-
PDF
- Split View
-
Views
-
Cite
Cite
Martine M. Goedendorp, Marlies E.W.J. Peters, Marieke F.M. Gielissen, J. Alfred Witjes, Jan Willem Leer, Constans A.H.H.V.M. Verhagen, Gijs Bleijenberg, Is Increasing Physical Activity Necessary to Diminish Fatigue During Cancer Treatment? Comparing Cognitive Behavior Therapy and a Brief Nursing Intervention with Usual Care in a Multicenter Randomized Controlled Trial, The Oncologist, Volume 15, Issue 10, October 2010, Pages 1122–1132, https://doi.org/10.1634/theoncologist.2010-0092
Close - Share Icon Share
Abstract
Two interventions for fatigue were given during curative cancer treatment. The aim of this multicenter randomized controlled trial (RCT) with three conditions was to demonstrate the efficacy and to determine the contribution of physical activity.
Recruited from seven hospitals, 220 patients with various malignancies participated in a RCT. The brief nursing intervention (BNI) consisted of two 1-hour sessions, 3 months apart, given by 12 trained nurses, focusing only on physical activity. Cognitive behavior therapy (CBT) consisted of up to ten 1-hour sessions, within 6 months, provided by two therapists, focusing on physical activity and psychosocial elements. The control group received only usual care (UC). Assessments took place before and at least 2 months after cancer treatment, when patients had recovered from acute fatigue. Fatigue was the primary outcome. Efficacy was tested using analyses of covariance. A nonparametric bootstrap approach was used to test whether the effect on fatigue was mediated by physical activity.
The CBT group was significantly less fatigued than the UC group. Between the BNI and the UC groups, no significant difference was found in fatigue. The mediation hypothesis was rejected.
CBT given during curative cancer treatment proved to be an effective intervention to reduce fatigue at least 2 months after cancer treatment. The BNI was not effective. Contrary to what was expected, physical activity did not mediate the effect of CBT on fatigue. Thus, the reduction in fatigue elicited by CBT was realized without a lasting increase in physical activity.
Introduction
Fatigue is one of the most common and distressing symptoms in cancer patients, and when severe it has a large impact on daily functioning and quality of life [1–3]. It is assumed that levels of fatigue are low before the start of cancer treatment and high during cancer treatment. Prevalence estimates of fatigue during treatment are in the range of 25%–75%, in different samples and measured with different questionnaires [4]. Several studies even reported a prevalence ≥90% [5–9]. Fatigue continues to be problematic for many patients after cancer treatment is finished, because the number of patients with substantial fatigue is higher than that in control groups [4, 10]. Therefore, it is important to intervene during active cancer treatment in order to reduce severe fatigue after cancer treatment. Because nearly all cancer patients experience fatigue during active cancer treatment, we assumed that most patients could benefit from an intervention for fatigue.
Exercise and psychosocial interventions have the strongest evidence base for managing fatigue during cancer treatment [11], but clearly not all interventions generate similar effects. Psychosocial interventions specifically aimed at fatigue during cancer treatment were found to be more effective than psychosocial interventions not aimed at fatigue [12–14]. However, the number of randomized controlled trial (RCT) interventions specifically for fatigue during cancer treatment is limited [13].
Reviews demonstrate that interventions for fatigue and assessments take place during different phases of cancer treatment [12–14]. For example, in some RCTs, participants still received chemotherapy after the postintervention assessment [15–17]. Such a design is unsuitable to demonstrate whether the level of fatigue after finishing cancer treatment returns to the pretreatment level. To the best of our knowledge, there is no fatigue interventional RCT during cancer treatment that assessed patients at clinically relevant moments—before the start of cancer treatment and shortly after finishing cancer treatment.
Exercise interventions are solely aimed at physical activity, whereas psychosocial interventions often have a physical activity component, such as activity management. Intervening with physical activity to reduce fatigue is based on the assumption that a lack of physical activity and deconditioning during cancer treatment can worsen fatigue [18]. When patients are diagnosed with cancer, their activity pattern changes and they become physically less active, possible leading to deconditioning [19]. This is the result of a negative spiral, because when patients become physically less active they become more easily fatigued, and when patients experience fatigue they react by becoming physically even less active. Exercise can break this cycle by improving tolerance for physical activity [11]. Therefore, increasing physical activity is an important element in reducing fatigue during cancer treatment. However, the mediating role of physical activity in interventions aimed at reducing fatigue during cancer treatment has never been demonstrated.
