-
PDF
- Split View
-
Views
-
Cite
Cite
Guoqing Zhang, Peiyi Xia, Shanshan Zhao, Lulu Yuan, Xiaosu Wang, Xiangnan Li, Jindong Li, Gefitinib Combined with Cetuximab for the Treatment of Lung Adenocarcinoma Harboring the EGFR–Intergenic Region (SEC61G) Fusion and EGFR Amplification, The Oncologist, Volume 26, Issue 11, November 2021, Pages e1898–e1902, https://doi.org/10.1002/onco.13921
Close - Share Icon Share
Abstract
EGFR fusions are rare genomic events in non-small cell lung cancer (NSCLC), and a total of nine types have been previously reported in lung adenocarcinoma: EGFR-RAD51, EGFR-PURB, EGFR-ANXA2, EGFR-ZNF713, EGFR-YAP1, USP42-EGFR, EGFR-SEPTIN14, EGFR-TNS3, and EGFR-ZCCHC6. EGFR fusion mutations combined with EGFR amplification are even rarer in NSCLC. The EGFR–intergenic region (IGR) fusion mutation is unreported, and thus, there are no studies targeting this fusion together with EGFR amplification in lung adenocarcinoma. Our brief study provides clinical evidence that combined targeted therapy with gefitinib and cetuximab could result in a significant antitumor response in patients with the EGFR-IGR fusion and EGFR amplification.
EGFR fusion mutations are rare, and EGFR fusion mutations combined with EGFR amplification are even rarer in non-small cell lung cancer (NSCLC). To the authors’ knowledge, there is no previous report on the coexistence of the EGFR–intergenic region (IGR) fusion and EGFR amplification.
This is the first report of a patient with NSCLC with the EGFR-IGR fusion and EGFR amplification who achieved a significant antitumor response from treatment with gefitinib combined with cetuximab.
Patient Story
A 68-year-old man with an approximately 100-pack-year smoking history complained of chest pain and was admitted to our hospital on September 12, 2020. Thoracic computed tomography (CT) and positron emission tomography (PET)–CT scans showed a 2.6-cm right upper lung mass and metastatic carcinoma of the left fourth rib (Fig. 1A; Fig. 2A). A CT-guided biopsy of the pulmonary lesion showed that the mass was a lung adenocarcinoma, and the patient was diagnosed with lung adenocarcinoma (cT1bN0 M1b stage IVA) with solitary bone metastasis. A biopsy tissue sample was obtained for next-generation sequencing (NGS) using a panel of 14 cancer-related genes (Geneseeq, Nanjing, China) on September 24, 2020.
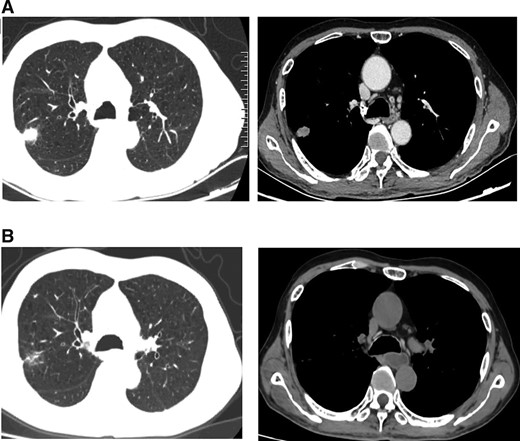
(A): Computed tomography (CT) scan before gefitinib and cetuximab treatment. (B): CT scan showing a partial response after 2 months of gefitinib and cetuximab treatment.
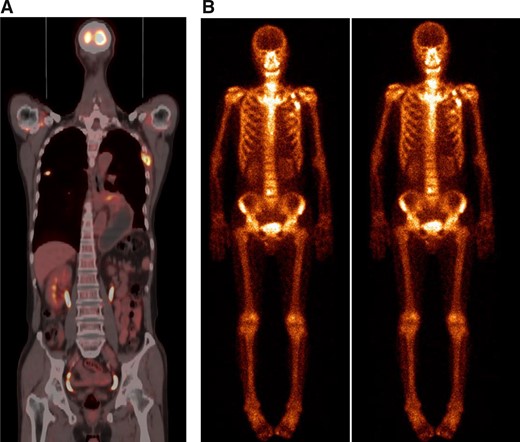
The foci of the left fourth rib turned out to be a fracture 2 months after diagnosis. (A): Positron emission tomography–computed tomography scan showed a right upper lung mass and metastatic foci of the left fourth rib. (B): The foci showed no response to treatment from two consecutive (November 2, 2020, and December 3, 2020) bone scintigraphies (and turned out to be benign, as revealed by bone biopsy).
