-
PDF
- Split View
-
Views
-
Cite
Cite
Xingxing Chen, Xiaoli Yu, Jiayi Chen, Zhaozhi Yang, Zhimin Shao, Zhen Zhang, Xiaomao Guo, Yan Feng, Radiotherapy Can Improve the Disease‐Free Survival Rate in Triple‐Negative Breast Cancer Patients with T1–T2 Disease and One to Three Positive Lymph Nodes After Mastectomy, The Oncologist, Volume 18, Issue 2, February 2013, Pages 141–147, https://doi.org/10.1634/theoncologist.2012-0233
Close - Share Icon Share
Abstract
Evaluate the effect of postmastectomy radiotherapy (PMRT) in terms of locoregional recurrence‐free survival and disease‐free survival in triple‐negative breast cancer (TNBC) patients.
Identify the subgroup of TNBC patients most likely to benefit from PMRT.
Assess the role of PMRT in TNBC patients with intermediate‐risk (T1/2N1) disease.
Several studies have demonstrated poor locoregional control in patients with triple‐negative breast cancer (TNBC), compared with other molecular subtypes of breast cancer. We sought to evaluate whether or not postmastectomy radiotherapy (PMRT) improves locoregional recurrence‐free survival (LRFS) and disease‐free survival (DFS) outcomes in TNBC patients.
Between January 2000 and July 2007, 553 TNBC patients treated with modified radical mastectomy from a single institution were analyzed retrospectively. Patients were categorized into three groups: low risk (stage T1–T2N0), intermediate risk (stage T1–T2N1), and high risk (stage T3–T4 and/or N2–N3). Cox proportional hazards models were used to evaluate the association between PMRT and LRFS and DFS times after adjusting for other clinicopathologic covariates.
With a median follow‐up of 65 months (range, 1–140 months), 51 patients (9.2%) developed locoregional recurrence and 135 patients (24.4%) experienced disease recurrence. On multivariate analysis, PMRT was associated with significantly longer LRFS and DFS times in the entire cohort. In the intermediate‐risk group, PMRT was associated with a longer DFS time but not with the LRFS interval. In the high‐risk group, PMRT was associated with significantly longer LRFS and DFS times.
PMRT is associated with longer LRFS and DFS times in high‐risk TNBC patients and a longer DFS time in intermediate‐risk TNBC patients. Prospective randomized studies are needed to investigate the best locoregional treatment approaches for patients with this molecular subtype of breast cancer.
摘要
目的. 若干研究已显示:与其他分子亚型乳腺癌相比,三阴性乳腺癌(TNBC)患者的局部控制欠佳。本研究旨在评估乳腺切除术后放疗(PMRT)能否改善TNBC患者的局部无复发生存(LRFS)和无病生存(DFS)转归。
材料和方法. 对2000年1月∼ 2007年7月于单一机构接受改良根治性乳腺切除术(MRM)的553例TNBC患者进行回顾性分析。患者分为3组:低危组(T1 ∼ T2N0期)、中危组(T1 ∼ T2N1期)和高危组(T3 ∼ T4和/或N2 ∼N3期)。校正其他临床病理协变量后,应用Cox比例风险模型评估PMRT与LRFS和DFS时间的相关性。
结果. 在65个月(范围1 ∼140个月)的中位随访期内,51例患者(9.2%)局部复发,135例患者(24.4%)疾病复发。多变量分析显示,PMBT与整个队列LRFS和DFS时间延长相关,有统计学意义。中危组患者中,PMRT与DFS时间延长相关,但与LRFS时间无关。高危组患者中,PMRT与LRFS和DFS时间延长均相关,有统计学意义。
结论. PMRT与高危TNBC患者LRFS和DFS时间延长相关,与中危TNBC患者DFS时间延长相关。有必要开展前瞻性随机研究,对该分子亚型乳腺癌的最佳局部治疗方案进行探讨。
Implications for Practice:
The role of radiation in triple‐negative breast cancer (TNBC) has not been adequately addressed. We retrospectively evaluated the outcomes associated with postmastectomy radiotherapy (PMRT) in a large cohort of TNBC treated with MRM in our study. PMRT was associated with improved locoregional recurrence‐free survival (LRFS) and disease‐free survival (DFS) in high‐risk TNBC (T3/4 and/or N2/3), and improved DFS in intermediate‐risk TNBC (T1/2N1) with a median follow‐up of 65 months. These findings provide evidence supporting the recommendation for PMRT for TNBC with intermediate‐ and high‐risk disease.
