-
PDF
- Split View
-
Views
-
Cite
Cite
Sotirios G. Papageorgiou, Christos K. Kontos, Vassiliki Pappa, Hellinida Thomadaki, Frida Kontsioti, John Dervenoulas, Efstathios Papageorgiou, Theofanis Economopoulos, Andreas Scorilas, The Novel Member of the BCL2 Gene Family, BCL2L12, Is Substantially Elevated in Chronic Lymphocytic Leukemia Patients, Supporting Its Value As a Significant Biomarker, The Oncologist, Volume 16, Issue 9, September 2011, Pages 1280–1291, https://doi.org/10.1634/theoncologist.2010-0349
Close - Share Icon Share
Abstract
BCL2L12 is a recently identified gene belonging to the BCL2 family, members of which are implicated in hematologic malignancies, including chronic lymphocytic leukemia (CLL). The aim of this study was to analyze the mRNA expression of the novel apoptosis‐related gene BCL2L12 in patients with CLL and to examine its prognostic and predictive value and potential clinical application as a novel molecular biomarker for CLL. For this purpose, total RNA was isolated from peripheral blood of 65 CLL patients and 23 healthy donors. An ultrasensitive quantitative real‐time polymerase chain reaction methodology for BCL2L12 and BCL2 mRNA quantification was developed using SYBR Green chemistry. After preparing cDNA by reverse transcription, relative quantification analysis was performed using the comparative CT (2−ΔΔCT) method. Furthermore, analysis of IGHV mutational status, CD38 expression, and detection of early apoptosis by double staining with Annexin V‐FITC and propidium iodide were performed. According to our findings, BCL2L12 mRNA expression is significantly higher in CLL patients than in healthy donors. Receiver operating characteristic analysis demonstrated that BCL2L12 expression had significant discriminatory value, distinguishing very efficiently CLL patients from the non‐leukemic population. Moreover, BCL2L12 expression predicts the presence of CLL, as demonstrated by both univariate and multivariate logistic regression analyses. Finally, high BCL2L12 mRNA levels are associated with advanced clinical stage and predict shorter overall survival in CLL patients.
摘要
BCL2L12是最近确定的一个BCL2家族成员,已知BCL2家族成员参与血液系统恶性肿瘤的形成,包括慢性淋巴细胞白血病(CLL)。本研究旨在分析新型凋亡相关基因BCL2L12在CLL患者中的mRNA表达水平,并观察其作为CLL的新型分子生物标志物的预后和预测价值以及潜在的临床意义。为了这一目的,从65例CLL患者和23例健康供者的外周血中分离总RNA。采用SYBR Green chemistry建立一种用于BCL2L12和BCL2 mRNA定量的超灵敏定量实时聚合酶链反应方法。通过逆转录制备cDNA后,采用比较CT(2-∆∆CT)法进行相对定量分析。此外,采用Annexin V‐FITC和碘化丙啶双染色法对早期凋亡进行检测,并对IGHV突变状态和CD38表达进行分析。根据本研究结果,BCL2L12 mRNA在CLL患者中的表达水平明显高于健康供者。受试者工作特征分析显示,BCL2L12表达具有重要的鉴别价值,能高效区分CLL患者与非白血病群体。此外,单变量和多元logistic回归分析显示BCL2L12表达预示存在CLL。最终,高BCL2L12 mRNA水平与临床晚期相关,并预示CLL患者的总生存时间较短。
Introduction
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world. It is characterized by accumulation of malignant, clonal CD5+/CD23+ B cells in peripheral blood, bone marrow, and lymphatic tissues. The malignant lymphocytes are long‐lived cells arrested in the G0/early G1 phase of the cell cycle, suggesting a role of impaired apoptosis in the pathogenesis of the disease [1, 2]. Recent studies have also demonstrated that in CLL increased proliferation and impaired malignant cell turnover take place on a daily basis [3, 4]. Furthermore, the role of microenvironment seems to be crucial since clonal B cells rapidly undergo apoptosis when cultured in vitro, whereas in vivo they accumulate progressively [5, 6].
The clinical course of individual CLL patients is extremely heterogeneous, with survival ranging from months to decades. Notably, an important subset of patients presenting with low‐grade CLL will develop a more aggressive and life‐threatening disease. Because these patients might potentially benefit from early treatment, it is critical to reliably predict patients' prognosis at diagnosis, especially those in an early disease stage, thus allowing personalized, risk‐adapted therapy [7]. During recent decades, many efforts have focused on the identification of novel prognostic markers in CLL, from clinical staging systems and serum markers over proliferation markers and cytogenetics to more recent markers like the IGHV mutational status and its possible surrogate markers such as CD38, ZAP70, and lipoprotein lipase expression, resulting in a high number of reports describing the predictive value of different parameters with regard to overall survival, disease progression, and response to treatment [8]. Rai and Binet staging systems are the most commonly used staging systems in CLL [9, 10]. However, neither system accurately identifies those patients in early stages that will progress from those who will remain indolent [11].
Apoptosis is a genetically controlled cell suicide program with a central role in the regulation of fundamental mechanisms such as tissue homeostasis, development, and differentiation, while its deregulation may lead to distinct pathologic processes and contribute significantly to the pathogenesis and progression of cancer, as well as to response of tumors to therapeutic intervention [12, 13]. Morphologic features of apoptosis usually entail chromatin condensation, DNA fragmentation, membrane blebbing, and disruption of the maintained integrity of organelle structures along with formation of apoptosomes [14, 15]. In recent years, the molecular machinery underlying apoptosis has been elucidated, thus revealing several proteins that are responsible, directly or indirectly, for the morphologic and biochemical changes that characterize this phenomenon. The apoptotic mechanism is executed by a family of cysteine proteases, known as caspases, the activation of which is mainly regulated by members of the BCL2 family [16].