In the current RCT, two interventions for fatigue during cancer treatment were compared with usual care (UC). The first intervention was a minimal intervention performed by nurses. The brief nursing intervention (BNI) was aimed at advising patients how to avoid deconditioning. There is evidence that such brief interventions for fatigue given by nurses are effective [15–17]. Furthermore, it is recognized that oncology nurses can play significant roles in the translation of cancer-related fatigue guidelines by teaching patients and decreasing barriers [19]. The second intervention was an extensive intervention aimed at fatigue based on cognitive behavior therapy (CBT). This CBT intervention was, in addition to avoiding deconditioning, based on elements such as changing dysfunctional cognitions about fatigue, changing a distorted sleep–wake rhythm, and coping with the consequences of having cancer.
The first aim of this RCT was to determine the efficacy of these two interventions compared with UC. The moment of postintervention assessment (T2) was chosen at a clinically relevant point. T2 was chosen postintervention and also after a recovery period from the direct effects of cancer treatment. A previous study found that the immediate effects of surgery, chemotherapy, or radiotherapy on fatigue disappear after 6 weeks [20]. Therefore, the postintervention assessment was completed at least 2 months after cancer treatment finished. It was expected that patients in these two intervention groups would be less fatigued at least 2 months after cancer treatment than patients given UC. In addition, it was expected that patients in the intervention groups would have higher levels of functioning, less psychological distress, and a better quality of life.
Our second aim was to determine the role of physical activity in reducing fatigue during cancer treatment. It was expected that a reduction in fatigue was mediated by enhanced physical activity.
Methods
Patients and Procedure
Sample
Patients were recruited from the Radboud University Nijmegen Medical Centre and six regional hospitals from November 2005 until August 2007. Patients were included after being diagnosed with a primary tumor and scheduled to receive treatment with curative intent. Patients had to be 18–75 years of age and able to speak, read, and write Dutch. To minimize dropout and exclusion during the study, patients with lung cancer and with head and neck cancer were excluded. Exclusion criteria were: comorbidities causing fatigue, seeking treatment for preexisting chronic fatigue, and receiving psychiatric or psychological treatment in the preceding 3 months. The ethics committees from all seven hospitals approved the study. Informed consent was obtained from all participants.
Design and Procedure
Eligible patients were approached by their physician or specialized nurse at the time they were informed about their diagnosis and treatment plan. The recruitment procedure is described in detail elsewhere [21]. Patients with initial interest received written information and supplementary information by telephone. Subsequently, patients who consented completed the baseline assessment (T1) by computer or paper and pencil depending on their preference. T1 was completed before the start of cancer treatment. Subsequently, participants were randomly allocated to one of the three groups: BNI, CBT, or UC. Randomization was performed in blocks separately for each hospital, using labeled cards in numbered closed envelopes prepared by a statistician not involved in the study. Test assistants blinded to the randomization sequence opened the envelopes and informed the participants. The follow-up assessment (T2) was initially planned for 6 months after T1. If patients received surgery, chemotherapy, or radiotherapy in the fifth or sixth month, they were assessed 2 months after these treatments were finished.
Interventions
The UC group received treatment for cancer as proposed by the multidisciplinary working party for their specific tumor group, conforming to the guidelines of the comprehensive cancer center. None of the hospitals already offered supportive care for fatigue during cancer treatment.
BNI
The BNI consisted of two 1-hour sessions and a booklet. In the first session, the nurse explained how to break the negative spiral of low physical activity and fatigue. To demonstrate this, the patient's level of physical activity was determined before diagnosis and in the previous week. These levels were estimated with the questionnaire physical activity (QPA). Consequently, patients were advised to increase their physical activity level stepwise (5 minutes per week, up to 1 hour per day, for 5 days a week, by walking or cycling) up to 300 minutes per week. Patients who were physically active at this level were encouraged to maintain it. Additionally, how to remain physically active during cancer treatment was discussed, and what to do if complications occurred. The second session was planned for 3 months later. During that session, the level of physical activity was determined again and difficulties and solutions for becoming active or maintaining activity were discussed. Information and recommendations on physical activity could be reread in the booklet. All nurses received a protocol. To improve integrity, nurses were trained and supervised by G.B. and C.V. about every 2 months, and they were requested to send a checklist to the researcher after each session. The checklist contained questions on how much time was spent at the current level of physical activity and on discussing difficulties.