Introduction
The role of postmastectomy radiotherapy (PMRT) in patients with breast cancer has been well established by large randomized trials and meta‐analysis [1–4], showing that PMRT can improve locoregional recurrence (LRR) in terms of survival times for properly selected patients. In general, the selection of patients for PMRT is made on the basis of established clinicopathological parameters such as tumor size and nodal status concerning the baseline risk for LRR without regard to the molecular subtype of breast cancer. However, an increasing amount of data has demonstrated the important prognostic and predictive value of molecular subtypes approximated by immunohistochemistry (IHC) in clinical practice [5–8]. In particular, triple‐negative breast cancer (TNBC), defined as tumors that are negative for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER‐2), accounts for 10%–15% of all cases of breast cancer [7, 8]. TNBC has drawn particular attention because of its aggressive features and poor prognosis, such as the young age at onset, large tumor size, and high proliferation rate [7, 8], and it also confers a higher risk for early recurrence and higher incidence of visceral metastases compared with other subtypes.
Several retrospective studies have shown a higher risk for LRR in patients with TNBC than in those with other subtypes [9–11], whereas results on the role of PMRT in patients with TNBC are conflicting [9, 12]. Data from the Danish Breast Cancer Cooperative Group showed that patients with TNBC received an appreciably lower benefit from PMRT than patients with luminal subtypes, which suggested that TNBC might be a radioresistant phenotype [9]. However, a more recent analysis in a cohort of TNBC patients treated with all kinds of locoregional treatments showed that the addition of radiation was the most beneficial factor to lower the LRR risk of TNBC patients [12], which highlights the need for radiotherapy in TNBC patients. Thus, additional data are needed to better understand the role of PMRT in TNBC treatment.
To address this question, we analyzed our institutional database on a large cohort of patients with TNBC treated with modified radical mastectomy (MRM) followed by PMRT or not. We evaluated the efficacy of PMRT in terms of the LRR‐free survival (LRFS) and disease‐free survival (DFS) times of different LRR risk subgroups to assess whether or not TNBC patients, and if so, then which subgroups, benefit from PMRT.
Methods and Materials
Patient Population
Between January 1, 2000 and July 31, 2007, in total, 553 female patients with invasive TNBC after MRM were identified in the present retrospective study at our institution. Review of data for this investigation was approved by the institutional review board of our hospital. The definition of TNBC was based on IHC staining of ER, PR, and HER‐2, which was routinely performed in the pathology department of our hospital as previously described [13]. ER and PR nuclear staining ≤1% were categorized as negative, whereas a score of 0–2+ for HER‐2 by IHC or a nonamplified HER‐2 result by fluorescence in situ hybridization (FISH) was considered HER‐2−. Patients negative for ER, PR, and HER‐2 were eligible for this study. All patients' clinicopathologic information was recorded in a computerized database at accrual and included details about age, menopausal status, tumor characteristics, treatment, and outcome. Patients with distant metastasis (DM) at diagnosis, carcinoma in situ, <1 month of follow‐up, or treatment with neoadjuvant chemotherapy were excluded.