Molecular Tumor Board
The EGFR–intergenic region (IGR) (SEC61G) fusion (the mutation abundance was 79.8%; Fig. 3) and EGFR amplification (copy number: 5.6) were detected. The breakpoint positions of EGFR- IGR were near EGFR 7p11.2, which includes EGFR exons 1–25 (1,005 amino acids) and 32 amino acids downstream of SEC61G.
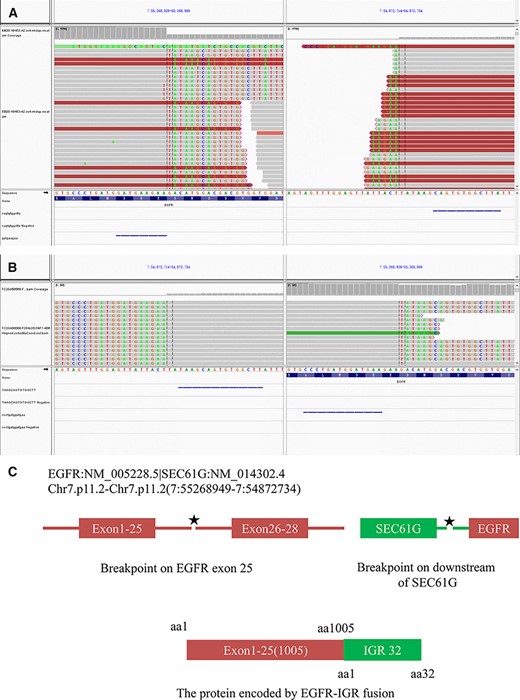
(A): Integrative Genomics Viewer snapshot of EGFR–intergenic region (IGR) fusion (DNA analysis). (B): Integrative Genomics Viewer snapshot of EGFR-IGR (RNA analysis). (C): Schematic representation of the EGFR-IGR fusion protein domain structure.
Limited evidence suggests that patients with EGFR-RAD51 [1–4], EGFR-PURB [1], and EGFR-SEPTIN14 [5] mutations exhibit a marked tumor response to EGFR tyrosine kinase inhibitor (TKI) therapies [1–5]. Herein, we report a case of lung adenocarcinoma with a novel EGFR fusion type (EGFR-IGR) as well as EGFR amplification, which added to the complexity of the treatment of this patient.
EGFR contains several autophosphorylation sites in the C-terminal tail of the receptor that positively regulate the transforming activity of EGFR by mediating downstream proliferative signaling. Previous studies have shown that autophosphorylation sites in the regulatory domain, which serve as docking sites for signaling adaptor proteins, are lacking in most EGFR fusion mutations [1]. In our case, the breakpoint positions of EGFR-IGR were near EGFR 7p11.2, which includes EGFR exons 1–25 (1,005 amino acids) and 32 amino acids downstream of SEC61G. That is, the kinase domain of EGFR-IGR contains tyrosine 845, tyrosine 920, and tyrosine 992 phosphorylation sites, which have been confirmed to be oncogenic [6]. Studies have shown that several types of EGFR fusions can be therapeutically targeted with EGFR TKIs. Thus, we assumed that our patient might benefit from EGFR TKIs.
EGFR overexpression leads to asymmetric dimer formation, increased downstream signaling activation, and consequent oncogenicity. However, the evidence supporting the application of this mutation as a biomarker for EGFR TKI therapy response is weak. Although studies have demonstrated that EGFR amplification is a biomarker for the response to EGFR TKI therapy in patients with advanced non-small cell lung cancer (NSCLC) [7], the predictive value of EGFR amplification may be driven by coexisting EGFR mutations [8]. Additionally, EGFR amplification has been shown to be one of the most common resistance mechanisms in patients undergoing treatment with third-generation EGFR TKIs [9].
