomic testing | 42-item self-administered questionnaire | Knowledge (objectively tested and self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary findings | Median knowledge score 8/12 (67%) true/false items correct; scores significantly associated with education level and income 48% reported having sufficient knowledge, and 34% indicated a need for formal genetic counseling to decide regarding testing 76% of patients were interested in learning more about testing 70% reported impact on treatment selection as most important reason to pursue testing 66% would consent to a needle biopsy and 39% to biopsy requiring general anesthetic if needed for testing 90% agreed to biobanking and sharing results for research purposes, 74% outside local hospitals 87% would want information about familial cancer risk 79% would want information about their own risk of developing noncancerous disease |
| Gray 2016 | U.S. | 167 | Advanced stage Lung: 53% Colorectal cancer: 47% | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary results | Moderately low genetic knowledge (mean score 4/7 correct) Interest in somatic testing correlated with interest in germline testing Almost all would wish to earn most cancer-related results including negative prognostic results Overall positive attitudes |
| Gray 2012 | U.S. | 69 | Stage not specified Multiple tumor types Tested after survey Patients were provided with baseline knowledge about test types (somatic and germline) | Semistructured interviews | Knowledge (objective) Attitudes and expectations regarding primary results Attitudes toward secondary findings | 74% had never heard of cancer genetic testing of whom 60% thought it was only to determine risk 96% expressed willingness to undergo selective somatic testing if predictive and 93% if prognostic 71% had concerns, particularly disclosure of unwanted information about poor prognosis and other psychological harms Only 62% would consent to whole genome sequencing; those willing hoped to help children avoid illness; those against feared information overload, concern about noncancerous disease |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire Knowledge assessed by a six-item previously validated true/false questionnaire | Knowledge (objective and self-perceived) Attitudes, concerns, and expectations regarding primary results Attitudes toward secondary findings | Average knowledge score of 88% 55%–60% indicated that they understood the study purpose, procedures, and risks and benefits 40% expected to receive direct benefits from testing including participation in clinical trials 84% expected notifications for relevant clinical trials 74% expected to learn more about the causes of their cancer Low levels of concern Despite explanations from study personnel to the contrary, most participants (67%–76%) presumed that incidental germline sequencing findings relevant to noncancerous health conditions would automatically be disclosed to them |
Abbreviation: NGS, next-generation sequencing.
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Best 2019 | Australia | 569 | Advanced stage, any tumor, no further treatment options, eight patients previously had germline genetic sequencing | Semistructured interviews (n = 20) Open ended questions on survey | Knowledge (self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary finding | Participants cognizant of lack of knowledge, but this did not deter them from consenting to testing Confusion regarding germline vs. somatic testing Strong readiness to participate, primarily because of desire for new treatments (42%) and altruism (32%) Expectations unrealistically high Receiving treatment to prolong life was the priority for all participants Greatest concern that results would be negative or reveal negative information Some saw discovery of germline mutations as a benefit, others as a burden given overall prognosis Noncancer information seen as irrelevant |
| Butow 2020 | Australia | 777 | Advanced stage, no further treatment options Multiple tumor types Either completing or had completed last line of effective treatment | Self-administered questionnaire | Attitudes and expectations regarding primary objectives | 89% would have testing for as little as a 1% actionable return rate, but fewer were willing to pay for it (24% unwilling to pay even A$1,000) Immigrants less willing to undergo testing and to pay for it |
| Davies 2020 | Australia | 1,074 | Advanced stage Multiple tumor types Provided with education material about possibility of incidental germline findings and low possibility of finding actionable somatic mutation | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary objectives | Poor to moderate knowledge of somatic testing, with an average correct response score of 43% Perceived somatic testing to have high importance 76% significantly overestimated the utility of testing for making decisions about future cancer risks Greater satisfaction with the decision to undergo somatic testing associated with higher knowledge scores |
| Bijlsma 2018a | The Netherlands | 24 | Early stage 29% Advanced stage, NGS inexperienced: 38% Advanced stage, NGS experienced: 33% (experienced means already participating in a tumor and germline NGS study in which patients had been informed about possibility of secondary findings) Multiple tumor types 66% highly educated | Semistructured interviews done before and after a video outlining four specific types of secondary findings | Attitudes toward secondary findings | Initially, almost all wanted to receive all secondary findings After video, including four categories of genetic results, <50% preferred to only receive subsets of information, primarily for own interest but some to prevent a noncancerous disease, for research, or to benefit family members Main concern was their own and family members’ ability to cope with results Request for support and information to help communicate secondary findings to family members |
| Bijlsma 2018b | The Netherlands | 24 | Same patients as in Bijlsma 2018a | Semistructured interviews done after the two interviews described in Bijlsma 2018a | Attitudes toward secondary findings | Desire of patients to know findings in order to control their lives (preventive measures, screening, foregoing childbearing to avoid a hereditary disease, preparing financially), but some did not want to know about something that would negatively affect their life Desire to know information that might help family members but also concerned about the emotional impact of this information on family Concepts and information difficult to understand and remember Emotional conflict between desire for knowledge and desire to avoid further stress |
| Blanchette 2014 | Canada | 98 | Advanced stage Multiple tumor types Referred for phase I trial or genomic testing | 42-item self-administered questionnaire | Knowledge (objectively tested and self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary findings | Median knowledge score 8/12 (67%) true/false items correct; scores significantly associated with education level and income 48% reported having sufficient knowledge, and 34% indicated a need for formal genetic counseling to decide regarding testing 76% of patients were interested in learning more about testing 70% reported impact on treatment selection as most important reason to pursue testing 66% would consent to a needle biopsy and 39% to biopsy requiring general anesthetic if needed for testing 90% agreed to biobanking and sharing results for research purposes, 74% outside local hospitals 87% would want information about familial cancer risk 79% would want information about their own risk of developing noncancerous disease |
| Gray 2016 | U.S. | 167 | Advanced stage Lung: 53% Colorectal cancer: 47% | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary results | Moderately low genetic knowledge (mean score 4/7 correct) Interest in somatic testing correlated with interest in germline testing Almost all would wish to earn most cancer-related results including negative prognostic results Overall positive attitudes |
