-
PDF
- Split View
-
Views
-
Cite
Cite
Ryo Ko, Takehito Shukuya, Yusuke Okuma, Kazunari Tateishi, Hisao Imai, Shunichiro Iwasawa, Eisaku Miyauchi, Akiko Fujiwara, Tomohide Sugiyama, Keisuke Azuma, Keiko Muraki, Masahiro Yamasaki, Hisashi Tanaka, Yuta Takashima, Sayo Soda, Osamu Ishimoto, Nobuyuki Koyama, Satoshi Morita, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi, North East Japan Study Group, Prognostic Factors and Efficacy of First‐Line Chemotherapy in Patients with Advanced Thymic Carcinoma: A Retrospective Analysis of 286 Patients from NEJ023 Study, The Oncologist, Volume 23, Issue 10, October 2018, Pages 1210–1217, https://doi.org/10.1634/theoncologist.2017-0586
Close - Share Icon Share
Abstract
The prognostic factors and the efficacy of first‐line chemotherapy remain unclear in patients with advanced thymic carcinoma.
We conducted a multi‐institutional retrospective study named NEJ023 for patients with advanced thymic carcinoma. All patients without any indication of curative treatment were treated with chemotherapy from 1995 to 2014 at 40 institutions of the North East Japan Study Group.
A total of 286 patients with advanced thymic carcinoma were analyzed. First‐line chemotherapy included platinum‐based doublets in 62.2% of the patients, monotherapy in 3.5%, and other multidrug chemotherapy (e.g., cisplatin, doxorubicin, vincristine, and cyclophosphamide [ADOC]) in 34.3%. The median follow‐up period was 55.5 months, and the median overall survival (OS) from the start of first‐line chemotherapy was 30.7 months (95% confidence interval, 25.9–35.9 months). There was no significant difference in OS among different first‐line chemotherapy regimens (e.g., between carboplatin/paclitaxel and ADOC, median OS: 27.8 vs. 29.9 months). Masaoka‐Koga stage IVa and volume reduction surgery were favorable prognostic factors for OS in the multivariate analysis using the Cox proportional hazards model.
The efficacy of each first‐line chemotherapy regimen for advanced thymic carcinoma did not vary significantly. Our results might support the adequacy of the use of carboplatin/paclitaxel as first‐line chemotherapy for these patients.
Because of its rarity, there is limited information about prognostic factors and efficacy of chemotherapy in patients with advanced thymic carcinoma. This is the largest data set for those patients treated with chemotherapy. This study suggests there is no significant difference in efficacy between carboplatin/paclitaxel and cisplatin/doxorubicin/vincristine/cyclophosphamide for advanced thymic carcinoma. This result can support the adequacy of the selection of platinum doublets as treatment for those patients, rather than anthracycline‐based multidrug regimen.
Abstract
摘要
背景?晚期胸腺癌患者的预后因素和一线化疗的疗效尚不清楚?
材料与方法?我们对晚期胸腺癌患者进行了一项名为 NEJ023 的多机构回顾性研究?1995‐2014 年间,所有无根治性治疗指征的患者在日本东北部研究小组的40个机构中接受了化疗?
结果?本文对286例晚期胸腺癌患者的临床资料进行分析?一线化疗包括铂类双药62.2%?单药治疗3.5%和其他多药化疗34.3% [如顺铂?阿霉素?长春新碱和环磷酰胺 (ADOC)] ?中位随访期为55.5个月,一线化疗开始后的中位总生存期(OS)为30.7个月(95% 置信区间,25.9–35.9月)?不同一线化疗方案的OS无显着差异(如卡铂/紫杉醇与ADOC,中位OS为27.8 vs. 29.9个月)?采用Cox比例风险模型进行多因素分析,Masaoka‐Koga分期IVa期和减容术是有利于OS的预后因素?
结论?各一线化疗方案对晚期胸腺癌的疗效无显着差异?我们的结果也许能支持卡铂/紫杉醇作为这些患者的一线化疗药物?
Introduction
Thymic epithelial tumors, comprising thymoma and thymic carcinoma, are rare cancers with an annual incidence of approximately 0.15 cases per 100,000 [1]. Of these, thymic carcinoma is a minor component of thymic epithelial tumors, which accounts for only 14.1% of cases [2]. Compared with thymoma, the tumor is highly atypical and tends to metastasize and invade surrounding tissues at an early stage. Thus, its prognosis is poor, with a 5‐year survival rate of only 30%–50% [2, 3].
Approximately half of the patients with thymic carcinoma have advanced‐stage disease at initial presentation [2-4]. Therefore, systemic chemotherapy is an important therapeutic modality for this malignant disease. However, due to the rarity and heterogeneity of biology, there is limited information about chemotherapy for advanced thymic carcinoma (Table 1) [5-14]. Hence, the optimal therapeutic strategy remains controversial. Presently, platinum combination chemotherapy, with or without anthracycline, is a widely used first‐line chemotherapy for thymic carcinoma. The National Comprehensive Cancer Network (NCCN) Guidelines recommend carboplatin/paclitaxel over the platinum combination regimen with anthracycline (e.g., cisplatin, doxorubicin, vincristine, cyclophosphamide [ADOC]) based on profile of toxicity [15]. However, there are no comparative trials, and the best regimen for thymic carcinoma is still unknown.
Previous reports for first‐line chemotherapy in patients with thymic carcinoma
| Author . | Year . | Study design . | Sample size . | Regimen . | RR, % . | mPFS, mos . | mOS, mos . |
|---|---|---|---|---|---|---|---|
| Yoh | 2003 | Retrospective | 12 | CODE | 42.0 | 5.6 | 46.0 |
| Agatsuma | 2011 | Retrospective | 34 | ADOC | 50.0 | N/A | 21.3 |
| Loehrer | 2001 | Prospective | 8 | VIP | 25.0 | N/A | N/A |
| Igawa | 2010 | Retrospective | 11 | CP | 36.4 | 7.9 | 22.0 |
| Lemmma | 2011 | Prospective | 23 | CP | 21.7 | 5.0 | 20.0 |
| Furugen | 2011 | Retrospective | 16 | CP | 37.5 | 8.6 | 49.4 |
| Okuma | 2011 | Retrospective | 9 | IP | 55.6 | 7.9 | 33.8 |
| Hirai | 2015 | Prospective | 39 | CP | 35.9 | 7.5 | N/A |
| Inoue | 2014 | Prospective | 33 | CA | 30.0 | 7.6 | 27.3 |
| Luo | 2016 | Retrospective | 13 | CG | 61.5 | 14.5 | 50.7 |
| Author . | Year . | Study design . | Sample size . | Regimen . | RR, % . | mPFS, mos . | mOS, mos . |
|---|---|---|---|---|---|---|---|
| Yoh | 2003 | Retrospective | 12 | CODE | 42.0 | 5.6 | 46.0 |
| Agatsuma | 2011 | Retrospective | 34 | ADOC | 50.0 | N/A | 21.3 |
| Loehrer | 2001 | Prospective | 8 | VIP | 25.0 | N/A | N/A |
| Igawa | 2010 | Retrospective | 11 | CP | 36.4 | 7.9 | 22.0 |
| Lemmma | 2011 | Prospective | 23 | CP | 21.7 | 5.0 | 20.0 |
| Furugen | 2011 | Retrospective | 16 | CP | 37.5 | 8.6 | 49.4 |
| Okuma | 2011 | Retrospective | 9 | IP | 55.6 | 7.9 | 33.8 |
| Hirai | 2015 | Prospective | 39 | CP | 35.9 | 7.5 | N/A |
| Inoue | 2014 | Prospective | 33 | CA | 30.0 | 7.6 | 27.3 |
| Luo | 2016 | Retrospective | 13 | CG | 61.5 | 14.5 | 50.7 |
Abbreviations: ADOC, cisplatin/doxorubicin/vincristine/cyclophosphamide; CA, carboplatin/amrubicin; CG, cisplatin/gemcitabine; CODE, cisplatin/vincristine/doxorubicin/etoposide; CP, carboplatin/paclitaxel; IP, cisplatin/irinotecan; mos, months; mOS, median overall survival; mPFS, median progression‐free survival; N/A, not analyzed; RR, response rate; VIP, etoposide/ifosfamide/cisplatin.