The BCL2 family consists of pro‐ and anti‐apoptotic proteins sharing structural homology, because they all contain at least one BCL2‐homology domain, namely, BH1, BH2, BH3, and/or BH4 [17]. The pro‐apoptotic members of the BCL2 family, including BAX, BAD, BID, and BCLXS, facilitate apoptosis, whereas the anti‐apoptotic members, such as BCL2, BCLXL, and BCLW, inhibit initiation of the apoptotic machinery and eventually impede this form of programmed cell death [18]. Interestingly, the relative ratios of pro‐ and anti‐apoptotic BCL2 family proteins dictate the ultimate sensitivity or resistance of cells to various apoptotic stimuli, including growth factor deprivation, hypoxia, irradiation, anti‐cancer drugs, oxidants, and Ca2+ overload, therefore presumably explaining why expression of a variety of BCL2 family members has a significant prognostic value for many types of cancer and leukemia treated by chemotherapy [19, 20]. In CLL, the BCL2 protein is overexpressed in about half of the patients [11] and is associated with decreased clonal cell apoptosis, resistance to chemotherapy (chlorambucil or fludarabine) [21–23], and advanced stages of the disease [24]. Other anti‐apoptotic members of the BCL2 family, like BCLXL and MCL1, are also overexpressed in CLL patients, whereas pro‐apoptotic proteins, such as BAX and BCLXS, are underexpressed [25].
BCL2L12 is a newly identified member of the BCL2 family, containing a highly conserved BH2 domain, a BH3‐like motif, and a proline‐rich region. The BCL2L12 gene maps to chromosome 19q13.3 and is localized between the IRF3 and PRMT1/HRMT1L2 genes, close to the RRAS oncogene. Currently, two alternative transcript variants of the BCL2L12 gene are known, one consisting of seven coding exons and producing a 334 amino acid polypeptide and another one resulting from alternative splicing. Expression of the full‐length mRNA transcript has been observed in many tissues, including breast, thymus, prostate, fetal liver, colon, placenta, pancreas, small intestine, spinal cord, kidney, and bone marrow, whereas the alternative splicing variant, named BCL2L12‐A, lacks exon 3 and is mainly expressed in fetal liver, spinal cord, and skeletal muscle [26].
In vitro studies in human leukemia cell lines have revealed notable alterations of BCL2L12 mRNA expression in HL‐60 acute promyelocytic leukemia cells after treatment with various chemotherapeutic drugs, including cisplatin, carboplatin, doxorubicin, methotrexate, etoposide, topotecan, vincristine, and taxol [27–31], as well as in SHI‐1 acute monocytic leukemia cells after treatment with bortezomib [32]. Recently, it has also been proposed that BCL2L12 and BCL2L12‐A may play an important role in cisplatin‐induced apoptosis in MDA‐MB‐231 breast adenocarcinoma cells [33, 34]. These important modulations in BCL2L12 mRNA levels seem to depend on both the apoptotic inducer and the induced apoptotic pathway, implying a strong relationship between changes in BCL2L12 mRNA levels and apoptosis [19]. Moreover, transfection of acute myeloid leukemia K562 cells with either the RUNX1‐p48 isoform or the CD56 (120 and 140 kDa) isoforms resulted in upregulation of BCL2L12 mRNA along with increased cell number, whereas transfection of the same cell line with either the RUNX1‐p38a or RUNX1‐p24 isoform resulted in downregulation of BCL2L12 mRNA along with decreased cell number and increased apoptosis [35]. However, there are no reports to date regarding BCL2L12 expression and its prognostic significance in patients with hematologic malignancies.
The purpose of the current study was to analyze the mRNA expression of the novel apoptosis‐related gene BCL2L12 in patients with CLL and healthy controls, using an ultrasensitive and highly accurate quantitative real‐time polymerase chain reaction (qRT‐PCR) methodology with the SYBR Green chemistry, and to examine its prognostic and predictive value and potential clinical application as a novel molecular biomarker for CLL.
Materials and Methods
Patients and Cell Line
Sixty‐five patients who were diagnosed with CLL at the 2nd Department of Internal Medicine in University General Hospital “Attikon”, Athens, Greece, were included. None of the patients received any treatment for at least 6 months prior to blood collection. All analyses were performed on patients' peripheral blood mononuclear cells (PBMCs), consisting of >90% of CLL lymphocytes, as confirmed by cell immunophenotyping. In addition, PBMCs including normal lymphocytes and monocytes from 23 healthy donors (14 men and 9 women), whose ages ranged from 48 to 83 years (median = 68 years), were used as normal controls. PBMCs were isolated from peripheral blood samples by centrifugation on a Ficoll‐Hypaque gradient. The current study was performed in accordance with the ethical standards of the 1975 Declaration of Helsinki as revised in 2000 and was approved by the institutional review board of University General Hospital “Attikon” (Athens, Greece). Moreover, written informed consent was obtained from CLL patients participating in the study.
Diagnosis of CLL was established according to the National Cancer Institute‐sponsored Working Group (NCI‐WG) recommended criteria [36]. The clinical stage of the disease was determined according to the Binet classification system [9]. Overall survival (OS) was calculated from the time of diagnosis up to death or last contact.
The human acute promyelocytic leukemia cell line HL‐60 was maintained in RPMI 1640 medium, adjusted to contain 10% fetal bovine serum, 100 kU/L penicillin, 0.1 g/L streptomycin, and 2 mM l‐glutamine. Cells were seeded at a concentration of 4 × 105 cells per mL and incubated for 48 hours at 37°C in a humidified atmosphere containing 5% CO2, before being collected for further use.