The efficacy of EGFR monoclonal antibodies (mAbs) has been evaluated in lung cancer for a long time, and the findings are highly controversial. Fortunately, the EGFR expression level or the EGFR gene copy number may be a predictive factor of the clinical benefit of the addition of cetuximab to the chemotherapy regimen [10–12]. As our patient exhibited high EGFR expression and EGFR amplification, we predicted that he might benefit from EGFR mAbs.
However, no investigation has focused on the EGFR-IGR fusion mutation combined with EGFR amplification, leaving the optimal therapy unclear. Recently, the combination of an EGFR TKI (afatinib) with cetuximab did not improve outcomes in previously untreated EGFR-mutant NSCLC [13]. We were interested in searching for a therapeutic regimen for our unique patient, and we sought to determine the effectiveness of EGFR TKIs combined with cetuximab against the EGFR-IGR fusion combined with EGFR amplification.
Case Report
After discussing the patient's case, the lung cancer multidisciplinary team (MDT) recommended treatment with gefitinib (250 mg every day) and cetuximab (500 mg/m2 every 2 weeks) on October 1, 2020. The risks and benefits of the combination of gefitinib with cetuximab treatment were fully explained to the patient. Informed consent was obtained from the patient, and this study was approved by the Zhengzhou University Institutional Review Board. After 2 months, a chest CT scan showed a decrease in tumor size (Fig. 1B). In particular, the metastatic foci of the left fourth rib diagnosed from PET-CT (Fig. 2A) turned out to be a fracture, as shown by two consecutive bone scintigraphies (Fig. 2B) and a bone biopsy. According to RECIST guidelines, the patient was considered to have a partial response to gefitinib combined with cetuximab. The side effect of the treatment was third-degree facial acne.
Another discussion with the lung cancer MDT after 2 months of treatment led to the conclusion that the patient should undergo surgical treatment. After adequate preoperative preparation, a right upper lobectomy with mediastinal lymphadenectomy was performed. The final pathology report revealed a 1.3-cm pyT1bN0 adenocarcinoma. Another NGS analysis of the tumor was conducted. Interestingly, the results illustrated that the former EGFR-IGR fusion mutation and EGFR amplification disappeared, and no drug-resistance–conferring mutations appeared.
Herein, we provide the first report of a patient with NSCLC with the EGFR-IGR fusion and EGFR amplification who achieved a significant antitumor response from treatment with gefitinib combined with cetuximab. In the future, we intend to explore fundamental research on such rare mutations in terms of the mechanism of tumorigenesis.
Acknowledgements
This study was supported by the Key Scientific Research Projects of Institutions of Higher Learning in Henan Province (No. 21A320032). The study protocol was reviewed and approved by the Ethics Committee of Zhengzhou University School of Medicine.
Glossary of Genomic Terms and Nomenclature
EGFR: epidermal growth factor receptor
IGR: intergenic region
Author Contributions
Conception/design: Guoqing Zhang, Peiyi Xia, Shanshan Zhao, Xiangnan Li, Jindong Li
Provision of study material or patients: Guoqing Zhang, Peiyi Xia, Lulu Yuan, Xiaosu Wang
Collection and/or assembly of data: Guoqing Zhang, Peiyi Xia, Lulu Yuan, Xiaosu Wang
Data analysis and interpretation: Guoqing Zhang, Peiyi Xia, Lulu Yuan, Xiaosu Wang
Manuscript writing: Guoqing Zhang, Peiyi Xia, Shanshan Zhao, Lulu Yuan, Xiaosu Wang, Xiangnan Li, Jindong Li
Final approval of manuscript: Guoqing Zhang, Peiyi Xia, Shanshan Zhao, Lulu Yuan, Xiaosu Wang, Xiangnan Li, Jindong Li
Disclosures
The authors indicated no financial relationships.
References
Author notes
Contributed equally.
Disclosures of potential conflicts of interest may be found at the end of this article.