Previous reports for first‐line chemotherapy in patients with thymic carcinoma
| Author . | Year . | Study design . | Sample size . | Regimen . | RR, % . | mPFS, mos . | mOS, mos . |
|---|---|---|---|---|---|---|---|
| Yoh | 2003 | Retrospective | 12 | CODE | 42.0 | 5.6 | 46.0 |
| Agatsuma | 2011 | Retrospective | 34 | ADOC | 50.0 | N/A | 21.3 |
| Loehrer | 2001 | Prospective | 8 | VIP | 25.0 | N/A | N/A |
| Igawa | 2010 | Retrospective | 11 | CP | 36.4 | 7.9 | 22.0 |
| Lemmma | 2011 | Prospective | 23 | CP | 21.7 | 5.0 | 20.0 |
| Furugen | 2011 | Retrospective | 16 | CP | 37.5 | 8.6 | 49.4 |
| Okuma | 2011 | Retrospective | 9 | IP | 55.6 | 7.9 | 33.8 |
| Hirai | 2015 | Prospective | 39 | CP | 35.9 | 7.5 | N/A |
| Inoue | 2014 | Prospective | 33 | CA | 30.0 | 7.6 | 27.3 |
| Luo | 2016 | Retrospective | 13 | CG | 61.5 | 14.5 | 50.7 |
| Author . | Year . | Study design . | Sample size . | Regimen . | RR, % . | mPFS, mos . | mOS, mos . |
|---|---|---|---|---|---|---|---|
| Yoh | 2003 | Retrospective | 12 | CODE | 42.0 | 5.6 | 46.0 |
| Agatsuma | 2011 | Retrospective | 34 | ADOC | 50.0 | N/A | 21.3 |
| Loehrer | 2001 | Prospective | 8 | VIP | 25.0 | N/A | N/A |
| Igawa | 2010 | Retrospective | 11 | CP | 36.4 | 7.9 | 22.0 |
| Lemmma | 2011 | Prospective | 23 | CP | 21.7 | 5.0 | 20.0 |
| Furugen | 2011 | Retrospective | 16 | CP | 37.5 | 8.6 | 49.4 |
| Okuma | 2011 | Retrospective | 9 | IP | 55.6 | 7.9 | 33.8 |
| Hirai | 2015 | Prospective | 39 | CP | 35.9 | 7.5 | N/A |
| Inoue | 2014 | Prospective | 33 | CA | 30.0 | 7.6 | 27.3 |
| Luo | 2016 | Retrospective | 13 | CG | 61.5 | 14.5 | 50.7 |
Abbreviations: ADOC, cisplatin/doxorubicin/vincristine/cyclophosphamide; CA, carboplatin/amrubicin; CG, cisplatin/gemcitabine; CODE, cisplatin/vincristine/doxorubicin/etoposide; CP, carboplatin/paclitaxel; IP, cisplatin/irinotecan; mos, months; mOS, median overall survival; mPFS, median progression‐free survival; N/A, not analyzed; RR, response rate; VIP, etoposide/ifosfamide/cisplatin.
The prognostic factors remain unclear in patients with advanced thymic carcinoma treated with chemotherapy. There have been some reports of the prognostic factors in thymic epithelial tumors, but most of the enrolled patients were with thymoma. There have been a few reports of the prognostic factors in patients with thymic carcinoma. In these limited reports, complete resection (resectability) [3,4,16,17], Masaoka‐Koga stage [3,4,17,18], sex [3], Karnofsky performance status (PS) [4], histology [4], lymph nodes metastasis [17], and response for first‐line chemotherapy [19] were reported as the prognostic factors. However, a majority of patients included in these studies had early‐stage disease. There are limited data for the prognostic factors in patients with advanced thymic carcinoma.
This study aims to evaluate the efficacy of chemotherapy in advanced thymic carcinoma and to compare the chemotherapy regimens and identify the promising candidates of chemotherapeutic regimen for further clinical investigation. The prognostic factors for patients with advanced thymic carcinoma treated with chemotherapy were also evaluated in this study. This study was registered with the University hospital Medical Information Network (UMIN) Clinical Trials Registry (ID: UMIN000015649).
Materials and Methods
Study Cohort
In this observational multicenter study, we retrospectively reviewed the medical files of patients diagnosed and treated between April 1995 and March 2014 in Japan. All the institutions in the North East Japan Study Group were invited to participate in this study. Inclusion criteria for this study were (a) histologic diagnosis of thymic carcinoma in each institution; (b) advanced stage without any indication for curative‐intent surgery or radiotherapy at diagnosis or recurrent thymic carcinoma without indication for curative‐intent treatment; and (c) treated with palliative‐intent chemotherapy.
Data Analysis
Data were initially obtained from 324 consecutive patients in 40 institutions. Thirty‐seven patients who did not meet the eligibility requirements and one patient for whom required data were unavailable were excluded from this analysis; thus, 286 patients were finally analyzed (supplemental online Fig. 1). The Institutional Review Boards of all the participating institutions approved the protocol of this retrospective study.
The following details were extracted from the medical records: date of diagnosis, sex, age, Eastern Cooperative Oncology Group (ECOG) PS, histology, Masaoka‐Koga staging [18], World Health Organization (WHO) TNM staging [20, 21], data of tumor markers (carcinoembryonic antigen, squamous cell carcinoma antigen, cytokeratin‐19 fragments, pro‐gastrin‐releasing peptide, neuron‐specific enolase, and α‐fetoprotein), data of blood tests (lactate dehydrogenase, hemoglobin, albumin, and calcium), metastatic sites, smoking history, past medical history, information of paraneoplastic syndrome, information of superior vena cava obstruction, history of surgery for primary site, history of radiotherapy for primary site, date of death/last follow‐up, regimen of chemotherapies, duration of chemotherapies, and efficacy of chemotherapies. Further, we collected the date of initiation and progressive disease of a part of chemotherapeutic regimens (carboplatin/paclitaxel, cisplatin/etoposide, carboplatin/etoposide, cisplatin/irinotecan, cisplatin/docetaxel, ADOC, cisplatin/doxorubicin/cyclophosphamide, S‐1 monotherapy, docetaxel monotherapy, and amrubicin monotherapy). The histologic subtypes were determined based on the 2004 World Health Organization classification [20]. For this study, the efficacy of chemotherapy was evaluated by RECIST version 1.1 [22] at each center. In this study, “volume reduction surgery” was defined as only debulking surgery without intention of curability.