Cell Immunophenotyping
Immunophenotyping of leukemic cells was performed using a routine panel of evaluated monoclonal antibodies, including anti‐CD3, ‐CD5, ‐CD10, ‐CD11c, ‐CD19, ‐CD20, ‐CD23, ‐FMC7, ‐Igκ, and ‐Igλ (Immunotech, Prague, Czech Republic), conjugated with fluorescein isothiocyanate (FITC), R‐phycoerythrin (PE), or cyanine‐5, with flow cytometry in a Coulter Epics XL‐MCL flow cytometer (Beckman Coulter, Inc., Miami, FL, USA). The diagnosis was confirmed by detecting the leukemic CD5+/CD19+/CD23+ clone. To assess CD38 expression, a monoclonal antibody against CD38 antigen was evaluated. According to the established thresholds [37], cases with >30% of CD38‐positive CLL cells were considered as positive.
Early Apoptosis Detection
Whole blood (2 mL) was lysed with ammonium chloride in a proportion of 1:7. After centrifugation of the solution, the supernatant was discarded and the remaining cell pellet was washed three times with phosphate‐buffered saline (pH 7.2 at 25°C). Cells were then simultaneously stained with Annexin V‐FITC (green fluorescence) and the nonvital dye propidium iodide, which allowed the discrimination of intact cells (FITC−/PI−), early apoptotic cells (FITC+/PI−), and late apoptotic or necrotic cells (FITC+/PI+) [38] and, therefore, the calculation of the percentage of leukemic cells at early stages of apoptosis (early apoptosis index). For this purpose, 1 μL of Annexin V‐FITC (Immunotech) solution and 5 μL of propidium iodide (Immunotech) were added in the resuspended cell pellet and mixed gently. Twenty microliters of anti‐CD19‐PE (Immunotech) antibody solution were added next. The tubes were kept for 15 minutes on ice and in the dark. After incubation, 400 μL of ice‐cold binding buffer were added. The cell preparation was analyzed by two‐color flow cytometry in a Coulter Epics XL‐MCL flow cytometer (Beckman Coulter, Inc.). At the FS/SS plot, the CD19+ cell population (B‐lymphocytes) was further analyzed.
IGHV Gene Sequencing Analysis
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) from isolated PBMCs of CLL patients and reverse‐transcribed into cDNA using the Superscript II reverse transcriptase (Invitrogen) and random examers as primers, according to the manufacturer's instructions. As a control for the presence of intact RNA, 5 μL of the synthesized cDNA were amplified by PCR using primers specific for the retinoic acid receptor alpha (RARA) gene, as previously described [39]. PCR amplification and sequence analysis of IGHV‐IGHD‐IGHJ gene rearrangements were performed as previously described [40, 41]. Clonal PCR products were purified with the QIAquick Gel Extraction Kit (Qiagen, Inc., Valencia, CA, USA), and both strands were sequenced by fluorescence dideoxy chain termination with a CEQ 8000 Genetic Analysis System (Beckman Coulter, Inc.). Sequence data were interpreted using IMGT, the International ImMunoGeneTics information system (http://imgt.cines.fr), and more particularly, the IMGT/V‐QUEST and IMGT/JunctionAnalysis tools [42]. IGHV genomic sequences were considered mutated if the homology with the closest germ line counterpart was <98%.
First‐Strand cDNA Synthesis
Total RNA was extracted using the TRIzol reagent (Invitrogen) from isolated PBMCs of CLL patients and healthy donors, and first‐strand cDNA was then synthesized using the Superscript II reverse transcriptase (Invitrogen) and oligo(dT)12–18 as primer, according to the manufacturer's instructions. The reaction mixture contained 2 μg total RNA diluted in sterile, distilled water, 500 ng of oligo(dT)12–18 primer, 4 μL of reaction buffer (5×, 250 mM Tris‐HCl, pH 8.3 at 25°C, 375 mM KCl, 15 mM MgCl2, 0.1 M DTT), 1 μL of dNTP mix (10 mM each), 20 U of RNaseOUT (40 U/μL, Invitrogen) RNase inhibitor, and 100 U of Superscript II reverse transcriptase (200 U/μL, Invitrogen). The final reaction volume was 20 μL. The initial reaction mixture containing only diluted RNA, oligo(dT)12–18 primer, and dNTPs was heated at 65°C for 5 minutes and then quickly chilled on ice, whereas the final reaction mixture was incubated at 42°C for 50 minutes, and the reverse transcription was terminated by heating the mixture at 70°C for 15 minutes.
qRT‐PCR
On the basis of the information of the BCL2L12, BCL2, and GAPDH cDNA sequences (GenBank Accession Nos.: NM_138639.1, NM_000633.2, NM_002046.3, respectively), three pairs of gene‐specific primers were designed. The sequences of all real‐time PCR primers and the lengths of the PCR amplicons are shown in supplemental online Table 1.
qRT‐PCR was performed using the SYBR Green chemistry, in MicroAmp Optical 96‐well reaction plates (Applied Biosystems, Foster City, CA, USA). PCR runs and fluorescence detection were carried out in a 7500 Real Time PCR system (Applied Biosystems). The increase in fluorescence emission (Rn) was measured during the course of PCR amplification, and the difference (ΔRn) between the fluorescence emission of the product and the baseline was calculated by the Sequence Detection System software (Applied Biosystems) and plotted versus the cycle number (supplemental online Figure 1). Threshold cycle values were then calculated by determining the point at which the emitted fluorescence exceeded the threshold, determined as 10 times the standard deviation of the baseline from cycles 3 to 15 [43].
Amplification Process and Dissociation Curve Analysis
The reaction mixture contained 10 ng of cDNA diluted in 2.5 μL of diethylpyrocarbonate‐treated water, 5 μL of Power SYBR Green PCR Master Mix (2×) (Applied Biosystems), and 2 μL of gene‐specific primers (final concentration = 50 nM each), in a final reaction volume of 10 μL. The reaction conditions were as follows: denaturation of the template and activation of AmpliTaq Gold DNA Polymerase LD, at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, for denaturation of the PCR products, and 60°C for 60 seconds, for primer annealing and extension. Each RT‐PCR reaction was performed in duplicate, to evaluate data reproducibility.
