-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Hastie, Lisa Stangl, Karen Deutsch, Jeffrey Yin, Andrew Hull, Stephen A Spector, Jill Blumenthal, Case Report: Erroneous Human Immunodeficiency Virus-1 Diagnosis in a Pregnant Woman Using Centers for Disease Control and Prevention Diagnostic Algorithm, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac402, https://doi.org/10.1093/ofid/ofac402
Close - Share Icon Share
Abstract
We describe a case of a pregnant cisgender woman diagnosed with human immunodeficiency virus (HIV)-1 using the current Centers for Disease Control and Prevention diagnostic algorithm who subsequently had her diagnosis overturned after additional testing outside of the algorithm, including an HIV-1 proviral deoxyribonucleic acid test that was negative.
CASE REPORT
An 18-year-old, cisgender woman was diagnosed with human immunodeficiency virus (HIV)-1 during routine prenatal laboratory tests at approximately 13 weeks of pregnancy. Her screening combination HIV-1 and HIV-2 antibody and HIV-1 p24 immunoassay were positive, and the confirmatory, differentiating antibody test was subsequently positive for HIV-1 antibodies. A viral load was ordered at this time but not drawn. Her community care provider prescribed her antiretroviral therapy (ART) with dolutegravir, emtricitabine, and tenofovir-disoproxil fumarate and referred her to an HIV specialty care clinic. She did not initiate the ART prescribed to her due to the high cost of the medication and concerns for harm to her baby. She also questioned the HIV-1 diagnosis because she believed she had no risk factors for HIV acquisition. She reported only ever having 1 sexual partner, the father of her baby, who she reported recently testing negative for HIV. She had tattoos, which she reported were obtained at reputable locations, and she denied any intravenous drug use.
During her first encounter at the HIV specialty care clinic, an HIV-1 viral ribonucleic acid (RNA) load and genotype were drawn, and she was prescribed dolutegravir, emtricitabine, and tenofovir alafenamide. She initiated this regimen after reassurance of fetal safety. She was also tested for chlamydia, gonorrhea, syphilis, hepatitis B virus, and hepatitis C virus, which were all negative. Four days after her laboratory tests were drawn, the HIV-1 viral RNA resulted undetectable. She was notified of this result and advised to continue taking ART while additional work-up was performed. At this time, we speculated whether the patient was an elite controller or had possibly been erroneously diagnosed with an HIV infection. Further work-up with an HIV-1 deoxyribonucleic acid (DNA) polymerase chain reaction (PCR) and HIV proviral DNA genotype (while on ART) were obtained. Although these were pending, the HIV-1 genotype from ARUP laboratories resulted indeterminate due to the viral load being below the lower limit of detection. She also had a repeat HIV-1 viral load and HIV antibody and P24 antigen assay drawn, both of which were negative.
About 6 weeks after her initial HIV-1 diagnosis, the HIV-1 DNA PCR resulted undetectable. She was informed that her HIV-1 diagnosis had been invalidated, and she was advised to stop ART. Events and laboratory studies from time of initial HIV-1 diagnosis to time of diagnosis being overturned are illustrated in Figure 1. She has been closely monitored off ART with HIV-1 viral RNA testing every 8 weeks, all of which have been negative to date. The patient had a successful, spontaneous vaginal delivery to a healthy baby. The baby was not tested for HIV as the mother was persistently negative on repeat testing.
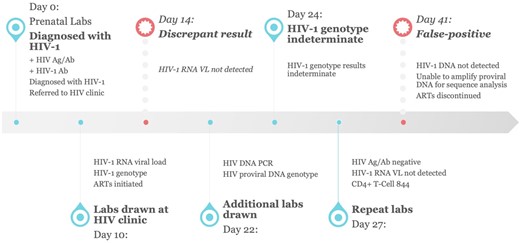
Timeline of diagnostic tests from initial diagnosis to its reversal. Ab, antibody; Ag, antigen; ART, antiretroviral therapy; DNA, deoxyribonucleic acid; HIV, human immunodeficiency virus; labs, laboratory tests; PCR, polymerase chain reaction; RNA, ribonucleic acid; VL, viral load.
DISCUSSION
The Centers for Disease Control and Prevention (CDC) first published guidelines for the diagnosis of HIV in 1989 after the screening test at that time, an HIV enzyme immunoassay, was recognized to have a high false-positive rate [1, 2]. Through 2014, their guidelines have consisted of only antibody tests, which were subsequently found to be limited in their ability to detect acute or early infections [1–3]. In 2014, the CDC issued new recommendations, most recently updated in 2018, which consists of antibody, antigen, and, in some situations, viral RNA testing.
The CDC’s most updated HIV testing algorithm (Supplemental Figure) starts with a combination HIV-1 and HIV-2 antibody and HIV-1 p24 antigen immunoassay. If positive, it is followed by a confirmatory, differentiating antibody immunoassay. Reactivity of both tests is interpreted as HIV-positive, with the recommendation for patients to be referred for HIV medical care and receive appropriate prevention counseling. It is notable that the diagnosis of HIV-1 with this algorithm does not require an HIV-1 nucleic acid test to confirm the presence of viral RNA. Viral load testing is only required for final interpretation when the initial combination immunoassay is reactive and the differentiation immunoassay is either indeterminate or negative [1, 4].