Statistical Analysis
All categorical variables were analyzed by the Fisher's exact test, as applicable. All continuous variables were analyzed using the Mann‐Whitney U test. The Kaplan‐Meier method was used to estimate overall survival (OS) and progression‐free survival (PFS) curves. The log‐rank test was used to evaluate the differences of OS and PFS among subgroups. Cox proportional hazards models were used to adjust for potential confounding factors. A p < .05 was considered statistically significant. All analyses were performed using JMP 10 for Windows statistical software (SAS Institute Japan Inc., Tokyo, Japan).
OS was defined as the period between the start of chemotherapy and the date of death from any cause; PFS was defined as the period between the start of chemotherapy and progressive disease or death from any cause.
Results
Patient Characteristics
The clinical characteristics of 286 patients with advanced thymic carcinoma are shown in Table 2. The study population comprised 202 men and 84 women, with the median age at diagnosis of 61 years (range, 13–84 years). Most patients in the study had good ECOG PS, and 181 (63.3%) patients were former/current smokers. The most frequent histological subtype was squamous cell carcinoma (66.4%), followed by undifferentiated carcinoma (10.5%) and poorly differentiated neuroendocrine carcinoma (10.1%). The Masaoka‐Koga stages III, IVa, and IVb were noted in 14 (4.9%), 75 (26.2%), and 144 (50.3%) patients, and the Union for International Cancer Control (UICC) TNM stages III and IV were noted in 11 (3.8%) and 222 (77.6%) patients, respectively. Fifty‐three (18.5%) patients were postoperative recurrence. Data of tumor markers are shown in supplemental online Table 1.
| Characteristics . | n (%) . |
|---|---|
| Age in years, median (range) | 61 (13–84) |
| Sex | |
| Male/female | 202/84 (70.6/29.4) |
| ECOG PS | |
| 0 | 102 (35.7) |
| 1 | 146 (51.0) |
| 2 | 29 (10.1) |
| 3 | 2 (0.7) |
| Unknown | 7 (2.4) |
| Smoking status | |
| Never | 101 (35.3) |
| Former/current | 181 (63.3) |
| Unknown | 4 (1.4) |
| Histology | |
| Squamous cell carcinoma | 190 (66.4) |
| Undifferentiated carcinoma | 30 (10.5) |
| Mucoepidermoid carcinoma | 2 (0.7) |
| Lymphoepithelioma‐like carcinoma | 1 (0.3) |
| Adenocarcinoma | 4 (1.4) |
| Sarcomatoid carcinoma | 2 (0.7) |
| Basaloid carcinoma | 1 (0.3) |
| Papillary adenocarcinoma | 1 (0.3) |
| Poorly differentiated neuroendocrine carcinoma | 29 (10.1) |
| Well‐differentiated neuroendocrine carcinoma | 8 (2.8) |
| Staging | |
| Masaoka‐Koga staging | |
| Stage III | 14 (4.9) |
| Stage IVa | 75 (26.2) |
| Stage IVb | 144 (50.3) |
| Postoperative recurrence | 53 (18.5) |
| WHO TNM staging | |
| Stage III | 11 (3.8) |
| Stage IV | 222 (77.6) |
| Postoperative recurrence | 53 (18.5) |
| Characteristics . | n (%) . |
|---|---|
| Age in years, median (range) | 61 (13–84) |
| Sex | |
| Male/female | 202/84 (70.6/29.4) |
| ECOG PS | |
| 0 | 102 (35.7) |
| 1 | 146 (51.0) |
| 2 | 29 (10.1) |
| 3 | 2 (0.7) |
| Unknown | 7 (2.4) |
| Smoking status | |
| Never | 101 (35.3) |
| Former/current | 181 (63.3) |
| Unknown | 4 (1.4) |
| Histology | |
| Squamous cell carcinoma | 190 (66.4) |
| Undifferentiated carcinoma | 30 (10.5) |
| Mucoepidermoid carcinoma | 2 (0.7) |
| Lymphoepithelioma‐like carcinoma | 1 (0.3) |
| Adenocarcinoma | 4 (1.4) |
| Sarcomatoid carcinoma | 2 (0.7) |
| Basaloid carcinoma | 1 (0.3) |
| Papillary adenocarcinoma | 1 (0.3) |
| Poorly differentiated neuroendocrine carcinoma | 29 (10.1) |
| Well‐differentiated neuroendocrine carcinoma | 8 (2.8) |
| Staging | |
| Masaoka‐Koga staging | |
| Stage III | 14 (4.9) |
| Stage IVa | 75 (26.2) |
| Stage IVb | 144 (50.3) |
| Postoperative recurrence | 53 (18.5) |
| WHO TNM staging | |
| Stage III | 11 (3.8) |
| Stage IV | 222 (77.6) |
| Postoperative recurrence | 53 (18.5) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; WHO, World Health Organization.
| Characteristics . | n (%) . |
|---|---|
| Age in years, median (range) | 61 (13–84) |
| Sex | |
| Male/female | 202/84 (70.6/29.4) |
| ECOG PS | |
| 0 | 102 (35.7) |
| 1 | 146 (51.0) |
| 2 | 29 (10.1) |
| 3 | 2 (0.7) |
| Unknown | 7 (2.4) |
| Smoking status | |
| Never | 101 (35.3) |
| Former/current | 181 (63.3) |
| Unknown | 4 (1.4) |
| Histology | |
| Squamous cell carcinoma | 190 (66.4) |
| Undifferentiated carcinoma | 30 (10.5) |
| Mucoepidermoid carcinoma | 2 (0.7) |
| Lymphoepithelioma‐like carcinoma | 1 (0.3) |
| Adenocarcinoma | 4 (1.4) |
| Sarcomatoid carcinoma | 2 (0.7) |
| Basaloid carcinoma | 1 (0.3) |
| Papillary adenocarcinoma | 1 (0.3) |
| Poorly differentiated neuroendocrine carcinoma | 29 (10.1) |
| Well‐differentiated neuroendocrine carcinoma | 8 (2.8) |
| Staging | |
| Masaoka‐Koga staging | |
| Stage III | 14 (4.9) |
| Stage IVa | 75 (26.2) |
| Stage IVb | 144 (50.3) |
| Postoperative recurrence | 53 (18.5) |
| WHO TNM staging | |
| Stage III | 11 (3.8) |
| Stage IV | 222 (77.6) |
| Postoperative recurrence | 53 (18.5) |
| Characteristics . | n (%) . |
|---|---|
| Age in years, median (range) | 61 (13–84) |
| Sex | |
| Male/female | 202/84 (70.6/29.4) |
| ECOG PS | |
| 0 | 102 (35.7) |
| 1 | 146 (51.0) |
| 2 | 29 (10.1) |
| 3 | 2 (0.7) |
| Unknown | 7 (2.4) |
| Smoking status | |
| Never | 101 (35.3) |
| Former/current | 181 (63.3) |
| Unknown | 4 (1.4) |
| Histology | |
| Squamous cell carcinoma | 190 (66.4) |
| Undifferentiated carcinoma | 30 (10.5) |
| Mucoepidermoid carcinoma | 2 (0.7) |
| Lymphoepithelioma‐like carcinoma | 1 (0.3) |
| Adenocarcinoma | 4 (1.4) |
| Sarcomatoid carcinoma | 2 (0.7) |
| Basaloid carcinoma | 1 (0.3) |
| Papillary adenocarcinoma | 1 (0.3) |
| Poorly differentiated neuroendocrine carcinoma | 29 (10.1) |
| Well‐differentiated neuroendocrine carcinoma | 8 (2.8) |
| Staging | |
| Masaoka‐Koga staging | |
| Stage III | 14 (4.9) |
| Stage IVa | 75 (26.2) |
| Stage IVb | 144 (50.3) |
| Postoperative recurrence | 53 (18.5) |
| WHO TNM staging | |
| Stage III | 11 (3.8) |
| Stage IV | 222 (77.6) |
| Postoperative recurrence | 53 (18.5) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; WHO, World Health Organization.