To distinguish between the main PCR products and primer‐dimers or other nonspecific products, dissociation curves of the PCR products were generated after amplification, by heating the reaction mixtures from 60 to 95°C with a heating rate of 0.1°C/s and continuously acquiring fluorescence emission data. The melting temperatures (Tm) of the BCL2L12, BCL2, and GAPDH amplicons are shown in supplemental online Table 1. Primer‐dimers and/or other nonspecific products are characterized by a much lower Tm (up to 75.0°C).
Calculations and Validation of the Comparative CT (2−ΔΔCT) Method for BCL2L12 and BCL2 mRNA Quantification
Calculations were made using the comparative CT (2−ΔΔCT) method. The application of this method is based on the assumptions that the PCR amplification efficiencies of the target and the reference genes are similar to each other and close to 1 as well [44]. The prerequisites for the application of the 2−ΔΔCT method were checked in a validation experiment, in which CT values of BCL2L12, BCL2, and GAPDH were measured in a dilution series of control cDNA over a 104‐fold range and then plotted against log cDNA dilution. RT‐PCR efficiency (E) for amplification of each gene was calculated using the following formula: E = −1 + 10(−1/α), where α is the slope of the corresponding amplification plot. As illustrated in Figure 1, the slopes of BCL2L12, BCL2, and GAPDH amplification plots are very similar (−3.392, −3.419, and −3.334, respectively), which clearly indicates similar efficiencies for the corresponding amplicons (97.2, 96.1, and 99.5%, respectively).
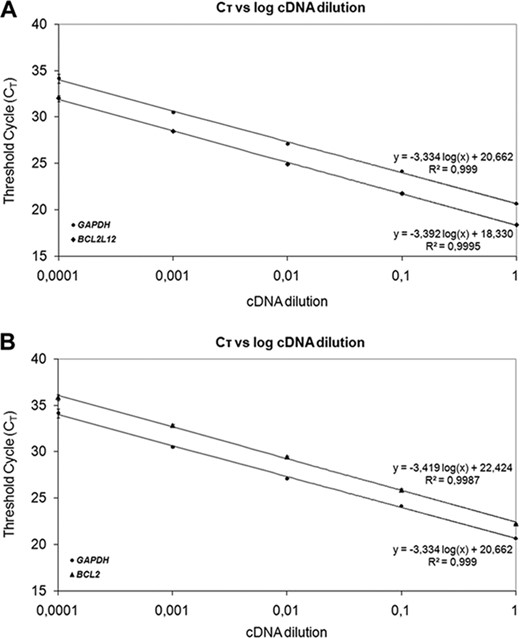
Validation of the comparative CT (2−ΔΔCT) method to assess the efficiency of amplification of the target genes, BCL2L12 (A), BCL2 (B), and GAPDH (A and B). CT was calculated for each gene and each cDNA dilution and plotted against log cDNA dilution. All data were fit using least‐squares linear regression analysis.
In our study, GAPDH was used as a reference gene so as to normalize all PCRs for the amount of RNA added to the reverse transcription reactions. Furthermore, the leukemic cell line HL‐60, in which expression of both BCL2L12 and BCL2 has been noticed, was used as a calibrator, thus allowing PCR comparison for distinct runs [44]. Normalized results were expressed as the ratio of the target gene (BCL2L12 and BCL2) mRNA copies to 1000 GAPDH mRNA copies (c/Kc), calculated for each CLL specimen, in relation to the same ratio calculated for HL‐60 cells.
Statistical Analysis
Because of the fact that the distributions of BCL2L12 and BCL2 expression levels in CLL patients were not Gaussian, the analysis of the differences in the two groups of patients was performed with the nonparametric Mann‐Whitney U test. Receiver operating characteristic (ROC) curves were constructed for BCL2L12 and BCL2 expression levels, by plotting sensitivity versus (1 − specificity), and the areas under the ROC curves (AUC) were analyzed by the Hanley and McNeil method. To further investigate the discriminatory value of the BCL2L12 and BCL2 expression in CLL, another logistic regression model (function combination = 1.16 × log BCL2L12 + 0.64 × log BCL2 − 3.31), adjusted only for these two variables, was developed. We calculated log likelihood scores for this multivariate logistic regression model, which incorporates both variables for each patient. To evaluate the predictive value of BCL2L12 and BCL2 mRNA expression in CLL, univariate and multivariate logistic regression analyses were conducted as well.
As the BCL2L12 gene is not a studied gene in CLL, there are no established cut points available. Therefore, for categorization of BCL2L12 expression levels, the X‐tile algorithm was used to generate an optimal cut point. Two methods of statistical correction for the use of minimal p‐value approach were utilized. First, the X‐tile program output includes calculation of a Monte Carlo p‐value for the optimal cut point generated. Cut points that yield Monte Carlo p‐values <.05 are considered robust and unlikely to represent type I error. Secondly, the Miller‐Siegmund minimal p‐value correction referenced by Altman et al. was utilized [45]. This process produced an optimal cutoff of 107 c/Kc for BCL2L12 expression levels, which is equal to the 30th percentile. Following the same procedure for BCL2 expression, the optimal cutoff of 9254 c/Kc, equal to the 50th percentile, was generated.
According to the aforementioned cutoffs, BCL2L12 and BCL2 mRNA expression values were classified as positive or negative. Associations between BCL2L12 or BCL2 status and patients' categorical clinicopathologic variables were analyzed using the Fisher exact test. Relationships between different continuous variables were also assessed by Spearman correlation coefficient (rs). Survival analysis was also performed by constructing Kaplan‐Meier overall survival curves, where differences between curves were evaluated by the log‐rank test. The level of significance was defined at a probability value of <.05 (p < .05).