Treatment
All patients underwent MRM including level I–II axillary dissection. The median number of axillary lymph nodes (LNs) removed was 14 (range, 1–39). Treatment with PMRT was determined by the patients and their physicians. The techniques of radiotherapy for breast cancer evolved from in‐house and sophisticated experience to evidenced‐based international guidelines around the year 2000 at our hospital, so we developed an in‐house institutional review board–approved protocol for individual treatment in patients with breast cancer in that transitional period.
At the discretion of the radiation oncologist, PMRT was delivered to 93 (13%) patients, applied with external‐beam irradiation of 46–50 Gy in 1.8‐ to 2.0‐Gy fractions to the chest wall and/or regional nodal areas, which generally included the internal mammary (IM) area and supraclavicular area (SA). Three‐dimensional (3‐D) treatment planning with the Pinnacle treatment planning system (Pinnacle, Philips Radiation Oncology Systems, Andover, MA) was performed for 52 (56.0%) patients. The chest wall was treated with an opposed tangent beam arrangement using cobalt‐60 or 4–6 MeV photons and the axillary was not routinely included in the PMRT field. The IM LNs were treated with an obliquely incident mixed photon/electron field, and an anterior photon/electron field was applied for the SA. In patients with 3‐D treatment planning, the optimal IM and SA electron field dimensions and energies were chosen on the basis of the IM‐SA LN target volume as delineated on computed tomography slices. The position of the tangential fields was adapted to match the IM‐SA fields.
Adjuvant chemotherapy was administered to 485 patients (89%) with different regimens for four to six cycles. Among them, 404 received an anthracycline‐based, a taxane‐based, or an anthracycline‐ and taxane‐based regimen and 81 patients received cyclophosphamide, methotrexate, and 5‐fluorouracil. No patient received adjuvant endocrine therapy or trastuzumab.
Endpoints
The primary endpoint of this study was LRR, defined as any clinical and biopsy‐proven tumor recurrence involving the ipsilateral chest wall and/or axillary, supraclavicular, intraclavicular, of internal mammary nodes. All LRRs were recorded regardless of their relation in time to DM. The secondary endpoint was disease recurrence (DR), defined as the first locoregional or distant failure or death during follow‐up. Both the LRFS and DFS intervals were measured from the date of surgery to the date of the occurrence of the event of interest. In the absence of the event of interest, the observation time was censored at the date on which follow‐up ended.
Statistical Analysis
Comparisons of tumor and treatment characteristics between the PMRT and non‐PMRT groups were performed using χ2 and Fisher's exact tests. Fisher's exact test was used when the cell counts were too low for χ2 tests. Current consensus guidelines recommend treatment with PMRT for high‐risk patients (tumor [T]stage T3–T4 and/or node [N] stage N2–N3), consideration of PMRT for intermediate‐risk patients (stage T1–T2N1), and omission of PMRT for low‐risk patients (stage T1–T2N0) [14, 15]. Patients were categorized into one of these three clinically relevant subgroups. Estimates of LRFS and DFS rates and adjusted survival curves were computed according to the method of Kaplan–Meier, with adjustment for the covariates included in the multivariate analysis. The multivariate analysis was performed using Cox logistic regression to test the following known variables: age, PMRT treatment, tumor size, LN status, lymphovascular invasion (LVI), grade, and chemotherapy regimens. Although a pathologic description of LVI and grade were not fully available in the present population, given that they have been reported to be additional useful prognostic indicators in assessing prognostic outcomes with invasive breast cancer [15, 16], we took them into account in the multivariate model. Tumor size and LN status (four or more positive LNs vs. one to three positive LNs) were investigated as categorical variables in multivariate models for the entire cohort but as continuous variables in the three subgroup models. All statistical analyses were carried out using STATA, version 11.0 (Stata Corporation, College Station, TX). All p‐values were two sided, and a p‐value ≤.05 was considered statistically significant.