Chemotherapy Regimens
The first‐line chemotherapy regimens are shown in Table 3. One hundred seventy‐eight (62.2%) patients received platinum‐based doublets. In this treatment, the most popular regimens were carboplatin/paclitaxel (70 patients), followed by cisplatin/etoposide (35 patients), carboplatin/amrubicin (17 patients), cisplatin/irinotecan (16 patients), and carboplatin/etoposide (15 patients). Other multidrug chemotherapies using more than two anticancer agents were conducted for 98 (34.3%) patients. Of these, most patients received ADOC (79 patients). Ten patients received single‐agent chemotherapy as first‐line chemotherapy. One hundred ninety patients (66.4%) received second‐line chemotherapy, and 104 patients (36.3%) were treated with third‐line chemotherapy.
| Regimen . | Patients, n . | RR, % . | mPFS, mos . | MST, mos . |
|---|---|---|---|---|
| Platinum‐based doublets | 178 | 38.2 | N/A | 30.7 |
| CBDCA + PTX | 70 | 40.0 | 9.1 | 27.8 |
| CDDP + VP‐16 | 35 | 48.6 | 8.1 | 35.0 |
| CBDCA + AMR | 17 | 11.8 | N/A | 51.9 |
| CDDP + CPT‐11 | 16 | 66.7 | 8.7 | 27.2 |
| CBDCA + VP‐16 | 15 | 30.8 | 8.4 | 16.8 |
| CDDP + DTX | 9 | 22.2 | 6.0 | 33.3 |
| Other | 16 | N/A | N/A | N/A |
| Other multidrug chemotherapy | 98 | 42.7 | N/A | 29.9 |
| ADOC | 79 | 41.0 | 6.7 | 29.9 |
| PAC | 8 | 37.5 | 30.8 | 42.4 |
| Other | 11 | N/A | N/A | N/A |
| Single agent | 10 | 44.4 | N/A | 54.9 |
| All regimens | 286 | 40.0 | N/A | 30.7 |
| Regimen . | Patients, n . | RR, % . | mPFS, mos . | MST, mos . |
|---|---|---|---|---|
| Platinum‐based doublets | 178 | 38.2 | N/A | 30.7 |
| CBDCA + PTX | 70 | 40.0 | 9.1 | 27.8 |
| CDDP + VP‐16 | 35 | 48.6 | 8.1 | 35.0 |
| CBDCA + AMR | 17 | 11.8 | N/A | 51.9 |
| CDDP + CPT‐11 | 16 | 66.7 | 8.7 | 27.2 |
| CBDCA + VP‐16 | 15 | 30.8 | 8.4 | 16.8 |
| CDDP + DTX | 9 | 22.2 | 6.0 | 33.3 |
| Other | 16 | N/A | N/A | N/A |
| Other multidrug chemotherapy | 98 | 42.7 | N/A | 29.9 |
| ADOC | 79 | 41.0 | 6.7 | 29.9 |
| PAC | 8 | 37.5 | 30.8 | 42.4 |
| Other | 11 | N/A | N/A | N/A |
| Single agent | 10 | 44.4 | N/A | 54.9 |
| All regimens | 286 | 40.0 | N/A | 30.7 |
Abbreviations: ADOC, cisplatin/doxorubicin/vincristine/cyclophosphamide; AMR, amrubicin; CBDCA, carboplatin; CDDP, cisplatin; CPT‐11, irinotecan; DTX, docetaxel; mos, months; mPFS, median progression‐free survival; MST, median survival time; N/A, not analyzed; PAC, cisplatin/doxorubicin/cyclophosphamide; PTX, paclitaxel; RR, response rate; VP‐16, etoposide.
| Regimen . | Patients, n . | RR, % . | mPFS, mos . | MST, mos . |
|---|---|---|---|---|
| Platinum‐based doublets | 178 | 38.2 | N/A | 30.7 |
| CBDCA + PTX | 70 | 40.0 | 9.1 | 27.8 |
| CDDP + VP‐16 | 35 | 48.6 | 8.1 | 35.0 |
| CBDCA + AMR | 17 | 11.8 | N/A | 51.9 |
| CDDP + CPT‐11 | 16 | 66.7 | 8.7 | 27.2 |
| CBDCA + VP‐16 | 15 | 30.8 | 8.4 | 16.8 |
| CDDP + DTX | 9 | 22.2 | 6.0 | 33.3 |
| Other | 16 | N/A | N/A | N/A |
| Other multidrug chemotherapy | 98 | 42.7 | N/A | 29.9 |
| ADOC | 79 | 41.0 | 6.7 | 29.9 |
| PAC | 8 | 37.5 | 30.8 | 42.4 |
| Other | 11 | N/A | N/A | N/A |
| Single agent | 10 | 44.4 | N/A | 54.9 |
| All regimens | 286 | 40.0 | N/A | 30.7 |
| Regimen . | Patients, n . | RR, % . | mPFS, mos . | MST, mos . |
|---|---|---|---|---|
| Platinum‐based doublets | 178 | 38.2 | N/A | 30.7 |
| CBDCA + PTX | 70 | 40.0 | 9.1 | 27.8 |
| CDDP + VP‐16 | 35 | 48.6 | 8.1 | 35.0 |
| CBDCA + AMR | 17 | 11.8 | N/A | 51.9 |
| CDDP + CPT‐11 | 16 | 66.7 | 8.7 | 27.2 |
| CBDCA + VP‐16 | 15 | 30.8 | 8.4 | 16.8 |
| CDDP + DTX | 9 | 22.2 | 6.0 | 33.3 |
| Other | 16 | N/A | N/A | N/A |
| Other multidrug chemotherapy | 98 | 42.7 | N/A | 29.9 |
| ADOC | 79 | 41.0 | 6.7 | 29.9 |
| PAC | 8 | 37.5 | 30.8 | 42.4 |
| Other | 11 | N/A | N/A | N/A |
| Single agent | 10 | 44.4 | N/A | 54.9 |
| All regimens | 286 | 40.0 | N/A | 30.7 |
Abbreviations: ADOC, cisplatin/doxorubicin/vincristine/cyclophosphamide; AMR, amrubicin; CBDCA, carboplatin; CDDP, cisplatin; CPT‐11, irinotecan; DTX, docetaxel; mos, months; mPFS, median progression‐free survival; MST, median survival time; N/A, not analyzed; PAC, cisplatin/doxorubicin/cyclophosphamide; PTX, paclitaxel; RR, response rate; VP‐16, etoposide.