Results
Clinical and Biologic Features of CLL Patients
The patients' group consisted of 41 men and 24 women, with a median age of 70 years (range = 50–87) at the time of diagnosis. According to the Binet classification system, 40 patients (62%) were diagnosed with stage A CLL, 10 (15%) with stage B, and 15 (23%) with stage C. Mutational status analysis was performed in 42 of 65 CLL specimens. Leukemic cells with IGHV mutations were detected in 23 (55%) patients, whereas 19 (45%) had clonal B cells with unmutated IGHV genes. Expression of CD38 was measured in 45 of 65 CLL samples. Leukemic cells were CD38− in 40 (89%) patients and CD38+ in 5 (11%) patients. The median early apoptosis index of CLL cells measured in vivo by the early apoptosis detection assay was 5.5, varying between 0.01 and 54.8. Patients' clinical and biologic characteristics are summarized in Table 1.
Clinical and biologic characteristics of chronic lymphocytic leukemia patients
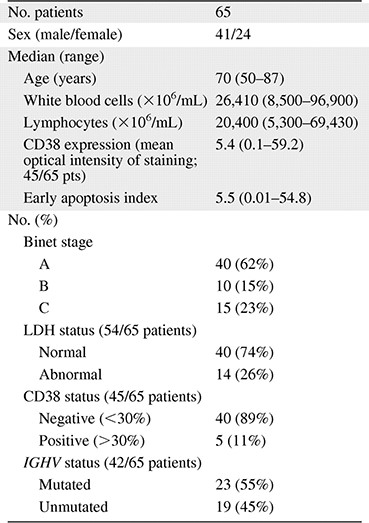
Abbreviation: LDH, lactate dehydrogenase.
Clinical and biologic characteristics of chronic lymphocytic leukemia patients

Abbreviation: LDH, lactate dehydrogenase.
BCL2L12 and BCL2 mRNA Expression Analysis in CLL
BCL2L12 mRNA expression was significantly higher in CLL than in normal blood samples (p < .001; Figure 2A), ranging from 0.14 to 729.5 c/Kc with a mean ± SEM of 204.7 ± 19.6 c/Kc in the former, while varying between 0.14 and 403.6 c/Kc with a mean ± SEM of 61.3±19.4 c/Kc in the latter (supplemental online Table 2). Similarly, BCL2 mRNA levels presented a slighter, although statistically significant (p < .001), increase in CLL patients, in comparison with healthy controls (Figure 2B). Therefore, BCL2 mRNA levels in CLL specimens varied between 204.0 and 46,559.0 c/Kc with a mean ± SEM of 12,734.2 ± 1,440.9, whereas in healthy controls they ranged from 27.0 to 45,887.0 c/Kc with a mean ± SEM of 5,563.1 ± 2,076.6 (supplemental online Table 2).
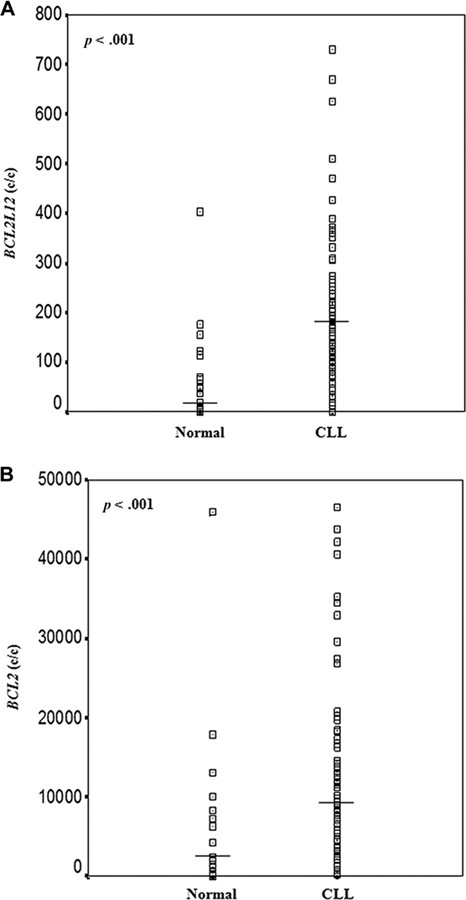
Distribution of BCL2L12 (A) and BCL2 (B) mRNA expression in the cohorts of non‐leukemic population and CLL patients. The line bars represent the median value (50th percentile) for each patient cohort. BCL2L12 and BCL2 mRNA expression levels were higher in CLL patients than in healthy controls. The p‐values were calculated using the Mann‐Whitney U test. Abbreviation: CLL, chronic lymphocytic leukemia.
Discriminatory Value of BCL2L12 and BCL2 mRNA Expression in CLL
To evaluate the potential of BCL2L12 expression as a predictive biomarker for the discrimination between CLL patients and non‐leukemic population, we performed ROC and logistic regression analyses. As illustrated by the ROC curve in Figure 3, BCL2L12 mRNA expression was found to distinguish very efficiently CLL patients from healthy controls (AUC = 0.833, 95% confidence interval [95% CI] = 0.731–0.935, p < .001). To investigate the discriminatory value of the combination of BCL2L12 and BCL2 expression in CLL, another logistic regression model, adjusted only for these two variables, was developed. As depicted in Figure 3, the combination of BCL2L12 and BCL2 mRNA expression enhances only slightly the discriminatory power of BCL2L12 expression (AUC = 0.840, 95% CI = 0.740–0.943, p < .001), while it increases notably the discriminatory power of BCL2 expression (AUC = 0.776, 95% CI = 0.656–0.896, p < .001).