CBT
Participants in the CBT group received up to ten 1-hour sessions during 6 months. The number of sessions and the time spend on each element varied among individual patients, depending on problems encountered. The methods used were: restructuring of cognitions and beliefs, education and behavioral instructions, and providing emotional support. The intervention focused on six elements. (a) Physical activity: patients received the same information and booklet as provided in the BNI; in addition, activity-related cognitions were disputed. (b) Fatigue-related cognitions: dysfunctional cognitions were changed to more helpful ones. (c) Sleep–wake rhythm: patients were motivated to maintain fixed bedtimes, taking the phase of cancer treatment into account; napping during the day was discouraged. (d) Effects of cancer and treatment: the consequences of having cancer and the side effects of cancer were discussed, aimed at helping patients to cope and accept these (e.g., stoma, amputation). (e) Cancer in contact with others: unhelpful cognitions were changed and coping strategies for dealing with having cancer in contact with others, such as family or colleagues, were discussed; for example, “With whom do you want to share your emotions?” or “How do I tell the kids?” (f) Plans for the future: patients were asked to think about the future, and to make a plan; for example, a concrete plan for returning to work. Obstacles, fears, and solutions were discussed. Therapists with previous CBT experience in treating chronically fatigued cancer survivors gave the CBT [22]. A protocol was developed and the therapists received training and supervision every 2 weeks by G.B., during which each case was discussed.
Instruments
Demographic characteristics were gathered by self-report using questionnaires. Information on diagnosis was obtained from the patient's physician.
Fatigue severity was the primary outcome and was assessed using the fatigue subscale of the Checklist Individual Strength (CIS) [23, 24]. The CIS is a well-validated instrument [25, 26]. The fatigue subscale (CIS-fat) consists of eight items with scores in the range of 8–56. A cutoff score ≥35 indicates severe fatigue [24]. It has been used in previous research investigating fatigue in cancer survivors and has shown sensitivity to detect change [3, 22, 27, 28].
As a secondary outcome, functioning was assessed using the Health Survey Short Form-36 (SF-36). The Dutch language version of the SF-36 has been proven to be a reliable and valid instrument in the general population and in chronic disease populations [29]. The Symptom Checklist-90 (SCL-90) was used to measure psychological distress. The SCL-90 has good reliability and discriminating validity [30]. Quality of life was assessed using the Quality of Life Questionnaire of the European Organization for Research and Treatment of Cancer (EORTC QLQ-C30), version 3.0. The EORTC-QLQ C30 is an internationally validated questionnaire [31, 32].
To test for mediation, physical activity was assessed using three different instruments. For all instruments, higher scores indicate higher levels of physical activity. Physical activity was measured with actigraphy using an actometer, which has been used in cancer survivors [33]. An actometer is a motion-sensing device based on a piezoelectric sensor, with highly reproducible readings [34]. It records the number of movements in 5-minute intervals. At baseline, participants wore an actometer from the assessment to the start of cancer treatment, for up to 12 days and nights. At T2, the actometer was worn for 12 consecutive days and nights. The mean daily physical activity score across all worn days and nights was the parameter used to assess the level of physical activity.
During the same period, participants were asked to complete the Daily Observed Activity (DOA), scoring their level of physical activity four times a day. A mean daily score was calculated, varying in the range of 0–16. The DOA has previously been used in cancer survivors [35].
To measure whether patients complied with advice concerning physical activity, the QPA was developed. Patients were asked whether they had practiced sports, walked, or cycled in the past week for at least 30 minutes. They were asked how many days and for how long they had performed these activities. The total duration was calculated in minutes. Criterion validity with the actometer was moderate (Spearman's ρ = .31); however, it was similar to the International Physical Activity Questionnaire with the actometer [36].
Statistical Methods
The data analysis was performed with SPSS, version 16.0 (SPSS Inc, Chicago, IL). Data were used from participants who met the eligibility criteria at both T1 and T2. According to Fergusson et al. [37], patient data can be excluded from analysis without risking bias when ineligible patients are mistakenly randomized into a trial.