Efficacy of First‐Line Chemotherapy Regimens
The median follow‐up period in this study was 55.5 months. The efficacy of first‐line chemotherapy is shown in Table 3. Baseline characteristics were well balanced between the patients treated with platinum‐based doublets and those with other multidrug chemotherapies. In the patients treated with single‐agent chemotherapy, there were more female and postoperative recurrence cases than the other two groups (supplemental online Table 2). The response rate (RR) was 40.0% in all regimens: 38.2% for patients treated with platinum‐based doublets, 42.7% for those treated with other multidrug chemotherapies (e.g., ADOC), and 44.4% for those treated with single‐agent chemotherapy.
The median OS for all patients was 30.7 months. The median OS for platinum‐based doublets, other multidrug chemotherapies, and single‐agent chemotherapy were 30.7, 29.9, and 54.9 months, respectively. There was no significant difference among these groups (p = .7081; Fig. 1A).
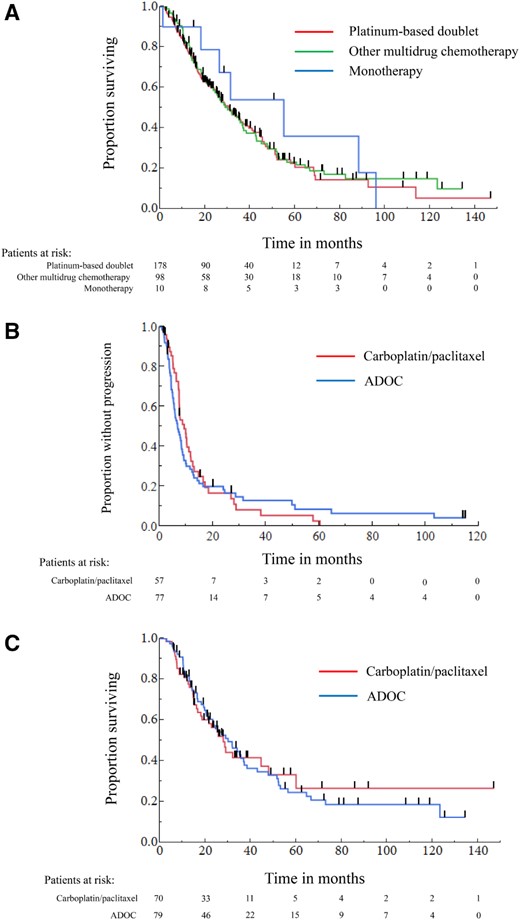
The Kaplan‐Meier curves of overall survival and progression‐free survival in patients with advanced thymic carcinoma treated by systemic chemotherapies. (A): Overall survival in patients treated with each first‐line regimen. (B): Progression‐free survival in patients treated with carboplatin/paclitaxel or ADOC. (C): Overall survival in patients treated with carboplatin/paclitaxel or ADOC.
Abbreviation: ADOC, cisplatin, doxorubicin, vincristine, and cyclophosphamide.
The patient characteristics stratified by carboplatin/paclitaxel or ADOC are summarized in supplemental online Table 3. Although there was a slight imbalance with more Masaoka‐Koga stage IVa and postoperative recurrence in carboplatin/paclitaxel group, the difference was not statistically significant (p = .6129). Other patient characteristics were well balanced between the two groups. The RR of carboplatin/paclitaxel was comparable with ADOC (40.0% vs. 41.0%, p = .9032). There was no significant difference in PFS and OS between carboplatin/paclitaxel and ADOC (median PFS: 9.1 vs. 6.7 months, p = .6603; median OS: 27.8 vs. 29.9 months, p = .9054; Fig. 1B, 1C).
Prognostic Factors
The results of univariate and multivariate analyses for OS are shown in Table 4. In the univariate analysis, sex, ECOG PS, Masaoka‐Koga stage, and volume reduction surgery were significantly predictive for OS. In the multivariate analysis, the prognostic factors associated with good survival were Masaoka‐Koga stage (IVa vs. IVb: hazard ratio [HR], 0.521; 95% confidence interval [CI], 0.356–0.751, p < .001; Fig. 2A) and volume reduction surgery for primary site (Yes vs. No: HR, 0.491; 95% CI, 0.253–0.867, p = .013; Fig. 2B). Twenty‐one of the patients with Masaoka‐Koga stage IVb had lymph node metastasis only. The OS was significantly longer in Masaoka‐Koga stage IVb patients with lymph node metastasis only (n = 21) than in the other stage IVb patients (n = 123; median OS 46.8 vs. 19.1 months, p = .019). In the multivariate analyses for OS in the patients with Masaoka‐Koga stage IVb, the prognostic factors associated with long survival were volume reduction surgery (Yes vs. No: HR, 0.276; 95% CI, 0.095–0.640, p = .002) and lymph node metastasis only (Yes vs. No: HR, 0.310; 95% CI, 0.135–0.625, p = .001; supplemental online Table 4). The median OS of the patients treated with multimodality therapy including volume reduction surgery was not reached in Masaoka‐Koga stage III patients (n = 3), 52.0 (95% CI, 17.9–123.2) months in stage IVa (n = 9), and 44.6 (95% CI, 28.5–123.2) months in stage IVb (n = 11), respectively. Twelve patients underwent volume reduction surgery before first‐line chemotherapy, and 11 patients underwent this procedure after chemotherapy.
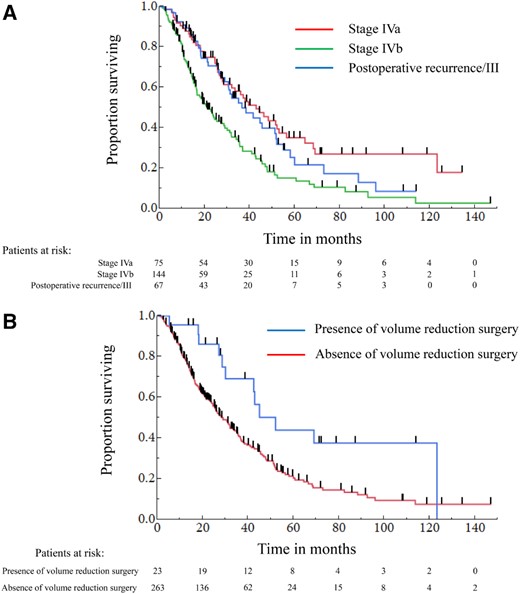
The Kaplan‐Meier curves of overall survival stratified according to the prognostic factors. (A): Overall survival stratified according to the Masaoka‐Koga stage. (B): Overall survival stratified according to the presence/absence of volume reduction surgery.