Results
Patient and Treatment Characteristics
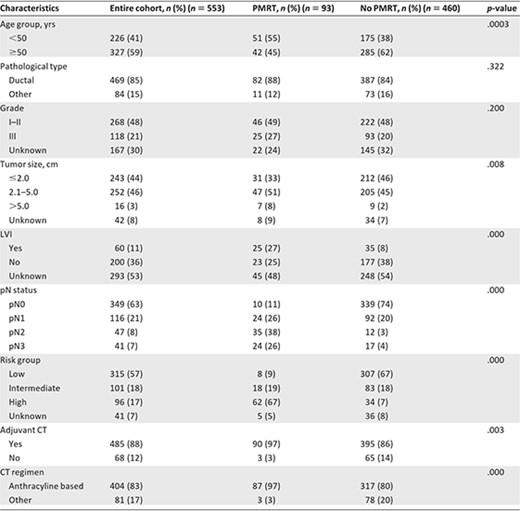
Of 553 patients identified with TNBC, the median age was 52 years (range, 28–80 years). The median tumor size was 2.2 cm (range, 0–10 cm), 469 patients (85%) had ductal histology, and 204 patients (36.9%) had pathologically involved LNs. As previously reported [12], this population had a high percentage of patients with small sized tumors (stage T1, 44%) and a low incidence of LN involvement (stage N0, 63%) (Table 1).
Clinicopathologic characteristics in the entire cohort and comparisons between patients treated with and without PMRT
Data presented as numbers, with percentages in parentheses.
Abbreviations: CT, chemotherapy; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy; pN, pathologic node.
Clinicopathologic characteristics in the entire cohort and comparisons between patients treated with and without PMRT

Data presented as numbers, with percentages in parentheses.
Abbreviations: CT, chemotherapy; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy; pN, pathologic node.
The associations between PMRT and key patient characteristics are presented in Table 1. Worse prognostic features were correlated with greater use of PMRT. Patients treated with PMRT were younger; had more advanced T stage, N stage, and American Joint Committee on Cancer stage; had greater LVI; and were more frequently treated with chemotherapy than those not treated with PMRT (all p < .05) (Table 1). The distributions of histologic type, tumor size, and grade were similar in the two groups (all p > .05) (Table 1).
In total, 315 patients (57%) were subclassified as low risk, 101 patients (18%) were classified as intermediate risk, and 96 patients (17%) were classified as high risk. Risk group was strongly associated with receipt of PMRT (p < .0001), with high‐risk patients most likely to receive PMRT (62 of 96; 67%) (Table 1).
For 93 patients (13%) who received PMRT, four (4.30%) received PMRT directed to the chest wall only, 65 (69.89%) received PMRT directed to the chest wall as well as the regional nodal areas, and 24 (25.81%) received PMRT directed to the regional nodal areas only. Details of radiation fields among the different risk groups are shown in Table 2.
Details of PMRT fields in 93 (13%) triple‐negative breast cancer patients treated with PMRT
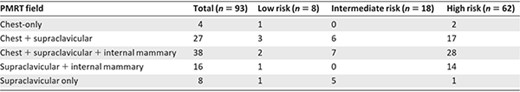
Abbreviation: PMRT, postmastectomy radiotherapy.
Details of PMRT fields in 93 (13%) triple‐negative breast cancer patients treated with PMRT

Abbreviation: PMRT, postmastectomy radiotherapy.
Multivariate Analysis: Entire Population
With a median follow‐up of 65 months (range, 1–140 months), 51 patients (9.2%) developed LRR and 135 patients (24.4%) experienced disease recurrence (12.7%). The 5‐year cumulative LRFS and DFS rates were 90.6% and 75.9%, respectively, for all patients. In the multivariate analysis of the LRFS time (Table 3) for all 553 patients, the lack of PMRT was associated with developing a LRR (adjusted hazard ratio [HR], 4.34; 95% confidence interval [CI], 1.08–17.45; p = .039) (Fig. 1A). Other factors found to be significant for developing LRR included the presence of four or more positive LNs (HR, 4.67; 95% CI, 1.34–16.32; p = .016) and LVI (HR, 3.51; 95% CI, 1.30–9.491; p = .013). In the multivariate analysis of the DFS time, the lack of PMRT (HR, 2.94; 95% CI, 1.16–7.47; p = .023) (Fig. 1C), the presence of four or more positive LNs (HR, 7.12; 95% CI, 2.82–17.92; p = .000), and LVI (HR, 2.75; 95% CI, 1.48–5.13; p = .001) were independent predictors of a shorter DFS interval.
