-
PDF
- Split View
-
Views
-
Cite
Cite
Anne‐Sophie Bats, Claude Nos, Chérazade Bensaïd, Marie‐Aude Le Frère‐Belda, Marie‐Anne Collignon, Marc Faraggi, Fabrice Lécuru, Lower‐Limb Drainage Mapping for Lymphedema Risk Reduction After Pelvic Lymphadenectomy for Endometrial Cancer, The Oncologist, Volume 18, Issue 2, February 2013, Pages 174–179, https://doi.org/10.1634/theoncologist.2012-0309
Close - Share Icon Share
Abstract
Identify lower‐limb drainage nodes preoperatively and during pelvic lymphadenectomy for endometrial cancer.
Map lower‐limb drainage nodes during pelvic lymphadenectomy for endometrial cancer, using the “reverse mapping” concept.
Preserve lower‐limb drainage nodes during pelvic lymphadenectomy for endometrial cancer in order to reduce the risk of lower limb lymphedema.
Pelvic lymphadenectomy is associated with a significant risk of lower‐limb lymphedema. In this proof‐of‐concept study, we evaluated the feasibility of identifying the lower‐limb drainage nodes (LLDNs) during pelvic lymphadenectomy for endometrial cancer. Secondary objectives were to map lower‐limb drainage and to assess the diagnostic value of our mapping technique.
This prospective study included patients with endometrial cancer requiring pelvic lymphadenectomy, without neoadjuvant radiotherapy or chemotherapy and without history of lower‐limb surgery. A radiopharmaceutical was injected into both feet on the day before surgery. LLDNs were identified using preoperative lymphoscintigraphy and intraoperative isotopic probe detection, then removed before complete pelvic lymphadenectomy. LLDNs and pelvic lymphadenectomy specimens underwent separate histological analysis.
Of the 12 patients with early‐stage endometrial cancer, 10 underwent preoperative lymphoscintigraphy, which consistently identified inguinal, femoral, and pelvic LLDNs (detection rate: 100%). The intraoperative detection rate was 83% (10/12). Median number of hot nodes per patient was 5 nodes (range: 3–7) on the right and 3 nodes (range: 2–6) on the left. Of 107 LLDNs, 106 were in the external iliac area, including 38 in the lateral group and 45 in the intermediate and medial groups. None of the patients had node metastases at any site. No early complications related to the technique occurred.
Our mapping technique appears feasible, safe, and associated with a high LLDN identification rate. LLDN mapping may allow the preservation of LLDNs, thereby decreasing the risk of lower‐limb lymphedema and improving quality of life.
摘要
目的. 盆腔淋巴结清扫术与下肢淋巴水肿的发生显著相关。本项概念验证研究旨在评估子宫内膜癌盆腔淋巴结清扫术中确定下肢引流淋巴结(LLDN)的可行性。次要目的是行下肢引流定位并对所采用定位技术的诊断价值进行评估。
方法. 本项前瞻性研究纳入需行盆腔淋巴结清扫术、未行新辅助放疗/化疗且无下肢手术史的子宫内膜癌患者。术前一日,双足行放射性药物注射。应用术前淋巴闪烁显像和术中同位素探针行LLDN检测,并于盆腔淋巴结清扫术完成前切除。分别对LLDN和盆腔淋巴结清扫标本行独立组织学分析。
结果. 12例早期子宫内膜癌患者中,10例行术前淋巴闪烁显像,该检查可完全检出腹股沟、股动脉和盆腔的LLDN(检出率100%)。术中检出率为83%(10/12)。每例患者的中位热淋巴结数如下:右侧5个(范围:3 ∼ 7个)、左侧3个(范围:2 ∼6个)。107个LLDN中,106个位于髂外区,其中38个位于外侧组,45个位于中内侧组。患者任何部位均无淋巴结转移。未发生与该技术相关的早期并发症。
结论. 本项定位技术安全,可行,LLDN检出率高。LLDN定位有助于保全LLDN,从而降低下肢淋巴水肿发生风险,提高生活质量。
Implications for Practice:
Pelvic lymphadenectomy is associated with substantial postoperative morbidity related to the removal of pelvic lower‐limb drainage nodes, which are not identified during the procedure. The sentinel node biopsy technique has been developed to decrease lymphadenectomy‐related morbidity. In some gynecological cancers, however, routine pelvic lymphadenectomy remains required. Our proof‐of‐concept study was designed to evaluate the feasibility of identifying the lower‐limb drainage nodes (LLDNs) during pelvic lymphadenectomy for endometrial cancer. Our results hold promise for preserving the lymphatic drainage pathways of the lower limbs. Sparing the LLDNs may decrease the risk of lower‐limb lymphedema, which is associated with both a decrease in quality of life and additional healthcare costs.