| Variants . | n . | mOS (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | p value . | HR (95% CI) . | p value . | |||
| Age, years | ||||||
| ≥65 | 104 | 31.2 (25.7–37.1) | 1 | 1 | ||
| <65 | 182 | 30.5 (23.2–36.7) | 0.972 (0.722–1.320) | .853 | 1.107 (0.805–1.540) | .536 |
| Sex | ||||||
| Female | 84 | 21.3 (16.4–35.5) | 1 | 1 | ||
| Male | 202 | 31.9 (27.2–41.5) | 0.713 (0.528–0.975) | .034 | 0.783 (0.567–1.094) | .150 |
| ECOG PS | ||||||
| 2–3 | 31 | 17.7 (11.3–21.3) | 1 | 1 | ||
| 0–1 | 248 | 32.0 (27.8–37.9) | 0.570 (0.375–0.908) | .019 | 0.719 (0.454–1.185) | .189 |
| Histology | ||||||
| NEC | 37 | 27.0 (16.3–45.0) | 1 | 1 | ||
| SQC | 190 | 31.9 (27.2–38.3) | 0.739 (0.494–1.146) | .308 | 0.898 (0.586–1.423) | .636 |
| Other | 59 | 21.3 (14.8–35.9) | 0.972 (0.597–1.607) | .912 | 1.093 (0.655–1.850) | .735 |
| Masaoka stage | ||||||
| IVb | 144 | 21.3 (16.3–28.5) | 1 | 1 | ||
| IVa | 75 | 42.8 (28.3–52.9) | 0.478 (0.332–0.677) | <.001 | 0.521 (0.356–0.751) | <.001 |
| Postoperative recurrence/III | 67 | 36.5 (28.9–51.7) | 0.581 (0.400–0.831) | .003 | 0.677 (0.252–1.590) | .393 |
| WHO TNM stage | ||||||
| IV | 222 | 27.2 (23.2–33.9) | 1 | |||
| Postoperative recurrence/III | 64 | 38.3 (30.5–51.9) | 0.730 (0.502–1.034) | .077 | 0.851 (0.349–2.297) | .741 |
| Volume reduction surgery | ||||||
| No | 263 | 28.9 (24.4–34.9) | 1 | 1 | ||
| Yes | 23 | 52.0 (28.5–123.2) | 0.469 (0.247–0.807) | .005 | 0.491 (0.253–0.867) | .013 |
| Volume reduction radiotherapy | ||||||
| No | 240 | 27.8 (23.9–31.9) | 1 | 1 | ||
| Yes | 47 | 42.4 (32.0–52.2) | 0.701 (0.452–1.041) | .080 | 0.815 (0.510–1.248) | .357 |
| First‐line chemotherapy regimen | ||||||
| Platinum doublet | 178 | 30.7 (24.5–37.9) | 1 | 1 | ||
| Monotherapy | 10 | 54.9 (1.1–95.9) | 0.735 (0.309–1.469) | .410 | 0.825 (0.331–1.774) | .641 |
| Other multidrug regimen | 98 | 29.9 (23.2–37.1) | 0.943 (0.695–1.271) | .702 | 0.960 (0.688–1.330) | .809 |
| Variants . | n . | mOS (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | p value . | HR (95% CI) . | p value . | |||
| Age, years | ||||||
| ≥65 | 104 | 31.2 (25.7–37.1) | 1 | 1 | ||
| <65 | 182 | 30.5 (23.2–36.7) | 0.972 (0.722–1.320) | .853 | 1.107 (0.805–1.540) | .536 |
| Sex | ||||||
| Female | 84 | 21.3 (16.4–35.5) | 1 | 1 | ||
| Male | 202 | 31.9 (27.2–41.5) | 0.713 (0.528–0.975) | .034 | 0.783 (0.567–1.094) | .150 |
| ECOG PS | ||||||
| 2–3 | 31 | 17.7 (11.3–21.3) | 1 | 1 | ||
| 0–1 | 248 | 32.0 (27.8–37.9) | 0.570 (0.375–0.908) | .019 | 0.719 (0.454–1.185) | .189 |
| Histology | ||||||
| NEC | 37 | 27.0 (16.3–45.0) | 1 | 1 | ||
| SQC | 190 | 31.9 (27.2–38.3) | 0.739 (0.494–1.146) | .308 | 0.898 (0.586–1.423) | .636 |
| Other | 59 | 21.3 (14.8–35.9) | 0.972 (0.597–1.607) | .912 | 1.093 (0.655–1.850) | .735 |
| Masaoka stage | ||||||
| IVb | 144 | 21.3 (16.3–28.5) | 1 | 1 | ||
| IVa | 75 | 42.8 (28.3–52.9) | 0.478 (0.332–0.677) | <.001 | 0.521 (0.356–0.751) | <.001 |
| Postoperative recurrence/III | 67 | 36.5 (28.9–51.7) | 0.581 (0.400–0.831) | .003 | 0.677 (0.252–1.590) | .393 |
| WHO TNM stage | ||||||
| IV | 222 | 27.2 (23.2–33.9) | 1 | |||
| Postoperative recurrence/III | 64 | 38.3 (30.5–51.9) | 0.730 (0.502–1.034) | .077 | 0.851 (0.349–2.297) | .741 |
| Volume reduction surgery | ||||||
| No | 263 | 28.9 (24.4–34.9) | 1 | 1 | ||
| Yes | 23 | 52.0 (28.5–123.2) | 0.469 (0.247–0.807) | .005 | 0.491 (0.253–0.867) | .013 |
| Volume reduction radiotherapy | ||||||
| No | 240 | 27.8 (23.9–31.9) | 1 | 1 | ||
| Yes | 47 | 42.4 (32.0–52.2) | 0.701 (0.452–1.041) | .080 | 0.815 (0.510–1.248) | .357 |
| First‐line chemotherapy regimen | ||||||
| Platinum doublet | 178 | 30.7 (24.5–37.9) | 1 | 1 | ||
| Monotherapy | 10 | 54.9 (1.1–95.9) | 0.735 (0.309–1.469) | .410 | 0.825 (0.331–1.774) | .641 |
| Other multidrug regimen | 98 | 29.9 (23.2–37.1) | 0.943 (0.695–1.271) | .702 | 0.960 (0.688–1.330) | .809 |
Abbreviations: CI, confidence interval; ECOG; Eastern Cooperative Oncology Group; HR; hazard ratio; mOS, median overall survival; NEC, neuroendocrine carcinoma; PS, performance status; SQC, squamous cell carcinoma; WHO, World Health Organization.