| Gray 2012 | U.S. | 69 | Stage not specified Multiple tumor types Tested after survey Patients were provided with baseline knowledge about test types (somatic and germline) | Semistructured interviews | Knowledge (objective) Attitudes and expectations regarding primary results Attitudes toward secondary findings | 74% had never heard of cancer genetic testing of whom 60% thought it was only to determine risk 96% expressed willingness to undergo selective somatic testing if predictive and 93% if prognostic 71% had concerns, particularly disclosure of unwanted information about poor prognosis and other psychological harms Only 62% would consent to whole genome sequencing; those willing hoped to help children avoid illness; those against feared information overload, concern about noncancerous disease |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire Knowledge assessed by a six-item previously validated true/false questionnaire | Knowledge (objective and self-perceived) Attitudes, concerns, and expectations regarding primary results Attitudes toward secondary findings | Average knowledge score of 88% 55%–60% indicated that they understood the study purpose, procedures, and risks and benefits 40% expected to receive direct benefits from testing including participation in clinical trials 84% expected notifications for relevant clinical trials 74% expected to learn more about the causes of their cancer Low levels of concern Despite explanations from study personnel to the contrary, most participants (67%–76%) presumed that incidental germline sequencing findings relevant to noncancerous health conditions would automatically be disclosed to them |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Best 2019 | Australia | 569 | Advanced stage, any tumor, no further treatment options, eight patients previously had germline genetic sequencing | Semistructured interviews (n = 20) Open ended questions on survey | Knowledge (self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary finding | Participants cognizant of lack of knowledge, but this did not deter them from consenting to testing Confusion regarding germline vs. somatic testing Strong readiness to participate, primarily because of desire for new treatments (42%) and altruism (32%) Expectations unrealistically high Receiving treatment to prolong life was the priority for all participants Greatest concern that results would be negative or reveal negative information Some saw discovery of germline mutations as a benefit, others as a burden given overall prognosis Noncancer information seen as irrelevant |
| Butow 2020 | Australia | 777 | Advanced stage, no further treatment options Multiple tumor types Either completing or had completed last line of effective treatment | Self-administered questionnaire | Attitudes and expectations regarding primary objectives | 89% would have testing for as little as a 1% actionable return rate, but fewer were willing to pay for it (24% unwilling to pay even A$1,000) Immigrants less willing to undergo testing and to pay for it |
| Davies 2020 | Australia | 1,074 | Advanced stage Multiple tumor types Provided with education material about possibility of incidental germline findings and low possibility of finding actionable somatic mutation | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary objectives | Poor to moderate knowledge of somatic testing, with an average correct response score of 43% Perceived somatic testing to have high importance 76% significantly overestimated the utility of testing for making decisions about future cancer risks Greater satisfaction with the decision to undergo somatic testing associated with higher knowledge scores |
| Bijlsma 2018a | The Netherlands | 24 | Early stage 29% Advanced stage, NGS inexperienced: 38% Advanced stage, NGS experienced: 33% (experienced means already participating in a tumor and germline NGS study in which patients had been informed about possibility of secondary findings) Multiple tumor types 66% highly educated | Semistructured interviews done before and after a video outlining four specific types of secondary findings | Attitudes toward secondary findings | Initially, almost all wanted to receive all secondary findings After video, including four categories of genetic results, <50% preferred to only receive subsets of information, primarily for own interest but some to prevent a noncancerous disease, for research, or to benefit family members Main concern was their own and family members’ ability to cope with results Request for support and information to help communicate secondary findings to family members |
| Bijlsma 2018b | The Netherlands | 24 | Same patients as in Bijlsma 2018a | Semistructured interviews done after the two interviews described in Bijlsma 2018a | Attitudes toward secondary findings | Desire of patients to know findings in order to control their lives (preventive measures, screening, foregoing childbearing to avoid a hereditary disease, preparing financially), but some did not want to know about something that would negatively affect their life Desire to know information that might help family members but also concerned about the emotional impact of this information on family Concepts and information difficult to understand and remember Emotional conflict between desire for knowledge and desire to avoid further stress |
| Blanchette 2014 | Canada | 98 | Advanced stage Multiple tumor types Referred for phase I trial or genomic testing | 42-item self-administered questionnaire | Knowledge (objectively tested and self-perceived) Attitudes and expectations regarding primary objectives Attitudes toward secondary findings | Median knowledge score 8/12 (67%) true/false items correct; scores significantly associated with education level and income 48% reported having sufficient knowledge, and 34% indicated a need for formal genetic counseling to decide regarding testing 76% of patients were interested in learning more about testing 70% reported impact on treatment selection as most important reason to pursue testing 66% would consent to a needle biopsy and 39% to biopsy requiring general anesthetic if needed for testing 90% agreed to biobanking and sharing results for research purposes, 74% outside local hospitals 87% would want information about familial cancer risk 79% would want information about their own risk of developing noncancerous disease |
| Gray 2016 | U.S. | 167 | Advanced stage Lung: 53% Colorectal cancer: 47% | Self-administered questionnaire | Knowledge (objectively tested) Attitudes and expectations regarding primary results | Moderately low genetic knowledge (mean score 4/7 correct) Interest in somatic testing correlated with interest in germline testing Almost all would wish to earn most cancer-related results including negative prognostic results Overall positive attitudes |
| Gray 2012 | U.S. | 69 | Stage not specified Multiple tumor types Tested after survey Patients were provided with baseline knowledge about test types (somatic and germline) | Semistructured interviews | Knowledge (objective) Attitudes and expectations regarding primary results Attitudes toward secondary findings | 74% had never heard of cancer genetic testing of whom 60% thought it was only to determine risk 96% expressed willingness to undergo selective somatic testing if predictive and 93% if prognostic 71% had concerns, particularly disclosure of unwanted information about poor prognosis and other psychological harms Only 62% would consent to whole genome sequencing; those willing hoped to help children avoid illness; those against feared information overload, concern about noncancerous disease |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire Knowledge assessed by a six-item previously validated true/false questionnaire | Knowledge (objective and self-perceived) Attitudes, concerns, and expectations regarding primary results Attitudes toward secondary findings | Average knowledge score of 88% 55%–60% indicated that they understood the study purpose, procedures, and risks and benefits 40% expected to receive direct benefits from testing including participation in clinical trials 84% expected notifications for relevant clinical trials 74% expected to learn more about the causes of their cancer Low levels of concern Despite explanations from study personnel to the contrary, most participants (67%–76%) presumed that incidental germline sequencing findings relevant to noncancerous health conditions would automatically be disclosed to them |
Abbreviation: NGS, next-generation sequencing.