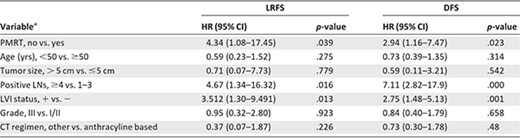
aUnknowns excluded.
Abbreviations: CI, confidence interval; CT, chemotherapy; DFS, disease‐free survival; HR, hazard ratio; LN, lymph node; LRFS, locoregional recurrence‐free survival; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy.

aUnknowns excluded.
Abbreviations: CI, confidence interval; CT, chemotherapy; DFS, disease‐free survival; HR, hazard ratio; LN, lymph node; LRFS, locoregional recurrence‐free survival; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy.
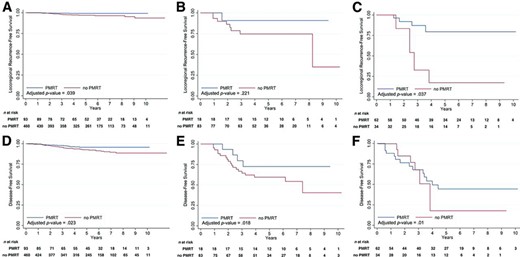
Adjusted Kaplan–Meier probability curves of locoregional recurrence‐free survival and disease‐free survival probabilities according to the use of PMRT in various risk groups. (A, D): Entire cohort. (B, E): Intermediate‐risk group (stage T1–T2N1). (C, F): High‐risk group (stage T3–T4 and/or N2–N3).
Abbreviation: PMRT, postmastectomy radiotherapy.
Multivariate Analysis: According to Risk Group
Patients with Low‐Risk Disease Without PMRT
The majority of the patients with low‐risk disease (n = 315) were not treated with PMRT, hence the small number of patients treated with PMRT (n = 8) were not considered in the analysis. The 5‐year actuarial LRFS and DFS rates were 92.9% and 84.0%, respectively. On multivariate analysis of patients with low‐risk disease without PMRT (Table 4), LVI was the only strong significant predictor of both the LRFS (HR, 9.48; 95% CI, 2.37–37.90; p = .001) and DFS (HR, 3.23; 95% CI, 1.03–10.11; p = .044) times. Patients with LVI (n = 60) had a significantly higher 5‐year LRR rate and 5‐year DR rate than those without LVI (n = 200) (5‐year LRR rate, 18.2% vs. 6.2%; 5‐year DR rate, 18.3% vs. 14.7%).
Multivariate analyses of LRFS and DFS probabilities stratified by risk group
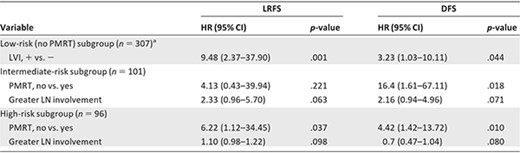
PMRT and other covariates with p ≤.1 were selected to show in this table. All HRs are adjusted for known covariates mentioned in methods and materials.
aOnly 8 patients with low‐risk disease were treated with PMRT, so they were excluded in this multivariate analysis of the low‐risk group.
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; LN, lymph node; LRFS, locoregional recurrence‐free survival; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy.
Multivariate analyses of LRFS and DFS probabilities stratified by risk group

PMRT and other covariates with p ≤.1 were selected to show in this table. All HRs are adjusted for known covariates mentioned in methods and materials.
aOnly 8 patients with low‐risk disease were treated with PMRT, so they were excluded in this multivariate analysis of the low‐risk group.