Introduction
Endometrial cancer is the most common gynecological malignancy in Western countries. Contrary to total hysterectomy with bilateral salpingo‐oophorectomy, which is the recommended surgical treatment, the place of lymphadenectomy is currently under debate. Although recent randomized trials and a meta‐analysis showed that pelvic lymphadenectomy had no affect on either overall or recurrence‐free survival rates for patients with early‐stage endometrial cancer [1–3], the positive affect of both pelvic and para‐aortic lymphadenectomy in patients with high‐risk endometrial cancer has been clearly demonstrated [4].
However, pelvic lymphadenectomy is associated with substantial intraoperative and postoperative morbidity, particularly lymphatic complications, including lymphocele and lymphedema. Although the risk of lymphedema has received little attention, the few available studies showed rates of up to 30% [5–7], with major quality‐of‐life impairments [8–10]. Pathophysiology of lymphedema has been demonstrated to be related to proximal obstruction of the lymphatic pathways [11], primarily at the pelvic and inguinal lymph nodes [12], explaining why surgical nodal staging contributes to these lymphatic damages.
To reduce the morbidity associated with lymphadenectomy, targeted lymph node sampling (or sentinel node biopsy) was recently evaluated in endometrial cancer. This technique consists of identifying the first node draining the tumor—that is, the node most likely to be metastatic. High detection rates and low false‐negative rates were found [13]. The morbidity related specifically to sentinel node biopsy has not yet been assessed, but this technique has the potential to decrease postoperative morbidity. For instance, for patients with breast cancer, sentinel node biopsy has considerably decreased the risk of arm lymphedema [14]. Indeed, the mapping technique used to identify sentinel nodes has been applied to the detection of nodes draining the arms of patients with breast cancer, with the goal of preventing arm lymphedema in patients who require extensive axillary lymphadenectomy. This method, known as axillary reverse mapping, allows preservation of the lymphatic drainage of the arm [15, 16].
We hypothesized that reverse mapping might be feasible for the lower limbs. Therefore, the primary objective of this pilot study was to evaluate the ability of lymphoscintigraphy and intraoperative gamma‐radiation probe examination to detect the pelvic nodes draining the lower limbs in patients undergoing surgery for early endometrial cancer. The secondary objectives were to describe the lower‐limb drainage pathways in the pelvis and to assess the diagnostic value of our detection technique.
Patients and Methods
This single‐center prospective study was approved by our institutional review board and by our multidisciplinary board. Written informed consent was obtained from each patient before study inclusion. We included patients undergoing surgery for endometrial cancer between February 2009 and February 2010. At the time, the French guidelines recommended total hysterectomy with bilateral salpingo‐oophorectomy and bilateral pelvic lymphadenectomy, irrespective of tumor stage and grade. Exclusion criteria were neoadjuvant chemotherapy or radiotherapy, contraindication to pelvic lymphadenectomy, past history of lower limb surgery, pre‐existing lower limb lymphedema, and patient age older than 70 years.