An a priori power analysis indicated that 48 patients would be required in each group, based on the following assumptions. A change of 8 points was expected on the CIS-fat [22]. An α of .017 (.05/3) and two-sided significance level were used to yield an 80% power. This study was overpowered as a result of the fact that fewer patients were excluded during the study than expected.
Baseline differences among the three conditions were tested with a t-test or χ2 test for independent samples. Significant differences were entered as covariates in all further analyses. To test for an overall significant difference among mean scores for the three conditions, analyses of covariance (ANCOVA) were performed for the outcome measures, with baseline scores entered as covariates and condition as a fixed factor. When an overall effect was significant, a contrast analysis was performed to compare the intervention groups (level 2 and 3) with the UC group (level 1). To test whether there was a clinically significant difference, the differences among the proportions of severely fatigued participants in the three groups were tested with a logistic regression analysis using the enter method. A two-sided p < .05 was considered significant.
Primary outcome data were missing from two participants at T2. To avoid overestimation of the effects of the interventions, missing data were substituted with the mean score of the UC group. A sensitivity analysis showed that entering the missing data with the mean score of the UC group added with one or two standard deviations (SDs) did not influence the results.
An intention-to-treat analysis was performed for all outcomes except for the actometer and the DOA. Completers were used for these measures, because less than half the participants wore the actometer and completed the DOA at both assessments.
The mediation hypothesis was tested with a nonparametric bootstrap approach. This approach was chosen because it gives more power to detect significant differences in small, non-normally distributed samples. A macro expansion, consisting of a syntax file for SPSS, was introduced by Preacher and Hayes [38] to test for mediation according to the guidelines of Baron and Kenney [39]. The macro generates a mean mediation effect with a 95% confidence interval (CI) by randomly resampling the observed dataset 5,000 times with replacement. The mediation hypothesis was accepted when the 95% CI included zero [38].
Results
Figure 1 illustrates the flow of participants. A total of 395 eligible cancer patients were approached, and 155 refused to participate. “Participating would take too much time” was the most common reason. Nonparticipants were older than participants, but no significant difference was found for sex or type of malignancy [21]. Because of the short time span between the diagnosis and start of treatment, not all participants could be assessed before the start of treatment. Twenty-six percent of the participants were assessed after surgery or the start of hormone therapy, but always before adjuvant chemotherapy or radiotherapy. Of the 240 participants, 77 were assigned to the BNI group, 82 were assigned to the CBT group, and 81 were assigned to the UC group. The majority were recruited from the university hospital (n = 158). Twenty patients were excluded postrandomization. Intention-to-treat analyses were based on 220 participants—72 in the BNI group, 76 in the CBT group, and 72 in the UC group. Two participants dropped out. T2 was completed by 162 participants 6 months after T1. Fifty-six participants who received cancer treatment for a longer period completed T2 2 months after their cancer treatment was finished.
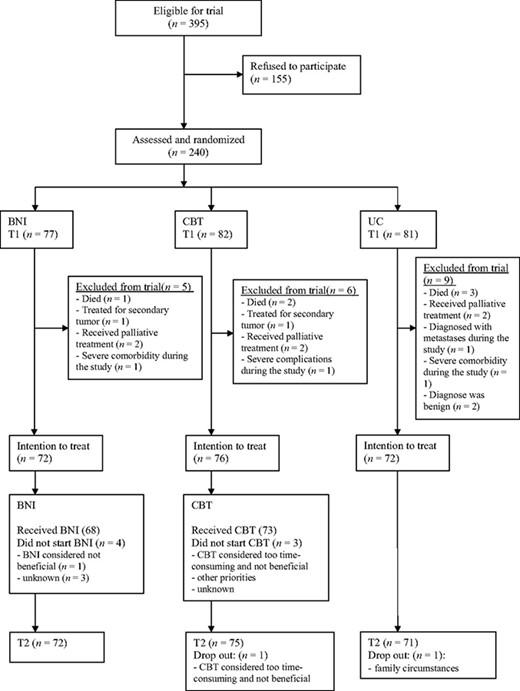
Participant flow diagram.
Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Intervention
BNI
Of the 68 patients who started the BNI, 66 attended both sessions. Most sessions were face to face; 10% were telephone sessions. All but two of the checklists were returned by the nurses. The mean time between the two sessions was 4.5 months (SD, 2.5 months). In total, 12 nurses gave the BNI. The mean hours of training and supervision were 7.8 (SD, 4.9 hours), varying in the range of 2–12 hours. In the university hospital, two nurses administered the BNI to 20 and 26 participants each. In the regional hospitals, nurses gave the BNI to one to seven participants. There was no significant difference between participants treated in the university hospital by the more experienced nurses and those treated in the regional hospitals for level of fatigue and physical activity at T2 (data not shown).
CBT
Seventy-three patients started with CBT. The mean duration was 7 months (SD, 2.6 months). The mean number of sessions was 6.2 (SD, 1.9; range, 2–11). One person received 11 sessions. Fifty-nine percent of the participants had only face-to-face sessions; 41% combined face-to-face sessions with telephone sessions. Most of the sessions (80%) were given face to face. No relationship was found between change in fatigue severity, and the number of sessions and type of contact. Two therapists treated 34 and 39 patients each. No therapist effect was found on fatigue at T1 (p = .937) or at T2 (p = .991), or on other outcome measures (data not shown).
Baseline Comparison
No baseline significant differences were found among the three groups in terms of diagnosis, cancer treatment, or fatigue (Tables 1, 2, and 3). Significantly more participants in the BNI group than in the UC group were married. For the secondary outcomes, a significant difference (p = .029) was found on the cognitive functioning subscale of the EORTC-QLQ C30 between the CBT group (mean, 86.0; SD, 19.5) and the UC group (mean, 92.8; SD, 12.5). In addition, a significant difference was found for the QPA between the BNI group and the UC group (Table 4). These three significant differences were entered as covariates in further analyses.
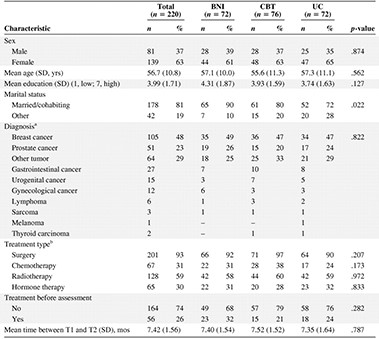
aTwo patients were diagnosed with both bladder and prostate cancer and were categorized as other tumor. One was allocated to the control group, the other to CBT.
bThe total is >100% because several combinations of treatment regimens were given to patients.
Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.

aTwo patients were diagnosed with both bladder and prostate cancer and were categorized as other tumor. One was allocated to the control group, the other to CBT.
bThe total is >100% because several combinations of treatment regimens were given to patients.
Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Mean (SD) fatigue at T1 and T2 and results of the analysis of covariance showing the overall effects and contrast analysis of the interventions on fatigue
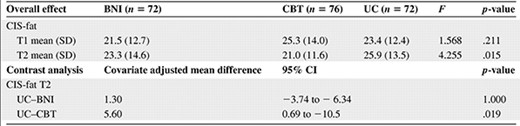
Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; CIS-fat, Checklist Individual Strength fatigue subscale; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Mean (SD) fatigue at T1 and T2 and results of the analysis of covariance showing the overall effects and contrast analysis of the interventions on fatigue

Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; CIS-fat, Checklist Individual Strength fatigue subscale; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Percentage of severe fatigue in the three conditions at T1 and T2 and results of the logistic regression analysis
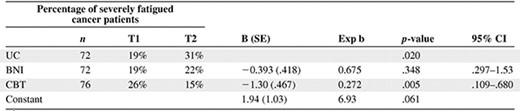
R2 values were 0.127 (Cox & Snell) and 0.195 (Nagelkerke). A two-sided p-value < .05 was considered significant.
Abbreviatons: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Percentage of severe fatigue in the three conditions at T1 and T2 and results of the logistic regression analysis

R2 values were 0.127 (Cox & Snell) and 0.195 (Nagelkerke). A two-sided p-value < .05 was considered significant.