Studies evaluating cancer patients who have undergone tumor next-generation sequencing but have not yet received results
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Hamilton 2017 | U.S. | 40 | Advanced stage Multiple tumor types Tumor profiling results but not secondary findings disclosed | Semistructured in-person and phone interviews | Attitudes toward secondary findings | 57% expressed interest in learning secondary germline findings Anticipated diverse benefits for themselves or their families (disease prevention or management), other patients, and society 53% did not anticipate any harms Concerns primarily related to emotional distress to family if increased risk of disease disclosed and to other patients with cancer A small number were concerned with privacy and insurance issues |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Attitudes toward secondary findings | 93% felt obligation to receive all the genetic information for benefit of family |
| Rohrmoser 2019 | Germany | 30 | Advanced stage Exhausted conventional treatment | Self-administered questionnaire Face-to-face semistructured interview | Attitudes and expectations regarding primary results | Expectation of improved treatment, a contribution to research, and/or additional insight about own cancer Reported feeling individually appreciated for participating in research Testing gave reason to maintain hope for cure or recovery Declined feelings of skepticism |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Hamilton 2017 | U.S. | 40 | Advanced stage Multiple tumor types Tumor profiling results but not secondary findings disclosed | Semistructured in-person and phone interviews | Attitudes toward secondary findings | 57% expressed interest in learning secondary germline findings Anticipated diverse benefits for themselves or their families (disease prevention or management), other patients, and society 53% did not anticipate any harms Concerns primarily related to emotional distress to family if increased risk of disease disclosed and to other patients with cancer A small number were concerned with privacy and insurance issues |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Attitudes toward secondary findings | 93% felt obligation to receive all the genetic information for benefit of family |
| Rohrmoser 2019 | Germany | 30 | Advanced stage Exhausted conventional treatment | Self-administered questionnaire Face-to-face semistructured interview | Attitudes and expectations regarding primary results | Expectation of improved treatment, a contribution to research, and/or additional insight about own cancer Reported feeling individually appreciated for participating in research Testing gave reason to maintain hope for cure or recovery Declined feelings of skepticism |
Studies evaluating cancer patients who have undergone tumor next-generation sequencing but have not yet received results
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Hamilton 2017 | U.S. | 40 | Advanced stage Multiple tumor types Tumor profiling results but not secondary findings disclosed | Semistructured in-person and phone interviews | Attitudes toward secondary findings | 57% expressed interest in learning secondary germline findings Anticipated diverse benefits for themselves or their families (disease prevention or management), other patients, and society 53% did not anticipate any harms Concerns primarily related to emotional distress to family if increased risk of disease disclosed and to other patients with cancer A small number were concerned with privacy and insurance issues |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Attitudes toward secondary findings | 93% felt obligation to receive all the genetic information for benefit of family |
| Rohrmoser 2019 | Germany | 30 | Advanced stage Exhausted conventional treatment | Self-administered questionnaire Face-to-face semistructured interview | Attitudes and expectations regarding primary results | Expectation of improved treatment, a contribution to research, and/or additional insight about own cancer Reported feeling individually appreciated for participating in research Testing gave reason to maintain hope for cure or recovery Declined feelings of skepticism |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions measured . | Main findings . |
|---|---|---|---|---|---|---|
| Hamilton 2017 | U.S. | 40 | Advanced stage Multiple tumor types Tumor profiling results but not secondary findings disclosed | Semistructured in-person and phone interviews | Attitudes toward secondary findings | 57% expressed interest in learning secondary germline findings Anticipated diverse benefits for themselves or their families (disease prevention or management), other patients, and society 53% did not anticipate any harms Concerns primarily related to emotional distress to family if increased risk of disease disclosed and to other patients with cancer A small number were concerned with privacy and insurance issues |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Attitudes toward secondary findings | 93% felt obligation to receive all the genetic information for benefit of family |
| Rohrmoser 2019 | Germany | 30 | Advanced stage Exhausted conventional treatment | Self-administered questionnaire Face-to-face semistructured interview | Attitudes and expectations regarding primary results | Expectation of improved treatment, a contribution to research, and/or additional insight about own cancer Reported feeling individually appreciated for participating in research Testing gave reason to maintain hope for cure or recovery Declined feelings of skepticism |
Studies evaluating cancer patients who had received results of tumor next-generation sequencing
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Halverson 2016 | U.S. | 39 | Advanced-stage cancer and conditions that were presumed to be genetic but without diagnosis Exhausted standard treatments | Semistructured interviews | Satisfaction with primary results and overall process | Found sequencing helpful because of the personal utility and ability to make more informed decisions about health care Gave significant weight to their own interpretations that differed from health care providers Found satisfaction in being able to contribute knowledge to a new field regardless of clinical utility of the results |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Satisfaction with primary results and overall process | Those who had hoped for novel and targeted treatment resulting from testing were challenged by nonfindings or limited access to relevant trials |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire | Satisfaction with primary results and overall process | Low levels of regret Mean satisfaction score 4.06 (1 = not at all satisfied; 5 = extremely satisfied), and most would make the same choice again if they had to do it over (mean score 4.37; 1 = strongly disagree; 5 = strongly agree) |
| Gornick 2018 | U.S. | 57 | Advanced stage Multiple tumor types | Mailed survey | Recollection of disclosure of results | 25% to whom there was documentation of results disclosure reported that disclosure had not taken place |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Halverson 2016 | U.S. | 39 | Advanced-stage cancer and conditions that were presumed to be genetic but without diagnosis Exhausted standard treatments | Semistructured interviews | Satisfaction with primary results and overall process | Found sequencing helpful because of the personal utility and ability to make more informed decisions about health care Gave significant weight to their own interpretations that differed from health care providers Found satisfaction in being able to contribute knowledge to a new field regardless of clinical utility of the results |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Satisfaction with primary results and overall process | Those who had hoped for novel and targeted treatment resulting from testing were challenged by nonfindings or limited access to relevant trials |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire | Satisfaction with primary results and overall process | Low levels of regret Mean satisfaction score 4.06 (1 = not at all satisfied; 5 = extremely satisfied), and most would make the same choice again if they had to do it over (mean score 4.37; 1 = strongly disagree; 5 = strongly agree) |
| Gornick 2018 | U.S. | 57 | Advanced stage Multiple tumor types | Mailed survey | Recollection of disclosure of results | 25% to whom there was documentation of results disclosure reported that disclosure had not taken place |
Studies evaluating cancer patients who had received results of tumor next-generation sequencing