Before surgery, a complete general and gynecological evaluation was performed to confirm the absence of lower‐limb lymphedema. On the day before the procedure, a radiopharmaceutical (2×60 MBq of Nanocoll (GE Healthcare SAS, Vélizy‐Villacoublay, France) labeled with 99m Tc) was injected into the first interdigital spaces of each foot. Lymphoscintigraphy of the lower limbs was performed 45 minutes later to identify the pelvic pathways draining the lower limb. When no inguinocrural node was identified, a second scan was performed 3 hours later to look for late drainage. The lymphoscintigraphy images and report were available to the surgeon (Fig. 1A, 1B).
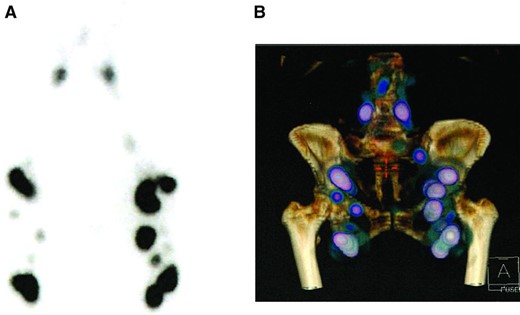
Preoperative single‐photon emission computed tomography lymphoscintigraphy with planar images (A) and three‐dimensional reconstruction of the lower limbs (B).
The surgical approach was at the surgeon's discretion. During surgery, before and after opening the peritoneum, an isotopic detection probe was used to detect lower‐limb drainage nodes (LLDNs) in the pelvis, including the first pelvic LLDN, usually located in the inguinal‐iliac area, and the secondary LLDN (Fig. 2). All the usual nodal territories were explored (external iliac with the lateral, intermediate, and medial groups; common iliac; promontory; and para‐aortic). Hot nodes were sampled electively and their locations were specified using the Marnitz classification scheme [17]. Pelvic lymphadenectomy was then performed. Hot nodes located outside the usual lymphadenectomy area were left in place, and the surgeon performed conventional ilio‐obturator pelvic lymphadenectomy (from the umbilical artery to the pelvic wall, under the external iliac vein, and above the obturator nerve).
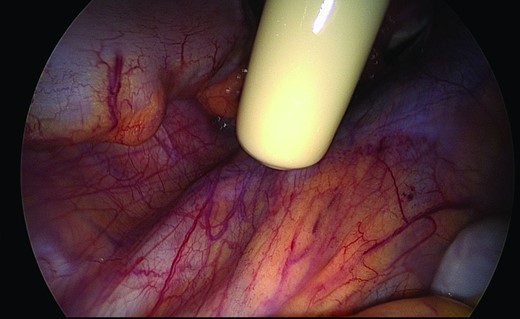
Intraoperative isotopic detection of lower‐limb drainage nodes in the pelvis.
LLDN radioactivity was checked ex vivo, as well as the absence of residual radioactivity in vivo. Hot nodes were sent to the laboratory separately from the rest of the lymphadenectomy specimen and were examined separately from the cold nodes. Hot nodes were cut in two, then serially sectioned into five 250‐μm slices, of which four were stained with hematoxylin‐eosin‐saffron and one was analyzed by immunohistochemistry. Cold nodes were examined by standard staining of the cut surface.
Patients were followed up 3 weeks after surgery (postoperative follow‐up) and later for their regular follow‐up visit, according to the stage of the disease. Recurrences as well as complications were assessed. Lymphedema was evaluated by looking for the following symptoms: feeling of heaviness, aching or discomfort, swelling of legs, restricted range of motion in the leg, and hardening and thickening of the skin on the leg.
Statistical Analysis
The analysis included all the study patients who underwent the investigations needed to assess the primary objective. We determined the percentages of patients, with the 95% confidence intervals (CIs), in whom LLDNs were identified by lymphoscintigraphy and dissected during surgery. To assess our secondary objectives, we described the lower‐limb drainage pathways in the common iliac and external iliac areas, and we computed the percentage of patients with metastatic LLDNs. The data are described as percentages or medians with interquartile ranges as appropriate. Analyses were performed using SAS software (SAS Institute, Cary, NC).