Abbreviatons: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Mean (SD) physical activity at T1 and T2 and results of the analysis of covariance showing the overall effects and contrast analysis of the interventions on physical activity
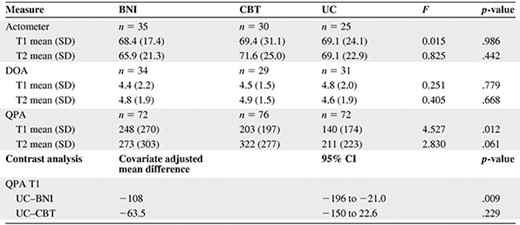
Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; DOA, Daily Observed Activity; QPA, Questionnaire Physical Activity; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Mean (SD) physical activity at T1 and T2 and results of the analysis of covariance showing the overall effects and contrast analysis of the interventions on physical activity

Abbreviations: BNI, brief nursing intervention; CBT, cognitive behavior therapy; CI, confidence interval; DOA, Daily Observed Activity; QPA, Questionnaire Physical Activity; SD, standard deviation; T1, baseline assessment; T2, follow-up assessment; UC, usual care.
Effect of the Intervention
The results of the ANCOVA on fatigue are shown in Table 2. Participants in the CBT group were significantly less fatigued than those in the UC group. From T1 to T2, fatigue increased in the UC group, whereas fatigue decreased in the CBT group. This resulted in a mean difference between the two conditions of 5.6 points on the CIS-fat. There was no significant difference in fatigue between the BNI group and the UC group (p = 1.000). The proportion of severely fatigued cancer patients was significantly lower in the CBT group than in the UC group (p = .019) (Table 3). No significant differences were found for the secondary outcomes (data available upon request).
Results of ANCOVA showed no significant differences in physical activity between the two intervention groups compared with the UC group (Table 4). The bootstrap approach showed that, at most, 3.4% of the effect of CBT on fatigue could be explained by physical activity (Table 5). The 95% CI of the mean mediation effect included zero, rejecting the mediation hypothesis.
Results of the mediation effect of physical activity in the CBT group compared with UC, according to the bootstrap approach

Abbreviations: CBT, cognitive behavior therapy; CI, confidence interval; DOA, Daily Observed Activity; QPA, Questionnaire Physical Activity; UC, usual care.
Results of the mediation effect of physical activity in the CBT group compared with UC, according to the bootstrap approach

Abbreviations: CBT, cognitive behavior therapy; CI, confidence interval; DOA, Daily Observed Activity; QPA, Questionnaire Physical Activity; UC, usual care.
Discussion
The first aim of this study was to evaluate two interventions for fatigue during curative cancer treatment—CBT and the BNI. Our results showed that CBT was effective. CBT significantly reduced fatigue shortly after cancer treatment. Also, significantly fewer participants were severely fatigued at least 2 months after cancer treatment, demonstrating its clinical relevance. The BNI did not reduce fatigue compared with UC. The uniqueness of this study was that the CBT intervention proved to be effective at a clinically relevant time, that is, after a recovery period from the direct effects of cancer treatment.
Contrary to our expectations, physical activity did not mediate the reduction in fatigue realized by CBT, whether physical activity was measured with actigraphy or questionnaires. The finding that there was no effect of the interventions on physical activity already showed that mediation was absent, but because mediation analyses require a large power, a bootstrap analysis was performed. The lack of mediation was a surprising finding because increasing physical activity is an important therapeutic component. Our findings indicate that, with CBT, it was possible to realize a significant reduction in fatigue without a lasting increase in physical activity.
A number of limitations should be considered. The majority of the participants were recruited from the university hospital. This could have raised the question of sample bias, but we found no difference between the university hospital and the regional hospitals for fatigue. The fact that this was a multicenter trial increases the generalizability of the findings.
Contamination could have occurred, although preventive actions were taken. The therapists and nurses who gave the interventions were not involved in recruiting participants or in UC.
No effect on secondary outcomes was found. This could be explained by the fact that the mean and SD for the SCL-90 and SF-36 were similar to those in the general Dutch population at baseline [29, 30]. Therefore, it was difficult to realize an improvement.
One could argue that an effect of the BNI failed to occur for several reasons.—because it consisted of only two sessions, because the time between the last session and T2 was longer than in the CBT condition, or because the nurses were less experienced than the therapists. However, the more intensive CBT also failed to show an increased level of physical activity.
A formal integrity check, such as recording of sessions, did not take place. Several actions were taken to ensure that nurses and therapists worked according to protocol, such as training and supervision. Almost all checklists were returned by the nurses, demonstrating good adherence.