| Variants . | n . | mOS (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | p value . | HR (95% CI) . | p value . | |||
| Age, years | ||||||
| ≥65 | 104 | 31.2 (25.7–37.1) | 1 | 1 | ||
| <65 | 182 | 30.5 (23.2–36.7) | 0.972 (0.722–1.320) | .853 | 1.107 (0.805–1.540) | .536 |
| Sex | ||||||
| Female | 84 | 21.3 (16.4–35.5) | 1 | 1 | ||
| Male | 202 | 31.9 (27.2–41.5) | 0.713 (0.528–0.975) | .034 | 0.783 (0.567–1.094) | .150 |
| ECOG PS | ||||||
| 2–3 | 31 | 17.7 (11.3–21.3) | 1 | 1 | ||
| 0–1 | 248 | 32.0 (27.8–37.9) | 0.570 (0.375–0.908) | .019 | 0.719 (0.454–1.185) | .189 |
| Histology | ||||||
| NEC | 37 | 27.0 (16.3–45.0) | 1 | 1 | ||
| SQC | 190 | 31.9 (27.2–38.3) | 0.739 (0.494–1.146) | .308 | 0.898 (0.586–1.423) | .636 |
| Other | 59 | 21.3 (14.8–35.9) | 0.972 (0.597–1.607) | .912 | 1.093 (0.655–1.850) | .735 |
| Masaoka stage | ||||||
| IVb | 144 | 21.3 (16.3–28.5) | 1 | 1 | ||
| IVa | 75 | 42.8 (28.3–52.9) | 0.478 (0.332–0.677) | <.001 | 0.521 (0.356–0.751) | <.001 |
| Postoperative recurrence/III | 67 | 36.5 (28.9–51.7) | 0.581 (0.400–0.831) | .003 | 0.677 (0.252–1.590) | .393 |
| WHO TNM stage | ||||||
| IV | 222 | 27.2 (23.2–33.9) | 1 | |||
| Postoperative recurrence/III | 64 | 38.3 (30.5–51.9) | 0.730 (0.502–1.034) | .077 | 0.851 (0.349–2.297) | .741 |
| Volume reduction surgery | ||||||
| No | 263 | 28.9 (24.4–34.9) | 1 | 1 | ||
| Yes | 23 | 52.0 (28.5–123.2) | 0.469 (0.247–0.807) | .005 | 0.491 (0.253–0.867) | .013 |
| Volume reduction radiotherapy | ||||||
| No | 240 | 27.8 (23.9–31.9) | 1 | 1 | ||
| Yes | 47 | 42.4 (32.0–52.2) | 0.701 (0.452–1.041) | .080 | 0.815 (0.510–1.248) | .357 |
| First‐line chemotherapy regimen | ||||||
| Platinum doublet | 178 | 30.7 (24.5–37.9) | 1 | 1 | ||
| Monotherapy | 10 | 54.9 (1.1–95.9) | 0.735 (0.309–1.469) | .410 | 0.825 (0.331–1.774) | .641 |
| Other multidrug regimen | 98 | 29.9 (23.2–37.1) | 0.943 (0.695–1.271) | .702 | 0.960 (0.688–1.330) | .809 |
| Variants . | n . | mOS (95% CI) . | Univariate . | Multivariate . | ||
|---|---|---|---|---|---|---|
| HR (95% CI) . | p value . | HR (95% CI) . | p value . | |||
| Age, years | ||||||
| ≥65 | 104 | 31.2 (25.7–37.1) | 1 | 1 | ||
| <65 | 182 | 30.5 (23.2–36.7) | 0.972 (0.722–1.320) | .853 | 1.107 (0.805–1.540) | .536 |
| Sex | ||||||
| Female | 84 | 21.3 (16.4–35.5) | 1 | 1 | ||
| Male | 202 | 31.9 (27.2–41.5) | 0.713 (0.528–0.975) | .034 | 0.783 (0.567–1.094) | .150 |
| ECOG PS | ||||||
| 2–3 | 31 | 17.7 (11.3–21.3) | 1 | 1 | ||
| 0–1 | 248 | 32.0 (27.8–37.9) | 0.570 (0.375–0.908) | .019 | 0.719 (0.454–1.185) | .189 |
| Histology | ||||||
| NEC | 37 | 27.0 (16.3–45.0) | 1 | 1 | ||
| SQC | 190 | 31.9 (27.2–38.3) | 0.739 (0.494–1.146) | .308 | 0.898 (0.586–1.423) | .636 |
| Other | 59 | 21.3 (14.8–35.9) | 0.972 (0.597–1.607) | .912 | 1.093 (0.655–1.850) | .735 |
| Masaoka stage | ||||||
| IVb | 144 | 21.3 (16.3–28.5) | 1 | 1 | ||
| IVa | 75 | 42.8 (28.3–52.9) | 0.478 (0.332–0.677) | <.001 | 0.521 (0.356–0.751) | <.001 |
| Postoperative recurrence/III | 67 | 36.5 (28.9–51.7) | 0.581 (0.400–0.831) | .003 | 0.677 (0.252–1.590) | .393 |
| WHO TNM stage | ||||||
| IV | 222 | 27.2 (23.2–33.9) | 1 | |||
| Postoperative recurrence/III | 64 | 38.3 (30.5–51.9) | 0.730 (0.502–1.034) | .077 | 0.851 (0.349–2.297) | .741 |
| Volume reduction surgery | ||||||
| No | 263 | 28.9 (24.4–34.9) | 1 | 1 | ||
| Yes | 23 | 52.0 (28.5–123.2) | 0.469 (0.247–0.807) | .005 | 0.491 (0.253–0.867) | .013 |
| Volume reduction radiotherapy | ||||||
| No | 240 | 27.8 (23.9–31.9) | 1 | 1 | ||
| Yes | 47 | 42.4 (32.0–52.2) | 0.701 (0.452–1.041) | .080 | 0.815 (0.510–1.248) | .357 |
| First‐line chemotherapy regimen | ||||||
| Platinum doublet | 178 | 30.7 (24.5–37.9) | 1 | 1 | ||
| Monotherapy | 10 | 54.9 (1.1–95.9) | 0.735 (0.309–1.469) | .410 | 0.825 (0.331–1.774) | .641 |
| Other multidrug regimen | 98 | 29.9 (23.2–37.1) | 0.943 (0.695–1.271) | .702 | 0.960 (0.688–1.330) | .809 |
Abbreviations: CI, confidence interval; ECOG; Eastern Cooperative Oncology Group; HR; hazard ratio; mOS, median overall survival; NEC, neuroendocrine carcinoma; PS, performance status; SQC, squamous cell carcinoma; WHO, World Health Organization.
Discussion
To our knowledge, this is the largest study evaluating the efficacy of chemotherapy and prognostic factors in patients with advanced thymic carcinoma. These results show that there are no significant differences in efficacy among different regimens used to treat advanced thymic carcinoma, including carboplatin/paclitaxel and ADOC. An exploratory multivariate analysis suggests that Masaoka‐Koga stage and volume reduction surgery for primary site are independent prognostic factors.