Results
We prospectively enrolled 12 consecutive patients (Table 1). Median age was 64.5 years (range: 56–73 years). All patients had early‐stage disease (International Federation of Gynecology and Obstetrics stage IA in nine patients and stage IB in three patients). The histological type was endometrioid adenocarcinoma in 10 patients (grade 1 in eight patients and grade 2 in two patients) and serous and endometrioid carcinoma in two patients. The surgical procedure consisted of total laparoscopic (two cases) or robot‐assisted (10 cases) hysterectomy with salpingo‐oophorectomy and pelvic lymphadenectomy.
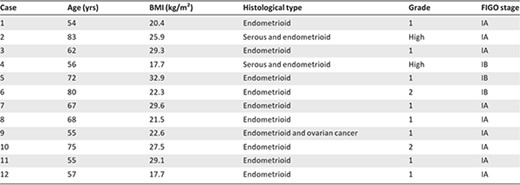
Abbreviations: BMI, body mass index; FIGO, Federation of Gynecology and Obstetrics.

Abbreviations: BMI, body mass index; FIGO, Federation of Gynecology and Obstetrics.
Preoperative Pelvic Lower‐Limb Drainage Node Detection
All 12 patients received intracutaneous injections of the radiopharmaceutical into both feet on the day before surgery. In two patients, lymphoscintigraphy was not performed for organizational reasons. Nine patients underwent preoperative lymphoscintigraphy on the day before surgery and one patient on the morning of surgery. Lymphoscintigraphy detected the nodes draining the lower limbs in all 10 patients (detection rate: 100%). All 10 patients had LLDNs in the inguinofemoral and pelvic regions. LLDNs were detected bilaterally in nine patients. Table 2 summarizes LLDNs location on lymphoscintigraphy.
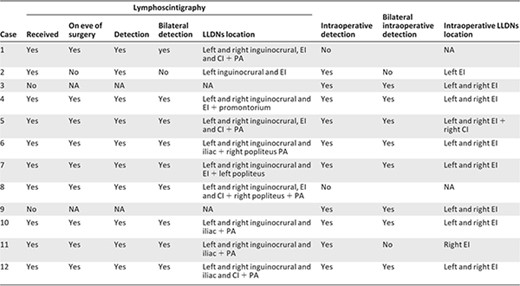
Abbreviations: CI, common iliac; EI, external iliac; LLDN, lower‐limb drainage node; NA, not applicable; PA, para‐aortic.

Abbreviations: CI, common iliac; EI, external iliac; LLDN, lower‐limb drainage node; NA, not applicable; PA, para‐aortic.
Intraoperative Pelvic Lower‐Limb Drainage Node Detection
Intraoperative detection using a handheld probe identified LLDNs in 10 (83%) of the 12 patients. Two patients had no LLDNs detected intraoperatively. These patients were respectively 54 and 68 years old. In both cases, lymphoscintigraphy had been performed on the eve of surgery, and one patient had a low dose of radiopharmaceutical (2×25 MBq). Both patients had several bilateral pelvic LLDNs by lymphoscintigraphy. Intraoperative LLDNs detection was bilateral in eight patients. Both patients without bilateral intraoperative detection received a low‐dose radioisotope (2×30 MBq), but one of them had the injection and lymphoscintigraphy the morning of surgery. In this case, a new injection had been done following lymphoscintigraphy because preoperative detection was unilateral. The median operating time was 215 minutes (range: 201–210 minutes). Table 2 summarizes preoperative and intraoperative detection of LLDNs.
Location of Pelvic Lower‐Limb Drainage Nodes
Of the 107 hot nodes, 106 were in the external iliac area, including 38 in the lateral group and 45 in the intermediate and medial groups. Location was not specified for the remaining 23 external iliac nodes. One hot node was in the common iliac area (Table 2).
Diagnostic Performance
Table 3 reports the main histological results. The median numbers of hot nodes per patient were 5 nodes (range: 3–7) on the right side and 3 nodes (range: 2–6) on the left side. The median numbers of cold nodes per patient were 7 nodes (range: 5–8) on the right and 6 nodes (range: 4–8) on the left. None of the hot or cold nodes showed evidence of metastasis.