We did not control for level of attention. It could be that part of the effect of CBT on fatigue can be explained by attention, but it is improbable that the effect is caused by attention alone. For example, we could not find a dose-response effect for CBT. Furthermore, no effect of an attention placebo group on fatigue was found in patients with chronic fatigue syndrome (CFS) [40, 41].
Actometer and daily self-observation data were not obtained from all participants. This could raise concerns about possible differences between those who did and those who did not complete these evaluations. However, no difference in fatigue at T1 and T2 or condition was found between completers and noncompleters.
The assumption that increasing physical activity reduces fatigue is widespread, but not always empirically supported. Some reviews found no effect of exercise on reducing fatigue [42, 43]. In addition, some exercise studies did not find an effect on fatigue, even though physical fitness increased [44–46]. Other intervention studies with mediation analyses support our findings, demonstrating that increasing physical activity is not necessary to reduce fatigue in CFS patients [47].
Because no intermediate assessments were performed, it could not be ruled out if a temporary increase in physical activity contributed to lower fatigue. However, it is more likely that other factors, such as fatigue- and cancer-related cognitions or stress reduction, and not physical condition, mediated the fatigue reduction. Results of a graded exercise RCT for CFS also demonstrated that symptom focusing, not physical condition, mediated the improvement in fatigue [48].
As expected, the number of patients with severe fatigue increased in the UC group. However, a finding not expected beforehand was that at T1 more participants than expected were already severely fatigued. This cannot be attributed to preceding cancer treatment, because no difference was found in fatigue between cancer treatment–naïve patients and patients assessed before adjuvant therapy. Type of malignancy was also not found to be a contributing factor to severe fatigue before the initiation of cancer treatment [21].
Although fatigue was not assessed during active cancer treatment, many patients in the UC group were not severely fatigued after cancer treatment finished. Apparently, the group of patients without severe fatigue managed without a specific intervention for fatigue, implying that not all cancer patients need CBT for fatigue during curative cancer treatment. Future studies should identify patients at risk for severe fatigue shortly after cancer treatment, and interventions should focus on these risk groups.
Summary
Until now, there was no interventional RCT for fatigue during curative cancer treatment that assessed patients before the start of cancer treatment and shortly after cancer treatment, after patients recovered from acute effects. Our RCT showed that participants who received CBT for fatigue during cancer treatment were less fatigued than patients who received UC at least 2 months after cancer treatment. The BNI was not effective.
Unexpectedly, physical activity did not mediate the reduction in fatigue. Thus, with CBT it was possible to realize an improvement in fatigue without a lasting increase in physical activity.
Acknowledgments
We express our gratitude to all people for participating during this phase of their lives, and for the contribution of participating physicians, nurses, and support staff. Special thanks to Thea B.M. Berends, M.Sc., Judith de Natris, M.Sc., Lianne C.A. Vermeeren, M.Sc., and Hein Voskamp, M.Sc.
We are grateful to the Dutch Cancer Society for funding this study.
Author Contributions
Conception/Design: Marieke F.M. Gielissen, Constans A.H.H.V.M. Verhagen, Gijs Bleijenberg
Provision of study material or patients: J. Alfred Witjes, Jan Willem Leer, Constans A.H.H.V.M. Verhagen
Collection and/or assembly of data: Martine M. Goedendorp, J. Alfred Witjes, Jan Willem Leer, Constans A.H.H.V.M. Verhagen
Data analysis and interpretation: Martine M. Goedendorp, Marlies E.W.J. Peters, Marieke F.M. Gielissen, Constans A.H.H.V.M. Verhagen, Gijs Bleijenberg
Manuscript writing: Martine M. Goedendorp, Marlies E.W.J. Peters, Marieke F.M. Gielissen, J. Alfred Witjes, Jan Willem Leer, Constans A.H.H.V.M.Verhagen, Gijs Bleijenberg
Final approval of manuscript: Martine M. Goedendorp, Marlies E.W.J. Peters, Marieke F.M. Gielissen, J. Alfred Witjes, Jan Willem Leer, Constans A.H.H.V.M. Verhagen, Gijs Bleijenberg
References
Author notes
Disclosures: Martine M. Goedendorp: None; Marlies E.W.J. Peters: None; Marieke F.M. Gielissen: None; J. Alfred Witjes: None; Jan Willem Leer: None; Constans A.H.H.V.M. Verhagen: None; Gijs Bleijenberg: None.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the authors or independent peer reviewers.