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Halverson 2016 | U.S. | 39 | Advanced-stage cancer and conditions that were presumed to be genetic but without diagnosis Exhausted standard treatments | Semistructured interviews | Satisfaction with primary results and overall process | Found sequencing helpful because of the personal utility and ability to make more informed decisions about health care Gave significant weight to their own interpretations that differed from health care providers Found satisfaction in being able to contribute knowledge to a new field regardless of clinical utility of the results |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Satisfaction with primary results and overall process | Those who had hoped for novel and targeted treatment resulting from testing were challenged by nonfindings or limited access to relevant trials |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire | Satisfaction with primary results and overall process | Low levels of regret Mean satisfaction score 4.06 (1 = not at all satisfied; 5 = extremely satisfied), and most would make the same choice again if they had to do it over (mean score 4.37; 1 = strongly disagree; 5 = strongly agree) |
| Gornick 2018 | U.S. | 57 | Advanced stage Multiple tumor types | Mailed survey | Recollection of disclosure of results | 25% to whom there was documentation of results disclosure reported that disclosure had not taken place |
| Study . | Country . | Sample size . | Study population . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Halverson 2016 | U.S. | 39 | Advanced-stage cancer and conditions that were presumed to be genetic but without diagnosis Exhausted standard treatments | Semistructured interviews | Satisfaction with primary results and overall process | Found sequencing helpful because of the personal utility and ability to make more informed decisions about health care Gave significant weight to their own interpretations that differed from health care providers Found satisfaction in being able to contribute knowledge to a new field regardless of clinical utility of the results |
| Miller 2014 | Canada | 29 | Advanced stage Multiple tumor types | Semistructured interviews | Satisfaction with primary results and overall process | Those who had hoped for novel and targeted treatment resulting from testing were challenged by nonfindings or limited access to relevant trials |
| Roberts 2018 | U.S. | 297 | Advanced stage Multiple tumor types | In-person or Web-based self-administered questionnaire | Satisfaction with primary results and overall process | Low levels of regret Mean satisfaction score 4.06 (1 = not at all satisfied; 5 = extremely satisfied), and most would make the same choice again if they had to do it over (mean score 4.37; 1 = strongly disagree; 5 = strongly agree) |
| Gornick 2018 | U.S. | 57 | Advanced stage Multiple tumor types | Mailed survey | Recollection of disclosure of results | 25% to whom there was documentation of results disclosure reported that disclosure had not taken place |
Table 1 includes three publications [7–9] from a single survey of 100 patients with breast cancer recruited during their first visit to the University of Texas MD Anderson Cancer Center who were asked to provide consent for tissue banking. Table 2 includes three publications [10–12] from the Psychosocial Issues in Genomics in Oncology (PiGeOn) Project, a substudy of the Molecular Screening and Therapeutics Program in Sydney, Australia, which provided tumor molecular profiling to 1,000 patients with advanced cancer for whom there are no further therapeutic options. Table 2 also includes two publications by Blijsma et al. [13, 14] who interviewed patients (twice) regarding attitudes toward secondary germline findings from tumor NGS and then, shortly thereafter, conducted a third set of more in-depth interviews with the same patients, summarized in a separate publication. In some studies, patients were interviewed before and after disclosure of NGS results [15]. In other studies, both patients and oncologists were surveyed [16, 17], and, accordingly, results are incorporated in more than one table. Patients enrolled between April 2014 and December 2016 in the University of Michigan Oncology Sequencing program, which sequences the genome, exome, and transcriptome of tumors, were surveyed at baseline and then 2 weeks after the referring oncologist received the results [15]. Oncologists who had referred patients to that program between June 2014 and March 2015 were surveyed online 1 week after testing results had been disclosed and potentially actionable findings discussed at an institutional precision tumor board [18].
Knowledge
Self-Perceived Knowledge. In two of three studies including patients considering or about to undergo somatic testing (Table 2), the majority of patients perceived their knowledge of somatic testing to be inadequate [10, 19]. The patients with end-stage disease in the PiGeOn study were so focused on the possibility that somatic testing might provide them with an additional line of treatment that they did not perceive their lack of knowledge as a deterrent to testing [10]. However, in the study by Blanchette et al. which included patients with advanced disease who had undergone variable amounts of previous treatment, over 50% of participants, including many who considered themselves to have a basic grasp of biological concepts, believed their genetics knowledge was insufficient to make an informed decision whether or not to pursue NGS [19]. Although up to 60% of patients in a study by Roberts et al. [15] indicated that they understood the purpose, procedures, risks and benefits of somatic testing, the patient population was highly educated (50% college graduates).
Objectively Assessed Knowledge. In five of six studies that objectively assessed patients’ knowledge of somatic testing (Tables 1 and 2), patients generally had moderate to poor knowledge of this concept, with the majority unaware of the distinction between somatic and germline testing [7, 12, 16, 19, 20]. Several of the validated questionnaires used in these studies had more questions about germline testing than about somatic testing, which may in some cases have added to the confusion. Overall scores generally correlated with education and income [8, 19]. The study by Roberts et al. [15] reported good overall knowledge, with an average score of 5.3 out of a maximum of 6.0 (88%) on a validated six-item genome sequencing scale designed for use in a cancer population. However, most of these very basic questions (e.g., “True or false: identification of cancer genes always leads to prevention or cure”) would require little if any cancer genomics knowledge to be answered correctly by the generally well-educated patients in this study. Rogith et al. [8] designed a 16-item multiple choice knowledge questionnaire with most of the questions directly targeting key concepts relevant to tumor NGS, such as “An individual's tumor molecular characteristics do not change over time. True, false or not sure.” Knowledge score was not higher in women who had previously undergone germline testing. Even among this highly educated breast cancer population (79% with college degree or higher), the mean score was only 8.7 out of 16, indicating the inability to guess the correct answer by these patients who were newly registered at the cancer center and had not yet been offered somatic testing.
Attitudes, Expectations, and Concerns About the Primary Results
In both studies that evaluated patients with early-stage or advanced cancer not actively being considered for tumor NGS (Table 1), the majority of patients expressed an interest in undergoing testing, even if there was only a small chance it would help with treatment, and welcomed the opportunity to contribute to research [21]. A minority of patients indicated less interest in testing if they would have to pay for it [9]. In their 2014 study, Rogith et al. [7] focused specifically on privacy issues, hypothesizing that, although patients generally have significant concerns about the privacy of germline genetic data, they might be less concerned about tumor somatic mutations. Indeed, most patients had few privacy concerns and would be willing to share even identified data with researchers who were not involved in their care. However, the investigators cautioned that their patient population comprised well-educated women with breast cancer, most of whom had been diagnosed within the previous year, and the results might not be generalizable to men, patients with other tumors, or less well-educated patient populations.