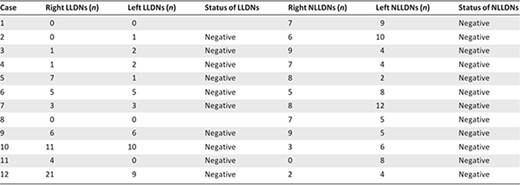
Abbreviations: LLDNs, lower‐limb drainage nodes; NLLDNs, non‐lower‐limb drainage nodes.

Abbreviations: LLDNs, lower‐limb drainage nodes; NLLDNs, non‐lower‐limb drainage nodes.
Complications and Recurrences
No intraoperative or early postoperative complications occurred in relation to the LLDN detection technique. There were three early postoperative complications (one lymphocele, one hemoperitoneum, and one vaginal vault dehiscence). After a median follow‐up of 27 months (range: 23–33 months), one patient had a lymphocele and a single patient (case 5) had lower‐limb lymphedema 4 months after initial surgery. This patient had a relapse 8 months after surgery, with involvement of a common iliac lymph node and of the vaginal vault. She presented with bilateral preoperative and intraoperative detection of LLDNs; both LLDNs and other removed pelvic nodes were free of metastases.
Discussion
In this preliminary prospective study in 12 patients with early endometrial cancer, we used an isotopic technique to detect LLDNs located in the pelvis. Detection rates were 100% (10/10) for lymphoscintigraphy and 83% (10/12) for intraoperative detection. Both patients without intraoperatively detected LLDNs had LLDNs visible by lymphoscintigraphy. Of the 107 hot nodes, 106 were located in the external iliac area (38 in the lateral group, 45 in the intermediate and medial groups, and 23 at unspecified locations) and one was in the common iliac area. No metastases were found in any of the hot or cold nodes. The absence of nodal metastases probably reflects the early stage of the disease in our patients.
To the best of our knowledge, this technique has not been previously reported. Our results suggest that it may hold promise for preserving the lymphatic drainage pathways of the lower limbs, provided further studies confirm its good diagnostic performance. Sparing the LLDNs may decrease the risk of lower‐limb lymphedema, which is associated with morbidity [8], anxiety and depression [9], decreased work ability, impairments in social and sexual function, a decrease in quality of life [10], and additional health care costs.
Our study sought to identify LLDNs with the goal of subsequently allowing LLDN preservation during pelvic lymphadenectomy for gynecological tumors, in the same way that axillary reverse mapping is used to preserve arm lymphatic drainage in patients requiring axillary lymphadenectomy for breast cancer [15, 16]. For axillary reverse mapping, a dye or an isotope is injected into the arm. The nodes draining the arm are then readily identified. Studies showed that these arm drainage nodes were not metastatic, except in patients with massive nodal involvement. At the lower limbs, reverse mapping was recently described in seven patients with vulvar cancer [18]. Injection of a radioisotope into the tumor and of isosulfan blue into the thigh 10 cm below the inguinal ligament ensured the detection of nodes draining the lower limbs. In three patients, hot nodes were metastatic.
The lymphatic drainage of the lower limbs has been well documented. There are two networks, one superficial and one deep, which are separated by a fascia and remain completely independent of each other until they converge in the superficial popliteal and inguinal regions. At the anterior part of the proximal thigh, there are two groups of lymph nodes, a superficial inguinal group and a deep inguinal group. The superficial group drains into the deep group, which drains into the femoral canal together with the lymph from the pelvis. The circumflex iliac node is a direct intrapelvic extension of the deep inguinal group, through the femoral canal, and is in contact with the lateral group of external iliac nodes [19]. During pelvic lymphadenectomy for gynecological tumors, the intermediate group of external iliac nodes is removed, as well as the medial group and, in some cases, the lateral group. The intermediate group is located in the volume bounded by the pelvic wall and, therefore, the psoas muscle, under the external iliac vein, lateral to the umbilical artery, and above the obturator nerve.