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; LN, lymph node; LRFS, locoregional recurrence‐free survival; LVI, lymphovascular invasion; PMRT, postmastectomy radiotherapy.
Patients with Intermediate‐Risk Disease
In patients with intermediate‐risk disease (n = 101), PMRT was not associated with the LRFS time (p = 0.221) (Fig. 1B) on multivariate analysis (Table 4). Greater LN involvement showed a marginally significant association with the LRFS interval (HR, 2.33; 95% CI, 0.96–5.70; p = .063). On multivariate analysis of the DFS time, PMRT was associated with a longer DFS time (HR, 16.41; 95% CI, 1.61–167.11; p = .018) (Fig. 1E). Greater LN involvement continued to show a marginally significant association with the DFS interval (HR, 2.15; 95% CI, 0.93–4.96; p = .071).
Patients with High‐Risk Disease
In patients with high‐risk disease (n = 96), PMRT was associated with a longer LRFS time (HR, 6.22; 95% CI, 1.12–34.45; p = .037) (Fig. 1C) on multivariate analysis (Table 4). Greater LN involvement showed a trend toward a shorter LRFS time (HR, 1.10; 95% CI, 0.98–1.22; p = .098). On multivariate analysis of the DFS time, PMRT was the only predictor associated with a longer DFS time (HR, 4.42; 95% CI, 1.42–13.71; p = .01) (Fig. 1F).
Discussion
This report is the first large series in a single institute investigating the efficacy of PMRT in patients with TNBC treated with MRM according to different risk subgroups. Our data showed that the addition of PMRT to the treatment of patients with high‐risk disease (stage T3–T4 and/or N2–N3) led to superior LRFS and DFS outcomes. For patients with intermediate‐risk disease (T1–T2N1), a superior DFS outcome but not LRFS outcome with the addition of PMRT was observed. Our findings provide evidence to support the recommendation of PMRT for intermediate‐ and high‐risk patients.
TNBC has consistently been suggested as a predictive factor for worse locoregional outcome in the setting of MRM [9–11]. A large retrospective study from Voduc et al. [10] analyzed 2,985 patients treated with all types of surgery, 291 of whom were subclassified as core basal TNBC using a validated six‐marker IHC panel (ER, PR, HER‐2, epidermal growth factor receptor, CK5/CK6, and Ki‐67). They found that core basal TNBC was an independent risk factor for local relapse (HR, 1.9) and regional relapse (HR, 4.22) in patients who received MRM. Another retrospective study by Wang et al. [17] that included 835 node‐positive patients treated with mastectomy with or without PMRT showed a 5‐year LRR rate of 13.5% in 141 patients with TNBC, which was significantly higher than in patients with other subtypes. Kyndi et al. [9] retrospectively examined the outcomes of 1,000 node‐positive breast cancer patients enrolled in the large Danish postmastectomy radiation trials, Danish Breast Cancer Cooperative Group (DBCTCG) 82b and DBCTCG 82c, including 152 patients with TNBC. They found that TNBC was strongly associated with a higher LRR risk in both randomization arms, with 15‐year LRR rates of 32% in the no‐PMRT cohort and 15% in the PMRT cohort.