One striking finding in the study by Yusuf et al. [9] was that the 28% of non-White patients in their study were significantly less willing to undergo molecular testing, minimally invasive procedures, or biospecimen banking than White patients, even if the results would guide their own treatment and if costs were covered. These findings could not be explained by education of income [9]. Similarly, Yushak et al. [21] found that White race was significantly associated with willingness to undergo tumor profiling.
Six studies focused on attitudes and expectations of patients with cancer who were considering or about to undergo tumor NGS (Table 2). The three publications from the PiGeOn Project all had similar findings. Patients generally had a positive attitude toward tumor NGS, and many anticipated access to clinical trials and targeted treatments on the basis of test results. Even in cases where patients were advised that it was unlikely that there would be improvements to their cancer care, they seemed eager to undergo testing for the possibility that they might derive personal benefit, to contribute to research, or to help their family. However, in two studies [10, 20] patients expressed concerns about receiving unhelpful results or learning negative information (e.g., poor prognosis). In the 2012 study by Gray et al. [20], Black patients were more than twice as likely as White patients (48% vs. 20%) to express worry about psychological harms of tumor NGS.
In the only study that focused on the attitudes and expectations of patients with advanced cancer who had undergone tumor multigene NGS but had not yet received results (Table 3), many patients reported realistic hope for better “individualized” treatment or contribution to research, but some expressed an unrealistic hope for cure [22].
Attitudes and Concerns Toward Secondary Findings
Among patients not being considered for tumor NGS, a significant portion of whom had early-stage disease (Table 1), the great majority wanted to know the risk of developing a new cancer or other preventable or treatable diseases [9], but only about 50% were interested in learning about serious nonpreventable diseases or genetic variants of uncertain significance [21]. Almost half were concerned about the potential impact of secondary findings on their ability to secure insurance [9].
Four studies assessed attitudes toward secondary NGS findings among advanced cancer patients who were considering or are about to undergo somatic testing (Table 2). The patients in the PiGeOn Project expressed little interest in secondary findings given the very advanced stage of their disease with no further conventional treatment options [10]. At the other extreme, 7 of the 24 patients interviewed by Blijsma et al. [13] had early-stage disease and, as a group, were very interested in exploring the benefits and risks of disclosure of secondary findings. Although almost all patients initially expressed a desire to receive all the possible secondary findings, most patients ultimately opted to receive only a subset of the findings after watching an educational video outlining four categories of secondary findings (mutation predisposing to preventable disease, mutation predisposing to disease for which prevention and treatment is unavailable, mutation that may affect health of family members, and a genetic variant of uncertain significance). The in-depth interviews revealed that patients found secondary findings to be cognitively and emotionally complex. On the one hand, information about secondary findings could potentially prevent disease in themselves or their at-risk relatives but, on the other hand, could lead to personal and/or familial distress. In contrast to the findings of the researchers in the PiGeOn Project, Blanchette et al. [19] reported that almost 80% of patients would wish to receive incidental results that would have an impact on their own risk of developing diseases other than cancer. In the study by Roberts et al. [15], 74% of patients wished to learn more about causes of their own cancer and 93% about genetic changes that might have implications for their relatives’ cancer risk.
Among patients to whom primary tumor NGS findings but not secondary findings had been disclosed [17, 23] (Table 3), patients’ attitudes toward secondary findings ranged from favorable (“I think any information you can learn about your health, if it could be good or bad, it's still good”) to unfavorable (“It's sort of enough right now to know that I have cancer … I would be upset to learn that I was, for example, at very, very high risk for some other dreadful disease”). Miller et al. [17] found that over 90% of patients wanted to know all the details of incidental germline information regarding disease risk in order to help their family.
Satisfaction with Testing
Three studies addressed satisfaction and experience of patients with cancer regarding tumor NGS testing after they received their results (Table 4). Many patients felt a sense of empowerment, despite, in many cases, not receiving clinical utility from the results [24]. Similarly, in the study by Roberts et al., most patients reported that they did not regret having undergone testing even though the majority of their expectations were not fulfilled [15]. However, Miller et al. [17] found that those who had hoped for novel and targeted treatment resulting from testing were challenged by nonactionable findings or limited access to relevant trials. These latter results are not surprising in that these patients with advanced cancer had all been referred, many from outside centers, for a study involving tumor biopsy followed by NGS that was likely perceived to be their “last hope” for effective treatment or a relevant clinical trial.
In the study by Gornick et al. [18], 34 of 57 patients, whose oncologist had indicated an intention to discuss sequencing results with them, reported that a results disclosure did not take place; in reality, results had been disclosed to at least 14 of these patients (25% of all respondents) based on their medical record.
Oncologists
Five studies surveyed oncologists regarding their knowledge, attitudes and/or concerns regarding somatic testing (Table 5). In two of the three studies evaluating self-perceived knowledge, the majority of oncologists, reported having low to moderate confidence in their genomic knowledge, particularly new technologies such as genetic sequencing [25, 26] even oncologists with extensive ordering tumor NGS were concerned about their ability to interpret the large volume of results and to determine the actionability of the findings [16]. The majority of medical oncologists envisioned that tumor genomic sequencing results would not have a significant impact on treatment decisions. Most of the oncologists surveyed did not favor limiting disclosure to results with clinical utility [16–18]. Most also identified concerns regarding cost of testing, the clinical utility, and patient literacy in the area of genomics [25]. In the 2016 study by Gray et al. [16], the primary concerns of the oncologists were managing patient expectations and determining what information should be disclosed.