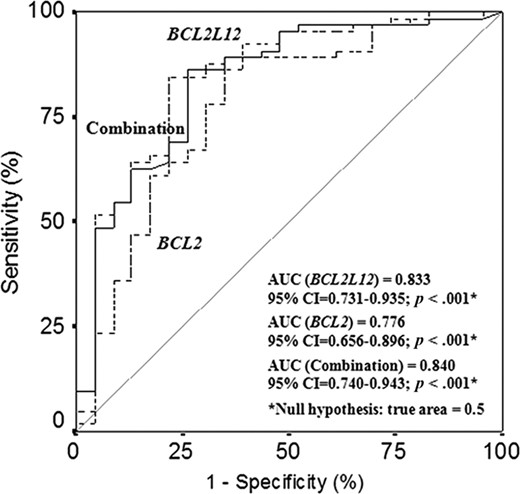
Receiver operating characteristic analysis for BCL2L12 and BCL2 mRNA expression. BCL2L12 expression (–‐–) was found to distinguish successfully chronic lymphocytic leukemia patients from healthy controls. Furthermore, the discriminatory power of BCL2 expression (‐‐‐) was significantly strengthened when combined with BCL2L12 expression (—). Abbreviation: AUC, areas under the receiver operating characteristic curves.
Univariate logistic regression analysis revealed that high BCL2L12 mRNA levels constitute a powerful predictor of the presence of CLL (crude odds ratio [OR] = 4.52, 95% CI = 2.11–9.65, p < .001). Furthermore, analysis of BCL2L12 expression as a dichotomous variable showed that BCL2L12‐positive people were at a quite sevenfold higher risk for CLL (crude OR = 6.71, 95% CI = 2.29–19.6, p = .001). With regard to BCL2, its high mRNA expression levels were also associated with high risk of CLL (crude OR = 4.79, 95% CI = 2.06–11.2, p < .001). Moreover, BCL2‐positive individuals were about 4.5 times more likely to suffer from CLL than BCL2‐negative ones (crude OR = 4.48, 95% CI = 1.50–15.9, p = .008; Table 2).
Logistic regression analysis for prediction of the presence of chronic lymphocytic leukemia
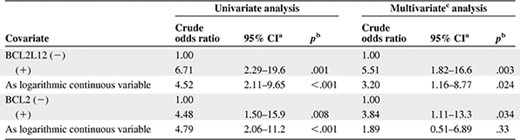
aConfidence interval of the estimated crude odds ratio.
bTest for trend.
cMultivariate models were adjusted for BCL2L12 and BCL2 mRNA expression.
Abbreviation: CI, confidence interval.
Logistic regression analysis for prediction of the presence of chronic lymphocytic leukemia

aConfidence interval of the estimated crude odds ratio.
bTest for trend.
cMultivariate models were adjusted for BCL2L12 and BCL2 mRNA expression.
Abbreviation: CI, confidence interval.
In multivariate analysis, the logistic regression models were adjusted for BCL2L12 and BCL2 mRNA expression (Table 2). BCL2L12 expression was found to be a significant and independent predictive marker for CLL when analyzed either as a dichotomous (crude OR = 5.51, 95% CI = 1.82–16.6, p = .003) or as a logarithmic continuous variable (crude OR = 3.20, 95% CI = 1.16–8.77, p = .024). On the other hand, mRNA expression of BCL2 was shown to have statistically significant predictive value only if used as a dichotomous variable (crude OR = 3.84, 95% CI = 1.11–13.3, p = .034).
Correlation Between BCL2L12, BCL2 mRNA Expression, and CLL Patients' Clinicopathologic Variables
Table 3 demonstrates the association between BCL2L12 mRNA expression status and several clinicopathologic parameters of the patients. Of 64 CLL cases examined, 45 (70%) were classified as positive for BCL2L12 expression and 19 (30%) were classified as negative. BCL2L12 mRNA expression was found to be significantly associated with Binet staging (p = .028), as patients in the advanced stage (Binet stage C) of the disease were more frequently BCL2L12‐positive, in contrast with early‐stage patients (Binet stages A/B). Remarkable associations were not observed between BCL2L12 mRNA expression status and lactate dehydrogenase (LDH), CD38 expression, or IGHV mutational status. Regarding BCL2 mRNA expression, it was significantly related to LDH and CD38 status. BCL2 positivity was detected more frequently in patients with normal LDH levels (p = .03) as well as in CD38− patients (p = .049; Table 3).
Associations between BCL2L12 status, BCL2 status, and other dichotomous variables
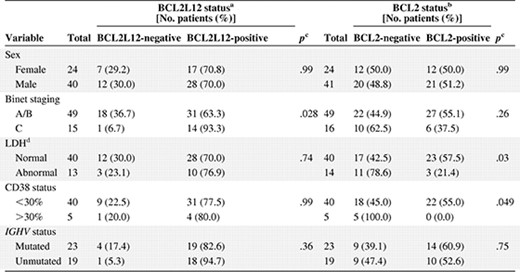
aCutoff point: 107 c/Kc, equal to the 30th percentile of BCL2L12 mRNA expression.
bCutoff point: 9254 c/Kc, equal to the 50th percentile of BCL2 mRNA expression.
cCalculated using the Fisher exact test.
Abbreviation: LDH, lactate dehydrogenase.
Associations between BCL2L12 status, BCL2 status, and other dichotomous variables

aCutoff point: 107 c/Kc, equal to the 30th percentile of BCL2L12 mRNA expression.
bCutoff point: 9254 c/Kc, equal to the 50th percentile of BCL2 mRNA expression.
cCalculated using the Fisher exact test.
Abbreviation: LDH, lactate dehydrogenase.