The previous reports for first‐line chemotherapy in patients with thymic carcinoma are shown in Table 1. There were no comparative trials, and only four prospective single‐arm trials. The number of patients enrolled in these retrospective trials was below 40. The most frequently investigated chemotherapeutic regimen is carboplatin/paclitaxel, which showed comparable RR to multidrug chemotherapy comprising more than two drugs [8-10,12]. Therefore, the NCCN Guidelines recommend carboplatin/paclitaxel as first‐line chemotherapy for patients with advanced thymic carcinoma [15]. On the other hand, anthracycline‐based multidrug regimens, such as ADOC, are also widely used as first‐line chemotherapy in clinical practice, which could be more effective than carboplatin/paclitaxel [6,15,23]. However, the toxicities of anthracycline‐based regimens, especially bone marrow suppression, are reportedly more severe than those of platinum‐based doublets [5,6,8,9,11-15]. Based on this background, the best regimen for advanced thymic carcinoma has been controversial. In this regard, our study suggests the equivalent of efficacy between carboplatin/paclitaxel and ADOC. The recommendation of carboplatin/paclitaxel for advanced thymic carcinoma as first‐line chemotherapy in the NCCN Guidelines could be supported by the results of our study.
There are several staging systems of thymic carcinoma. The Masaoka‐Koga staging system is the most widely used in the world and is also recommended to use for thymic carcinoma in the NCCN Guidelines [15,18,24]. Another notable staging system is the WHO TNM staging system [20, 21]. However, it is less commonly used than the Masaoka‐Koga staging system. There is a difference in stage IV disease between these staging systems. In the Masaoka‐Koga staging system, stage IV disease is divided into two categories: IVa and IVb. Stage IVa comprises serosal dissemination, that is, involvement of the pleura and pericardium. Stage IVb comprises metastasis via lymphogenous and hematogenous routes. On the other hand, stage IVa and IVb were categorized in the same stage IV disease in the WHO TNM staging system. There were few data to evaluate the utility of separating stage IV disease by the Masaoka‐Koga staging system in patients with thymic carcinoma treated with chemotherapy. In our study, there was a considerable difference in prognosis between stage IVa and stage IVb according to the Masaoka‐Koga staging system. These data also suggest that the Masaoka‐Koga staging system is more useful than the WHO TNM staging system for patients with advanced thymic carcinoma.
Surgery is reported as one of the most favorable prognostic factors in several reports on thymic carcinoma [3,4,16,17]. However, in these reports, most patients had early‐stage thymic carcinoma. Several reports have shown that volume reduction surgery (subtotal resection) improved the prognosis of patients with advanced thymoma [2, 25]. However, it is unclear whether there is improvement in patients with advanced thymic carcinoma. In our study, volume reduction surgery for primary site was determined as an independent favorable prognostic factor in patients with advanced thymic carcinoma treated with chemotherapy for the first time. Because this was a retrospective study, there may have been a selection bias in its result. Therefore, we conducted a multivariate analysis to minimize the effect of the selection bias. Based on our study, volume reduction surgery could be considered a treatment option for advanced thymic carcinoma; however, further studies, including prospective studies, are warranted to confirm our results.
Lymph node metastasis was reported as one of the prognostic factors in thymic carcinoma [17]. However, previous reports included early‐stage thymic carcinoma. Therefore, it is unclear whether lymph node metastasis is a prognostic factor in patients with Masaoka‐Koga stage IVb. In our study, the patients with lymph node metastasis only showed a significantly longer survival than the other patients with Masaoka‐Koga stage IVb. This result suggests that we should separately classify the Masaoka‐Koga stage IVb patients with lymph node metastasis only. Recently, UICC propounded a new TNM staging system [26]. In this staging system, N factors are used to categorize the patients more precisely into stages IVa and IVb, unlike the Masaoka‐Koga staging system. Further studies using the new UICC TNM staging system are warranted to evaluate the utility of lymph node metastasis as a prognostic factor in patients with advanced thymic carcinoma.
The limitation of our study is its retrospective design. However, it is difficult to develop a large, prospective clinical study for such a rare malignancy. The sample size of our study is by far the largest among clinical studies conducted for advanced thymic carcinoma.
Conclusion
There are no significant differences in efficacy among the chemotherapeutic regimens for advanced thymic carcinoma as a first‐line treatment, including carboplatin/paclitaxel and ADOC. Masaoka‐Koga stage and volume reduction surgery for primary site are independent prognostic factors in patients with advanced thymic carcinoma treated with chemotherapy.
Acknowledgments
This study was funded by Department of Respiratory Medicine, Juntendo University Graduate School of Medicine. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Author Contributions
Conception/design: Ryo Ko, Takehito Shukuya, Kunihiko Kobayashi, Kazuhisa Takahashi
Provision of study material or patients: Ryo Ko, Takehito Shukuya, Yusuke Okuma, Kazunari Tateishi, Hisao Imai, Shunichiro Iwasawa, Eisaku Miyauchi, Akiko Fujiwara, Tomohide Sugiyama, Keisuke Azuma, Keiko Muraki, Masahiro Yamasaki, Hisashi Tanaka, Yuta Takashima, Sayo Soda, Osamu Ishimoto, Nobuyuki Koyama, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi
Collection and/or assembly of data: Ryo Ko, Takehito Shukuya, Yusuke Okuma, Kazunari Tateishi, Hisao Imai, Shunichiro Iwasawa, Eisaku Miyauchi, Akiko Fujiwara, Tomohide Sugiyama, Keisuke Azuma, Keiko Muraki, Masahiro Yamasaki, Hisashi Tanaka, Yuta Takashima, Sayo Soda, Osamu Ishimoto, Nobuyuki Koyama
Data analysis and interpretation: Ryo Ko, Takehito Shukuya, Satoshi Morita
Manuscript writing: Ryo Ko, Takehito Shukuya
Final approval of manuscript: Ryo Ko, Takehito Shukuya, Yusuke Okuma, Kazunari Tateishi, Hisao Imai, Shunichiro Iwasawa, Eisaku Miyauchi, Akiko Fujiwara, Tomohide Sugiyama, Keisuke Azuma, Keiko Muraki, Masahiro Yamasaki, Hisashi Tanaka, Yuta Takashima, Sayo Soda, Osamu Ishimoto, Nobuyuki Koyama, Satoshi Morita, Kunihiko Kobayashi, Toshihiro Nukiwa, Kazuhisa Takahashi
Disclosures
Ryo Ko: Nippon Boehringer Ingelheim, AstraZeneca, Taiho Pharmaceutical (H); Takehito Shukuya: Chugai Pharmaceutical (RF), AstraZeneca, Ono Pharmaceutical, Nichi‐iko Pharmaceutical, Sanofi, Pfizer, Chugai Pharmaceutical (H); Shunichiro Iwasawa: Ono Pharmaceutical (RF); Kunihiko Kobayashi: AstraZeneca, Ono Pharmaceutical (H); Kazuhisa Takahashi: Novartis, Nippon Boehringer Ingelheim, AstraZeneca, Novelpharma, Shionogi Pharma, Taiho Pharmaceutical, Tsumura & Co., GlaxoSmithKline, Kyowa Hakko Kirin, Astellas Pharma, Toyama Chemical Co., Torii Pharmaceutical, Nippon Shinyaku, Nipro Corporation (RF); Novartis, Nippon Boehringer Ingelheim, AstraZeneca, Novelpharma, Shionogi Pharma, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Sumitomo Dainippon, Bristol‐Myers Squibb, Otsuka, Meiji Seika Kaisha (H); Toshihiro Nukiwa: Nippon Boehringer Ingelheim (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