Studies evaluating knowledge and attitudes of oncologists regarding tumor next-generation sequencing
| Study . | Country . | Sample size . | Physicians . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Chow-White 2017 | Canada | 31 | Medical oncologists (actively involved in clinical genomics trial) | Web-based questionnaire | Knowledge (self-perceived) Attitudes, concerns, and expectations regarding primary results | Low to moderate level of genomic literacy 42% thought medical training programs do not offer enough genomic training Majority thought genomics will have a major impact on drug discovery and treatment selection in the next 5 years Major challenges considered to be cost, patient genomic literacy, and clinical utility of genomics |
| Gray 2016 | U.S. | 27 | Medical oncologists with extensive experience ordering tumor NGS (median 100 per year) | Self-administered questionnaire (in person or by telephone) Follow-up interview | Knowledge (self-perceived) Attitudes about return of results | 97% moderately to very confident in their ability to interpret somatic results in their disease area, to explain concepts to patients, and to make treatment recommendations based on somatic information 78% wanted to disclose results if they have clinical utility, 67% if no clinical utility Some expressed concern about management of patients’ expectations and how much of the information should be shared with patients |
| Gray 2014 | U.S. | 160 | Medical, radiation, and surgical oncologists | Web survey | Knowledge (self-perceived) Attitudes and expectations regarding primary results Attitude toward secondary findings | Considerable variation in genomic confidence with 22% reporting low confidence in genomic knowledge Higher genomic confidence was associated with wanting to test a majority of patients Variation in attitudes about value of testing Between 70% and 90% endorsed disclosure of actionable or potentially actionable results <40% endorsed disclosure of uncertain genomic findings Respondents noted significant variation in language physicians use to describe genomic testing |
| Miller 2014 | Canada | 14 | Medical oncologists | Semistructured interviews | Attitudes and expectations regarding primary results Attitudes toward secondary findings | Optimistic about long-term potential but cautious about immediate benefits and mindful of elevated patient expectations Consent and counseling expected to mitigate challenges from incidental findings |
| Gornick 2018 | U.S. | 43 | Medical oncologists who had referred 112 patients to a genomic sequencing program | Online survey sent after each patient enrolled | Intended use of sequencing information | Intended to share results with 84% of patients Planned to make treatment changes for 24 (22%) of patients based on results, but only 9 of 24 actually had changes |
| Study . | Country . | Sample size . | Physicians . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Chow-White 2017 | Canada | 31 | Medical oncologists (actively involved in clinical genomics trial) | Web-based questionnaire | Knowledge (self-perceived) Attitudes, concerns, and expectations regarding primary results | Low to moderate level of genomic literacy 42% thought medical training programs do not offer enough genomic training Majority thought genomics will have a major impact on drug discovery and treatment selection in the next 5 years Major challenges considered to be cost, patient genomic literacy, and clinical utility of genomics |
| Gray 2016 | U.S. | 27 | Medical oncologists with extensive experience ordering tumor NGS (median 100 per year) | Self-administered questionnaire (in person or by telephone) Follow-up interview | Knowledge (self-perceived) Attitudes about return of results | 97% moderately to very confident in their ability to interpret somatic results in their disease area, to explain concepts to patients, and to make treatment recommendations based on somatic information 78% wanted to disclose results if they have clinical utility, 67% if no clinical utility Some expressed concern about management of patients’ expectations and how much of the information should be shared with patients |
| Gray 2014 | U.S. | 160 | Medical, radiation, and surgical oncologists | Web survey | Knowledge (self-perceived) Attitudes and expectations regarding primary results Attitude toward secondary findings | Considerable variation in genomic confidence with 22% reporting low confidence in genomic knowledge Higher genomic confidence was associated with wanting to test a majority of patients Variation in attitudes about value of testing Between 70% and 90% endorsed disclosure of actionable or potentially actionable results <40% endorsed disclosure of uncertain genomic findings Respondents noted significant variation in language physicians use to describe genomic testing |
| Miller 2014 | Canada | 14 | Medical oncologists | Semistructured interviews | Attitudes and expectations regarding primary results Attitudes toward secondary findings | Optimistic about long-term potential but cautious about immediate benefits and mindful of elevated patient expectations Consent and counseling expected to mitigate challenges from incidental findings |
| Gornick 2018 | U.S. | 43 | Medical oncologists who had referred 112 patients to a genomic sequencing program | Online survey sent after each patient enrolled | Intended use of sequencing information | Intended to share results with 84% of patients Planned to make treatment changes for 24 (22%) of patients based on results, but only 9 of 24 actually had changes |
Studies evaluating knowledge and attitudes of oncologists regarding tumor next-generation sequencing
| Study . | Country . | Sample size . | Physicians . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Chow-White 2017 | Canada | 31 | Medical oncologists (actively involved in clinical genomics trial) | Web-based questionnaire | Knowledge (self-perceived) Attitudes, concerns, and expectations regarding primary results | Low to moderate level of genomic literacy 42% thought medical training programs do not offer enough genomic training Majority thought genomics will have a major impact on drug discovery and treatment selection in the next 5 years Major challenges considered to be cost, patient genomic literacy, and clinical utility of genomics |
| Gray 2016 | U.S. | 27 | Medical oncologists with extensive experience ordering tumor NGS (median 100 per year) | Self-administered questionnaire (in person or by telephone) Follow-up interview | Knowledge (self-perceived) Attitudes about return of results | 97% moderately to very confident in their ability to interpret somatic results in their disease area, to explain concepts to patients, and to make treatment recommendations based on somatic information 78% wanted to disclose results if they have clinical utility, 67% if no clinical utility Some expressed concern about management of patients’ expectations and how much of the information should be shared with patients |
| Gray 2014 | U.S. | 160 | Medical, radiation, and surgical oncologists | Web survey | Knowledge (self-perceived) Attitudes and expectations regarding primary results Attitude toward secondary findings | Considerable variation in genomic confidence with 22% reporting low confidence in genomic knowledge Higher genomic confidence was associated with wanting to test a majority of patients Variation in attitudes about value of testing Between 70% and 90% endorsed disclosure of actionable or potentially actionable results <40% endorsed disclosure of uncertain genomic findings Respondents noted significant variation in language physicians use to describe genomic testing |
| Miller 2014 | Canada | 14 | Medical oncologists | Semistructured interviews | Attitudes and expectations regarding primary results Attitudes toward secondary findings | Optimistic about long-term potential but cautious about immediate benefits and mindful of elevated patient expectations Consent and counseling expected to mitigate challenges from incidental findings |
| Gornick 2018 | U.S. | 43 | Medical oncologists who had referred 112 patients to a genomic sequencing program | Online survey sent after each patient enrolled | Intended use of sequencing information | Intended to share results with 84% of patients Planned to make treatment changes for 24 (22%) of patients based on results, but only 9 of 24 actually had changes |
| Study . | Country . | Sample size . | Physicians . | Type of survey . | Dimensions and measures . | Main findings . |
|---|---|---|---|---|---|---|
| Chow-White 2017 | Canada | 31 | Medical oncologists (actively involved in clinical genomics trial) | Web-based questionnaire | Knowledge (self-perceived) Attitudes, concerns, and expectations regarding primary results | Low to moderate level of genomic literacy 42% thought medical training programs do not offer enough genomic training Majority thought genomics will have a major impact on drug discovery and treatment selection in the next 5 years Major challenges considered to be cost, patient genomic literacy, and clinical utility of genomics |