In CLL patients, BCL2L12 and BCL2 mRNA expression levels were positively correlated (rs = 0.345, p = .005). BCL2 mRNA expression was also found to co‐vary with white blood cell (rs = 0.367, p = .003) and lymphocyte numbers (rs = 0.338, p = .006), whereas it was negatively correlated with CD38 expression (rs = −0.423, p = .004). mRNA expression of both genes was negatively correlated with the early apoptosis index, although only BCL2 correlation was statistically significant (rs = −0.342, p = .025; supplemental online Table 3).
BCL2L12 Expression and Overall Survival of CLL Patients
Follow‐up information was available for 58 patients. There were 15 (25.9%) deaths recorded, 12 of which were due to causes related to CLL and 3 to unrelated causes. The median overall survival (OS) was 72 months (range = 6–225 months). Kaplan‐Meier analysis revealed significantly reduced OS of BCL2L12‐positive CLL patients, as compared to BCL2L12‐negative patients (p = .043) (Figure 4A). On the other hand, no difference was found between Kaplan‐Meier OS curves of BCL2‐positive and BCL2‐negative CLL patients (Figure 4B).
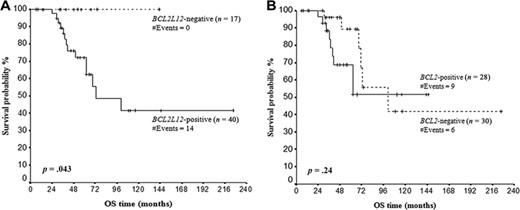
Kaplan‐Meier curves for OS of BCL2L12 (A) and BCL2 (B) mRNA‐positive and mRNA‐negative chronic lymphocytic leukemia patients. BCL2L12 mRNA expression has an unfavorable prognostic value for chronic lymphocytic leukemia, as BCL2L12‐positive patients have significantly shorter OS time, in comparison with BCL2L12‐negative patients. Abbreviation: OS, overall survival.
Discussion
Chronic lymphocytic leukemia is the most common form of leukemia in Europe and North America and mainly, although not exclusively, affects older individuals. Life expectancy of CLL patients ranges from months to decades. Advances in molecular biology have enhanced our understanding of the pathophysiology of the disease and, together with development of new therapeutic agents, have made management of CLL more rational and more effective than previously. Yet CLL remains an incurable disease, because available treatments generally induce remission but nearly all patients relapse [8]. Over the past years, major progress has been made in identification of molecular and cellular markers that could predict disease progression in CLL patients. In particular, the mutational profile of IGHV genes and some cytogenetic abnormalities have been described as important predictors of prognosis. However, no definitive prognostic system has been validated and established in clinical practice, so far [7]. Hence, novel prognostic markers are needed to stratify patients into treatment groups.
Analysis of BCL2L12 mRNA expression revealed that both splicing variants of this apoptosis‐related gene are overexpressed in colon cancer samples as compared to their paired normal mucosa [46, 47]. Furthermore, expression of BCL2L12‐A, the alternative transcript of BCL2L12, is associated with Dukes' stage and lymph node status [46], whereas higher expression of the full‐length BCL2L12 variant is associated with less aggressive forms of intestinal cancer [47]. In accordance with this finding, colon cancer patients showing BCL2L12 overexpression have significantly longer disease‐free survival and OS [47], supporting the notion that BCL2L12 mRNA overexpression is related to favorable prognosis in colon cancer patients and may represent a useful tissue biomarker. Interestingly, it has recently been suggested that BCL2L12 may be regarded as a novel, independent favorable tissue biomarker in breast cancer as well, since BCL2L12‐positive breast cancer patients have a lower probability of relapse and/or death, as compared to BCL2L12‐negative patients [48, 49]. Moreover, BCL2L12 is overexpressed more often in breast tumors with a high degree of differentiation as well as in patients at the initial stages of the disease [48]. It is worth mentioning that an association between BCL2L12 and BCL2 mRNA expression has been noticed in breast tumors [49]. High expression of BCL2L12 has also been linked with favorable outcome in patients with gastric cancer [50]. On the other hand, frequent mRNA upregulation and robust protein expression of this apoptosis‐related gene has been observed in primary glioblastoma specimens, relative to surrounding normal brain tissue [51]. Recent studies have identified and validated BCL2L12 as a potent glioma oncoprotein with multiple targets in apoptosis regulatory networks, such as effector caspase‐3, caspase‐7, and the tumor suppressor protein p53 [51–55].
In lymphoid malignancies and more specifically in CLL, there has been scarcity of data so far regarding the expression status of this gene. Our results reveal a significant overexpression of BCL2L12 mRNA in CLL patients as compared to healthy controls. The mechanisms underlying BCL2L12 overexpression have not yet been clarified. Known pathogenetic mechanisms such as gene amplifications, translocations, or hypomethylation of promoters of the genetic locus 19q13.3 have not been reported in CLL so far. Other apoptosis‐regulating members of the BCL2 family have been consistently reported to be implicated in the pathogenesis of CLL [21, 22, 56], with anti‐apoptotic BCL2 protein overexpression being a hallmark of the disease [23, 57, 58]. More specifically, it has been suggested that this very frequent overexpression is caused by hypomethylation of the promoter region of the BCL2 gene [59]. Additionally, it has been shown that miR‐15 and miR‐16, which negatively regulate BCL2 expression at the post‐transcriptional level, are deleted or downregulated in many CLL patients [60–62]. In agreement with published data, we have also found that BCL2 mRNA is overexpressed in the group of CLL patients, in comparison with the group of healthy controls [23, 57]. Additionally, the mRNA expression levels of BCL2L12 and BCL2 showed a positive intercorrelation, whereas both of them were negatively correlated with the early apoptosis index. On the basis of our findings, a possible anti‐apoptotic role of BCL2L12 could be implied in CLL.