Results from these studies collectively show that TNBC is an adverse prognostic factor for locoregional control (LRC). Because PMRT accounts for a considerable proportional reduction in the LRR risk for selected patients, it is reasonable to hypothesize that the relatively high risk for LRR after MRM in TNBC patients may be a result of its low radiosensitivity. This hypothesis is supported by the study from the Danish randomized PMRT trials [9], in which the impact of molecular subtype on the response to PMRT was assessed retrospectively. They showed a significantly lower LRR rate after PMRT in patients with TNBC; however, the degree of benefit obtained from PMRT was relatively lower in TNBC patients than in patients with the luminal subtypes. These data suggest some radioresistance of TNBC in contrast to luminal subtypes. However, a recent study from Abdulkarim et al. [12] did not detect an inferior benefit from PMRT in terms of the LRR rate in a separate analysis of 768 patients with TNBC. By stratifying the series according to locoregional management (breast‐conserving therapy [BCT], MRM without PMRT, MRM with PMRT), they found that MRM without PMRT was associated with a higher LRR rate in patients with TNBC, compared with BCT on multivariate analysis. In addition, MRM without PMRT was found to be the only independent prognostic factor associated with a higher LRR rate in patients with stage T1–T2N0 TNBC, compared with BCT. The authors thus suggested that the radioresistance of TNBC observed in the Danish study might be a result of the intrinsic aggressive behavior of TNBC, and that the addition of radiation had a significant beneficial impact on the LRFS rate in patients with early TNBC. The benefit of the addition of PMRT to the treatment of patients with TNBC was also seen in a multicenter prospective analysis by Wang et al. [18] in which 681 early‐stage TNBC patients were randomized to receive adjuvant chemotherapy plus radiation or adjuvant chemotherapy alone after mastectomy. In that study, the DFS and overall survival (OS) times were significantly longer in patients treated with adjuvant chemotherapy plus radiation than in those treated with chemotherapy alone.
The current study demonstrates that patients with TNBC derive a significant benefit in terms of the LRFS and DFS outcomes with the addition of PMRT. For the entire cohort, multivariate analysis revealed that the lack of PMRT had an HR of 4.34 associated with developing LRR after adjusting for other potential confounders. In patients with high‐risk disease in particular, the HR for LRR was 6.22 when no PMRT was performed. This lower LRR probability with PMRT use in the present study is in keeping with the data from randomized trials and meta‐analysis of PMRT trials, which demonstrated a one half to two thirds lower LRR risk with the use of PMRT [1–4]. Furthermore, our results also corroborate the previous data showing that the lack of PMRT is a strong predictor of a shorter DFS interval for the entire cohort, and PMRT remained the only significant discriminator of the DFS time among the high‐risk subgroup.
The incidence of LRR, and thus the value of PMRT in patients with stage T1–T2N1 disease, remains controversial. Some retrospective studies identified subsets of patients who have a significant risk for LRR, and for whom PMRT should be considered. These include patients with ER− disease, which is considered to be a powerful discriminator of LRR in various studies [19–21]. The high postmastectomy LRR risk observed in ER− patients with stage T1–T2N1 disease highlights that this subgroup of TNBC patients also might be a proper subgroup that would benefit from PMRT. However, until now, no study performed multivariate analyses restricted specifically to TNBC patient cohorts with stage T1–T2N1 disease. In our study, this subgroup of TNBC patients experienced a substantially higher DFS rate with PMRT on multivariate analysis, and there was a trend toward a higher 5‐year adjusted LRFS rate with the addition of PMRT; however, this association did not reach statistical significance. The role of radiation demonstrated in the current series corroborates large randomized trials reporting that radiation had an advantageous effect on the DFS outcome [1–3]. Conceptually, irradiation of locoregionally occult disease, which could serve as a source of DM, has the theoretical advantage of decreasing the subsequent incidence of DM and improving the DFS and OS rates. Our result is somewhat consistent with this theory in demonstrating that PMRT is effective in improving disease outcomes in TNBC patients of the intermediate‐risk subgroup. However, it is prudent that these observations be interpreted with caution because of the small sample size of this subgroup (n = 101) and the fact that only 17.8% of them received PMRT. In addition, our series documented baseline LRR rates of 11.5% at 5 years and 24.2% at 10 years for patients with stage T1–T2N1 TNBC in the absence of PMRT. This relatively low rate, which can be attributed to the aggressive axillary clearance, with a mean number of 16 (range, 2–33) axillary LNs dissected, could be one potential explanation for the small LRC benefit from PMRT. Truly, more data from clinical trials are needed to guide treatment recommendations regarding this subgroup of patients.