| Gray 2016 | U.S. | 27 | Medical oncologists with extensive experience ordering tumor NGS (median 100 per year) | Self-administered questionnaire (in person or by telephone) Follow-up interview | Knowledge (self-perceived) Attitudes about return of results | 97% moderately to very confident in their ability to interpret somatic results in their disease area, to explain concepts to patients, and to make treatment recommendations based on somatic information 78% wanted to disclose results if they have clinical utility, 67% if no clinical utility Some expressed concern about management of patients’ expectations and how much of the information should be shared with patients |
| Gray 2014 | U.S. | 160 | Medical, radiation, and surgical oncologists | Web survey | Knowledge (self-perceived) Attitudes and expectations regarding primary results Attitude toward secondary findings | Considerable variation in genomic confidence with 22% reporting low confidence in genomic knowledge Higher genomic confidence was associated with wanting to test a majority of patients Variation in attitudes about value of testing Between 70% and 90% endorsed disclosure of actionable or potentially actionable results <40% endorsed disclosure of uncertain genomic findings Respondents noted significant variation in language physicians use to describe genomic testing |
| Miller 2014 | Canada | 14 | Medical oncologists | Semistructured interviews | Attitudes and expectations regarding primary results Attitudes toward secondary findings | Optimistic about long-term potential but cautious about immediate benefits and mindful of elevated patient expectations Consent and counseling expected to mitigate challenges from incidental findings |
| Gornick 2018 | U.S. | 43 | Medical oncologists who had referred 112 patients to a genomic sequencing program | Online survey sent after each patient enrolled | Intended use of sequencing information | Intended to share results with 84% of patients Planned to make treatment changes for 24 (22%) of patients based on results, but only 9 of 24 actually had changes |
In 2014, Gray et al. [26] found that the majority of oncologists wanted to disclose secondary findings that were actionable or potentially actionable; however, few wanted to disclose results that were uncertain. On the other hand, in the study of Gornick et al. [18], oncologists intended to share NGS results with 84% of patients whom they referred for sequencing even though a change in treatment was anticipated for only 22%; ultimately a change in clinical management occurred for only 8% of patients.
Pinheiro et al. [27] surveyed 28 oncologists, all of whom were about to discuss tumor NGS testing or results with a patient, regarding which topics they thought were most important in an NGS discussion; 66 patients who had undergone such a conversation with their oncologist within the previous week also participated in this study. Although the great majority of patients and physicians agreed that the purpose of testing and how it determines treatment were the most important topics, patients were much more likely than physicians to list the implications for family in their top choices (70% vs. 26%; p > .001). Physicians were significantly more likely than patients to list limitations of testing (70% vs. 44%; p = .021) and cost of testing (59% vs. 24%; p = .001) as important discussion points. In terms of methods of receiving information, 85% of patients wanted to discuss NGS results with their physician or nurse, and 67% indicated interest in receiving written information with less preference for Internet-based (29%) or video-based (12%) resources. Physicians preferred to use pamphlets (67%), Web sites explaining key facts (44%), videos (41%), and standardized scripts (26%) to communicate results of NGS testing.
Discussion
This narrative review summarizes the current literature on knowledge and attitudes of patients and oncologists regarding tumor multigene NGS. The great majority of patients favored the concept of NGS, but for many this was due to unrealistically high expectations regarding its potential to identify additional treatment options and possibly even a cure. Such unrealistic expectations were even found among some patients who had already received counseling regarding their NGS results.
Many patients also had unrealistic expectations regarding the potential benefits of secondary findings for themselves and their family members, given opportunities for screening and secondary prevention in some cases. Others expressed significant concerns regarding the emotional impact of such findings on themselves and their children as well as practical issues such as insurability. Study participants who chose not to learn their secondary findings generally still chose to receive the primary results of their tumor genomic profiling. Not surprisingly, patients with advanced disease for whom there were no further conventional treatment options were much less interested in the implications of secondary findings than those with earlier stage disease.
For a significant minority of patients surveyed, barriers to undergoing tumor NGS included the need for a biopsy [12], cost [9], and, for a small minority, worries about information privacy [21].
Of concern was the finding in some studies of general reluctance among non-White patients to undergo tumor NGS and to participate in related research. If widespread, this could lead to further disparities in cancer outcomes, as fewer non-White patients would benefit from advances in personalized medicine. Moreover, molecular data derived predominantly from White populations might not be applicable to other races. Further research is needed to identify tumor NGS hesitancy in the non-White population [9].
It is interesting to note the similarity between our results and those of studies focused on germline testing. Among those patients undergoing germline testing, there was generally a positive attitude toward NGS [28, 29], a frequent feeling of familial responsibility to get tested [30], and high satisfaction and low distress after receiving their results [28]. These studies also found that patients generally preferred receiving some but not all incidental germline findings, mainly because of concern regarding inability to prevent a future disease for which a high risk had been identified [31].
Undoubtedly the unrealistically high expectations of patients regarding tumor NGS are related, at least in some cases, to poor knowledge. Patients to whom the genetics vocabulary is foreign may already be so confused at the onset of any discussion prior to testing, or at the time of results disclosure, that they are unable to absorb information about the very low clinical utility of tumor NGS. This hypothesis is supported by the finding of Gornick et al. [18], who identified that 25% of patients could not recall disclosure of test results despite clear documentation that such disclosure had occurred. Moreover, in the few studies in which participants were generally highly educated and had greater knowledge of genetic concepts, there was greater awareness of the low potential for personal benefit and more interest in undergoing testing for altruistic purposes such as furthering scientific research. It is quite likely that the written and verbal information that is currently being given to patients prior to their consenting to tumor NGS is only comprehensible to highly educated individuals. It has been recommended that patient education materials should not exceed a sixth grade reading level [32]. In designing and evaluating such tools it would be most helpful to have a standardized instrument for assessing patient knowledge of tumor NGS, as most cancer validated genetics knowledge assessment tools reported in the literature were designed for patients undergoing germline testing. The questionnaire developed by Rogith et al. [8] assesses more relevant concepts and deserves further validation testing. Furthermore, given the differences between oncologists and patients in their perception of the importance of specific topics and methods of communication [27], it is essential that patients as well as health care professionals be involved in developing NGS discussion materials and guidelines. For example, a tool called Genomics ADvISER has recently been developed to help patients understand incidental findings that might be revealed by germline testing. This tool familiarizes patients with five categories of potential incidental sequencing results and gives patients a chance to choose for themselves which subset(s) of information they would like to receive. The Genomics ADvISER begins with an educational whiteboard video and then engages users in a values clarification exercise, knowledge quiz, and final choice step [33]. Such a tool could quite likely be adapted for a population undergoing tumor NGS to aid with decision-making regarding disclosure of secondary findings.