In the United States, HIV testing is recommended as a part of routine prenatal screening [5]. It is estimated that less than 5000 cisgender women with HIV give birth each year, and in the United States in 2018, less than 1% of the 37 968 new HIV diagnoses were from perinatal transmission [6, 7]. However, approximately 3.8 million births were registered in the United States in 2018 [7], indicating that there is a high volume of HIV testing being done in this low incidence population. It is therefore no surprise that false-positive screening results are commonly reported in pregnant women [8–12], justifying the CDC’s multistep algorithm. To our knowledge, no cases have been published of a false-positive HIV-1 diagnosis made using this multistep algorithm at the time of this report.
In this case, an 18-year-old cisgender woman was diagnosed with HIV-1 after a combination antibody and antigen screen was reactive and confirmatory immunoassay was positive on prenatal laboratory tests. She did not report having any risk factors for HIV acquisition and questioned her diagnosis, but her provider appropriately prescribed her ART and referred her for specialized care. A subsequent HIV RNA viral load, notably drawn before her initiating ART, was negative. This negative viral load was what prompted her care team to pursue additional testing.
The current CDC guidelines emphasize that no testing algorithm is 100% accurate and that “inconsistent or conflicting test results should be investigated with follow-up testing on a newly collected specimen” [1]. However, no guidance is provided in terms of what follow-up testing should be pursued in general or specifically for this scenario of an undetectable HIV-1 viral RNA after a positive screening and confirmatory test. The results in this pregnant person indicated that the patient was either an elite-controller or had a false-positive result. Each outcome had high stakes for the patient. If we incorrectly assumed the patient was an elite controller and did have HIV, she would be on ART for the duration of her pregnancy and potentially longer, with the possibility of side effects and associated stigma. However, if we incorrectly determined that she had false-positive HIV testing, she and her developing fetus would not be on HIV therapy, which could result in utero HIV transmission. In this latter scenario, the psychological and emotional toll around a questionable HIV diagnosis with the need for frequent monitoring should not be underestimated.
We performed a literature review from which we found a case report published in the Journal of Clinical Microbiology in 2017 of a pregnant woman diagnosed with HIV via these CDC guidelines who also had undetectable HIV-1 viral RNA [8]. An HIV-1 proviral DNA was performed to resolve the discordant results, a decision they made based on the fact that proviral DNA is a product of early infection and may be present even before HIV-1 RNA. In this case, HIV-1 proviral DNA was detected confirming their patient’s diagnosis of HIV-1. The authors theorized that the patient was a long-term nonprogressor or elite-controller. Having found this case report, we too decided to obtain HIV-1 proviral DNA testing. Once the HIV-1 proviral DNA resulted undetectable, we felt comfortable in our decision to inform the patient her diagnosis was false and to stop ARTs. We have subsequently developed a follow-up plan (HIV-1 viral testing every 8 weeks) based on our best clinical judgement. To date, her repeat testing has been negative.
CONCLUSIONS
We present a case of a false-positive HIV-1 diagnosis made using the current CDC testing algorithm that was overturned after a series of additional tests were obtained after an undetectable HIV-1 RNA viral load. As her care providers, our decision to pursue additional testing, and, in particular, HIV-1 proviral DNA testing after her HIV-1 viral RNA resulted undetectable was based on multidisciplinary decision making and literature review, because there are no current, generally accepted recommendations for this scenario.
There are some limitations to this case worth mentioning. First, although this patient denied any risk factors for HIV acquisition, it is known that people underreport high-risk behaviors, especially those that are sensitive and socially stigmatized [13]. Second, having been referred from an outside facility, we were unable to follow-up with the laboratory that performed her initial positive HIV screen. A rare but possible scenario that could account for her false-positive result is a laboratory error, and this could not be explored. In addition, knowing the details of the type of assay used at the laboratory would be useful because the different combined antigen and antibody assays have different sensitivities and specificities [14, 15]. Third, the baby was not tested for HIV because it was deemed unnecessary given the patient consistently tested negative. Knowing the HIV test results of the baby would provide additional useful information for this case.
We suggest providers consider obtaining HIV-1 proviral DNA testing in low-risk patients who test positive via the CDC’s HIV diagnostic algorithm and subsequently have a negative HIV-1 RNA viral load. This recommendation is based on our experience with this case and literature review of a prior case also demonstrating the utility of HIV-1 proviral DNA testing in resolving this diagnostic dilemma [8]. We feel this recommendation is further justified by the unnecessary medications and anxiety that can result from a false-positive HIV diagnosis. Additional advice for providers, should they encounter this situation, is to repeat the screening combination HIV-1 and HIV-2 antibody and HIV-1 p24 antigen immunoassay.
This case report is a reminder that although the newer, fourth-generation, combined assay has a higher sensitivity and specificity than the third-generation assay, it is still being used in a low prevalence population, which reduces its positive predictive value [16]. We recommend that future societal HIV diagnostic guidelines consider incorporating HIV-1 proviral DNA testing into their algorithms or to at least consider providing guidance on the additional clinical questions posed here.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions. E. H. wrote the first draft of the case report and created the figure. All other authors reviewed and edited the report.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.





Comments