Abu‐Rustum et al., following their study of the lymphedema risk, pointed out a number of anatomic features of the lymphatic drainage of the pelvis and lower limbs [20]. The external iliac lymph nodes drain the ipsilateral leg, anterior abdominal wall, bladder, uterus, and vagina. The medial group of the common iliac nodes drains the pelvic viscera, whereas the lateral group drains the lower limbs and pelvis. The most distal external iliac nodes, known as the circumflex iliac nodes, are almost consistently present and are typically large because they contain an abundance of adipose tissue. The circumflex iliac nodes are routinely removed during pelvic lymphadenectomy, although they are usually free of metastases. Studies have shown that circumflex iliac nodes never contained metastases in patients without metastatic node involvement at other sites [21, 22]. The circumflex iliac nodes, also called distal external iliac nodes, lateral deep inguinal nodes, and suprainguinal nodes, may be a direct extension of the deep inguinal Cloquet nodes draining the lower limbs. Therefore, their removal may contribute to the occurrence of lower limb lymphedema [23].
Numerous cadaver and surgical studies have assessed the location of the sentinel nodes of uterine cancers, and none showed metastases in the inguinal or circumflex iliac nodes. In a retrospective study of 508 patients with intermediate‐ or high‐risk endometrial cancer requiring bilateral pelvic lymphadenectomy and para‐aortic dissection, only 2.8% of patients had circumflex iliac node involvement; furthermore, none of the patients with grade 1 or 2 disease and negative pelvic nodes had positive circumflex iliac nodes. These data suggest that leaving the circumflex iliac nodes in place to reduce the lymphedema risk may be safe [22].
Our study has several limitations. It is a single‐center feasibility study in a small number of patients who were at low risk for node metastases. Future studies should include patients at high risk for node involvement. Furthermore, LLDNs were found in territories containing nodes for the pelvic organs and not specifically in the circumflex iliac territory. This point is crucial and suggests that the role ascribed to the circumflex iliac nodes in draining the lower limbs may be a misconception. Further studies are required to improve the technique. The injection of blue dye into the uterus may allow differentiation of the tumor drainage nodes (blue ± hot) from the LLDNs (hot).
Conclusion
We conducted a pilot study to assess the identification of LLDNs in patients with endometrial cancer. The results show that the technique is feasible and safe and that it provides a high detection rate. Further studies are required to confirm the good diagnostic performance of the technique. If confirmed, this technique may improve the pelvic lymphadenectomy technique for gynecological cancers by allowing the preservation of LLDNs, thereby decreasing the risk of lymphedema.
Acknowledgments
We thank Antoinette Wolfe, M.D., for editorial assistance.
Author Contributions
Conception and design: Anne‐Sophie Bats, Claude Nos, Fabrice Lécuru
Provision of study materials or patients: Anne‐Sophie Bats, Chérazade Bensaïd, Marie‐Aude Le Frère‐Belda, Marie‐Anne Collignon, Marc Faraggi, Fabrice Lécuru
Collection and/or assembly of data: Anne‐Sophie Bats
Data analysis and interpretation: Anne‐Sophie Bats, Marie‐Aude Le Frère‐Belda, Marie‐Anne Collignon, Fabrice Lécuru
Manuscript writing: Anne‐Sophie Bats
Final approval of manuscript: Anne‐Sophie Bats, Claude Nos, Chérazade Bensaïd, Marie‐Aude Le Frère‐Belda, Marie‐Anne Collignon, Marc Faraggi, Fabrice Lécuru
Disclosures
The authors indicated no financial relationships.
Section Editors: Dennis Chi: None; Peter Harper: sanofi‐aventis, Roche, Imclone, Pfizer, GlaxoSmithKline, Lilly, Genentech (C/A); Lilly, Novartis, sanofi‐aventis, Roche (H)
Reviewer “A”: None
Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