One of the greatest barriers to educating patients about tumor genomic profiling is the limited knowledge of most oncologists in this area. Specific knowledge translation interventions are necessary, as are standardized measures for evaluating the utility of these interventions. Although the American College of Medical Genetics and Genomics has published guidelines regarding disclosure of secondary findings [34], oncologists would most likely be unaware of these guidelines and how to incorporate them into their practice. With respect to attitudes, there was also considerable variation among oncologists regarding the appropriateness of disclosing nonactionable somatic testing results and incidental germline findings. A consensus conference focusing on these issues would be most welcome. Educational efforts must also be directed toward nurses and other oncology health care professionals to whom patients frequently turn for clarification of information. Genetic counselors, who are almost always involved in counseling patients prior to germline testing and upon disclosure of test results, are often not part of the discussion when tumor NGS is being considered. Given their expertise, their involvement prior to tumor NGS testing would be most valuable to patients and well as oncologists.
One almost certain source of confusion for patients and health care professionals alike is the significant variation in terminology used when referring to tumor genomic testing. Synonymous terms used in the articles we reviewed included “tumor profiling,” “tumor genomic profiling,” “molecular profiling,” “molecular testing,” “genomic sequencing,” “genomic testing in cancer,” “genetic testing,” “tumor genomic testing,” “molecular diagnostics,” “(tumor multigene) next-generation sequencing (NGS),” and “whole-exome sequencing (WES).” Standardization of the nomenclature would be a simple but enormously helpful step.
There are several additional major challenges for cancer programs wishing to design appropriate educational materials pertaining to tumor NGS. One such challenge is the great variability in the number and nature of “actionable” mutations for the various tumor sites and subtypes. The European Society for Medical Oncology (ESMO) has recently developed a framework to categorize tumor genomic alterations, the ESMO Scale of Clinical Actionability of Molecular Targets (ESCAT). ESCAT defines six levels of clinical evidence for molecular targets according to the implications for patient management. Tier I targets are ready for implementation in routine clinical practice based on data from randomized controlled clinical trials, such as EGFR inhibitors for non-small cell lung cancer. Tier II alterations likely confer benefit from a targeted drug, but additional data are necessary. Tier III mutations include those that were detected in a yet unstudied tumor type but that have been previously proven to confer benefit from a targeted agent in another tumor type, as well as mutations predictive of benefit from a targeted agent effective against other alterations in the same pathway. Tier IV mutations are potential drug targets supported only by preclinical evidence, and Tier V mutations have shown evidence of antitumor activity without clinical benefit but may be candidates for combination therapy in the future. For Tier X alterations, no clinical or preclinical evidence supporting their utility exists to date [35]. The second challenge is that for each tumor type and specific target, the availability of targeted treatments varies according to insurance funding policies and access to compassionate drug programs or clinical trials.
Given the complexities of genomic concepts and testing, a modular approach to developing patient education materials would seem most appropriate. One or more general modules describing concepts such as basic genetics, differences between germline and somatic testing, general description of the ESCAT categories, and the nature of secondary findings would be suitable for most patients, independent of tumor type or institution. A standardized module that can be individualized for that patient, taking into account any testing costs, biopsy requirements, ESCAT mutation frequency for that patient's tumor type and testing panel, and local treatment /clinical trial availability, would be extremely useful for pretest counseling. Finally, a standardized, individualized module should be used for disclosure of test results.
Strengths of this review include the use of broad search terms, which allowed us to conduct a comprehensive search, and identification of studies of patients with diverse tumor types in countries with varying health care systems. However, our review may have been limited by the single database searched, the relatively small number of articles that fulfilled our search criteria, and the small sample size of some of the studies. Yet the relative uniformity of findings across these studies is reassuring. Also, because tumor NGS is still relatively expensive, the studies were all done in wealthy developed countries where such testing is currently available.
Conclusion
These results point to the need for improved patient education regarding tumor genomic profiling so that the decision to undergo testing is based on accurate understanding of the potential benefits and limitations, thereby leading to realistic expectations. Modular educational materials, based on consensus guidelines and developed in conjunction with patients, might consist of written brochures or booklets, videos, or infographics presented in language that is clear and understandable even to patients with lower levels of literacy. Also, of great importance is educating oncology health care professionals to enable them to knowledgeably discuss testing with appropriate patients and thereby optimize shared decision-making. Finally, our results highlight the need to improve access to NGS testing and related research for patients of varying socioeconomic backgrounds and ethnicities.
Acknowledgments
We thank Henry Lam for his assistance with the literature search.
Author Contributions
Conception/design: Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner
Collection and/or assembly of data: Melika Shirdarreh, Orly Aziza, Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner
Data analysis and interpretation: Melika Shirdarreh, Orly Aziza, Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner
Manuscript writing: Melika Shirdarreh, Orly Aziza, Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner
Final approval of manuscript: Melika Shirdarreh, Orly Aziza, Rossanna C. Pezo, Katarzyna J. Jerzak, Ellen Warner
Disclosures
Rossanna C. Pezo: Merck (RF), Novartis, Pfizer, AstraZeneca, Mylan, Bristol-Myers Squibb, Exact Sciences, Myria, Eli Lilly & Co. (C/A); Katarzyna J. Jerzak: Amgen, AstraZeneca, Apo Biologix, Eli Lilly & Co., Esai, Genomic Health, Knight Therapeutics, Merck, Pfizer, Roche, Novartis, Purdue Pharma (C/A), Amgen, AstraZeneca, Eli Lilly & Co., Genomic Health, Knight Therapeutics, Merck, Pfizer, Roche, Novartis, Purdue Pharma (SAB), AstraZeneca, Eli Lilly & Co. (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Contributed equally as co-first authors.
Disclosures of potential conflicts of interest may be found at the end of this article.