The second novel finding of this study is that overexpression of BCL2L12 mRNA appears as an independent predictive marker of the presence of the disease, showing a greater potential than BCL2 mRNA expression. This suggestion is supported by the ROC analysis that showed a very high AUC of the BCL2L12 curve (0.833)—which was not substantially improved by the combinatorial curve of BCL2L12 and BCL2—and also by the logistic regression models, where BCL2L12 mRNA expression was analyzed both as a continuous and a dichotomous variable, uncovering a 5.5‐fold higher risk of CLL, independently from BCL2 mRNA expression. Therefore, although the presence of absolute lymphocytosis in the peripheral blood of at least 5 × 109/L mature‐appearing lymphocytes with a CD5+/CD19+/CD23+ immunophenotype is the gold standard for the clinical diagnosis of CLL [63], we suggest that BCL2L12 mRNA expression could serve as a biomarker for the selection of cases with potential clonal lymphocytosis that need further diagnostic workup and/or as a surrogate marker for minimal residual disease, the eradication of which is a necessary condition to cure CLL [64]. However, this finding needs confirmation by larger prospective studies testing also the specificity of this marker for CLL against other lymphoid malignancies.
CLL patients with mutated immunoglobulin genes have good prognosis, and those with unmutated genes show poor prognosis [65–67]. The mutational profile of immunoglobulin genes delineates prognostic groups within all Binet stages [67, 68]. Because the determination of the IGHV mutational status is laborious, expensive, and time‐consuming, and this method is difficult to be implemented in the routine hematologic laboratory because of the need for specialized equipment, detection of appropriate, reliable surrogate markers for the IGHV mutational status has attracted worldwide attention [8]. CD38 expression was the first marker that was found to correlate with the IGHV mutational status [69]. Our study did not reveal any correlation between BCL2L12 mRNA expression status and established prognostic parameters examined, such as IGHV mutational status, CD38 expression, or LDH levels. However, BCL2L12 mRNA expression was significantly associated with Binet staging, as patients in advanced stage were more frequently BCL2L12‐positive than early‐stage patients. This finding possibly reflects the increased size of the leukemic compartment in the advanced stages of the disease, which may further be linked to a more pronounced suppression of the apoptotic process. With regard to BCL2 mRNA expression status, it was significantly related to LDH and CD38 expression status. On the other hand, Faderl et al. have shown that high expression levels of the BCL2 protein correlate with most of the poor prognostic factors in CLL, including high white blood cell count, peripheral blood lymphocytosis, advanced Rai or Binet stage, decreased hemoglobin, low platelet counts, and high levels of serum beta‐2‐microglobulin. These seemingly different results may be due to post‐transcriptional regulation of BCL2 levels, resulting from downregulation or deletion of miR‐15 and miR‐16, which is common in many CLL patients [60–62], as mentioned before.
In our study, the unfavorable prognostic value of BCL2L12 mRNA overexpression in patients with CLL in terms of OS has been shown. Our finding is in contrast with the favorable prognostic value of this gene in patients with solid tumors including breast, colon, and gastric cancer [46–50], which might be due to the different role of BCL2L12—either pro‐ or anti‐apoptotic—in different types of cancer. On the other hand, BCL2 mRNA expression did not have any prognostic impact on OS of patients, in contrast with BCL2 protein expression, which was shown in another study to constitute an unfavorable prognostic biomarker in CLL [22]. In addition, the differences observed in our data between BCL2L12 and BCL2 mRNA expression in terms of OS may result from the fact that BCL2 and BCL2L12, although they belong to the same family, have different properties and are most likely involved in distinct pathways.
Conclusion
To the best of our knowledge, this is the first study showing that the BCL2L12 gene, a novel member of the BCL2 family, is significantly overexpressed in CLL patients, in comparison with healthy controls. Interestingly, BCL2L12 mRNA expression possesses important discriminatory value, distinguishing very efficiently CLL patients from non‐leukemic population, and bears a powerful and independent predictive potential. Finally, BCL2L12 mRNA overexpression is associated with the clinical stage of the disease and constitutes an unfavorable prognostic biomarker in CLL, in terms of OS. Undoubtedly, further studies are needed to confirm the present findings and establish its application in clinical practice. Our future goals include also comparison of BCL2L12 mRNA expression profile of purified CLL cells to that of normal memory B cells, to elucidate the molecular pathways in which BCL2L12 is involved.
Acknowledgments
This work was partly supported by the Hellenic Cooperative Oncology Group (HECOG), Athens, Greece. We thank Dr. Chrysoula Belessi for performing IGHV gene sequencing analysis and Dr. Katerina Spyridaki for cell immunophenotyping and assessment of apoptosis. S.G.P. and C.K.K. contributed equally to this work.
Author Contributions
Conception/Design: Sotirios G. Papageorgiou, Andreas Scorilas
Provision of study material or patients: Sotirios G. Papageorgiou, Vassiliki Pappa
Collection and/or assembly of data: Sotirios G. Papageorgiou, Christos K. Kontos, Vassiliki Pappa, Hellinida Thomadaki, Frida Kontsioti
Data analysis and interpretation: Christos K. Kontos, Frida Kontsioti, Andreas Scorilas
Manuscript writing: Sotirios G. Papageorgiou, Christos K. Kontos, Hellinida Thomadaki
Final approval of manuscript: Vassiliki Pappa, John Dervenoulas, Efstathios Papageorgiou, Theofanis Economopoulos, Andreas Scorilas
References
Author notes
Disclosures: Sotirios G. Papageorgiou: None; Christos K. Kontos: None; Vassiliki Pappa: None; Hellinida Thomadaki: None; Frida Kontsioti: None; John Dervenoulas: None; Efstathios Papageorgiou: None; Theofanis Economopoulos: None; Andreas Scorilas: None.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers.