Although current international consensuses and guidelines do not recommend the routine use of PMRT for patients with stage T1–T2N0 disease, there is accumulating information that this is a clinically heterogeneous group and that some subgroup of these patients may benefit from radiotherapy [22, 23]. Our results are concordant with the previous studies finding that, among patients with stage T1–T2N0 TNBC treated without PMRT, LVI causes a higher risk for LRR after MRM, with a 5‐year LRR rate of 18.2% and a 10‐year LRR rate >30%. These findings may have some implications for designing prospective studies of PMRT in patients with stage T1–T2N0 TNBC, with special interest in those with LVI.
There are certain limitations to this study. First, it has inherent selective biases in patient selection and treatment assignment because of the retrospective nature of the design. Although we endeavored to mitigate several risk variables by controlling for them in a multivariate model, it is possible that other unmeasured confounders that we did not take into account may account, in part, for our current results. Second, potential misclassification of TNBC could happen based on IHC. Information on the 2+ HER‐2 score in the majority of patients in our study could not be re‐evaluated using FISH. In an effort to further validate the present findings, we conducted a separate analysis that excluded the 102 patients with a 2+ HER‐2 score. This analysis continued to show the same results as with our prior findings. Finally, a larger sample size and longer follow‐up duration may help to assess the present outcomes and define subgroups of patients who can benefit from PMRT with respect to survival times. Despite these limitations, our study offers LRR risk and DFS estimates and provides evidence supporting the beneficial effect of PMRT in selected patients with TNBC.
Conclusion
This study confirms the beneficial impact of PMRT on the LRFS and DFS outcomes and supports the use of PMRT in patients with TNBC with high‐risk (stage T3–T4 and/or N2–N3) and with intermediate‐risk disease (stage T1–T2N1). For low‐risk TNBC patients not treated with PMRT, LVI is associated with a significantly greater LRR risk. Prospective randomized studies are warranted to confirm our findings and further define optimal locoregional treatment approaches for patients with this unique subtype of breast cancer.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81072164). This work was presented in part at the 53rd Annual American Society for Therapeutic Radiology and Oncology Meeting, Miami Beach, October 2011.
Xingxing Chen and Xiaoli Yu contributed equally to this work.
Author Contributions
Conception/Design: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Jiayi Chen, Zhaozhi Yang, Zhimin Shao, Zhen Zhang, Yan Feng
Provision of study material or patients: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Jiayi Chen, Zhimin Shao, Zhen Zhang, Yan Feng
Collection and/or assembly of data: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Zhaozhi Yang, Zhimin Shao, Zhen Zhang, Yan Feng
Data analysis and interpretation: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Jiayi Chen, Zhaozhi Yang, Zhimin Shao, Zhen Zhang, Yan Feng
Manuscript writing: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Jiayi Chen, Zhaozhi Yang, Yan Feng
Final approval of manuscript: Xingxing Chen, Xiaomao Guo, Xiaoli Yu, Jiayi Chen, Zhaozhi Yang, Zhimin Shao, Zhen Zhang, Yan Feng
Disclosures
The authors indicated no financial relationships.
Section Editors: Gabriel Hortobágyi: Antigen Express, Galena Biopharma, Novartis, Rockpointe (C/A); Novartis (RF); Taivex, (O); founder and member of the board of directors for Citizen's Oncology Foundation; Kathleen Pritchard: Novartis, Roche, AstraZeneca, Pfizer, Abraxis, Boehringer‐Ingelheim, GlaxoSmithKline, Sanofi, Ortho‐Biotech, YM Biosciences, Amgen, Bristol‐Myers Squibb, Bayer Schering Pharma (C/A), (H)
Reviewer “A”: None
Reviewer “B”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
Author notes
Disclosures of potential conflicts of interest may be found at the end of this article.



