-
PDF
- Split View
-
Views
-
Cite
Cite
Kathryn Grace Kompa, Caitlin A Trottier, Charles L Hyman, Rakhi Kohli, Disseminated Mycobacterium avium Complex Myositis in a Patient With Graft-Versus-Host Disease, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac385, https://doi.org/10.1093/ofid/ofac385
Close - Share Icon Share
Abstract
Mycobacterium avium complex (MAC) is a ubiquitous environmental pathogen that was infrequently reported as a cause of disease before the human immunodeficiency virus (HIV)/acquired immune deficiency syndrome epidemic. We present a case of MAC pyomyositis and bacteremia in a 59-year-old man with chronic lymphocytic leukemia in remission after an allogenic stem cell transplant. His posttransplant course was complicated by graft-versus-host disease, requiring treatment with oral steroids and ruxolitinib. In this report, we review the literature on disseminated MAC infection in patients with and without HIV. We also propose a potential mechanism by which this patient may have developed disseminated disease. Disseminated MAC myositis is uncommon in persons without HIV and requires a high index of suspicion for timely diagnosis.
CASE SUMMARY
A 59-year-old man with a past medical history of chronic lymphocytic leukemia who underwent matched related donor allogenic stem cell transplant 14 years prior was admitted with progressive right arm swelling and pain without associated erythema. He was cured of leukemia but remained on prednisone, mycophenolate mofetil, and intermittent ruxolitinib due to the development of graft-versus-host disease of the eyes, skin, and oral mucosa.
Three months before admission, he sustained an injury to his right arm and shoulder due to sudden pulling of the arm while trying to catch himself to prevent a fall. Thereafter, he suffered right arm and shoulder pain that limited his use of the extremity. A few weeks after the injury, he received a right shoulder steroid injection with minimal improvement in pain. Approximately 2 weeks before admission, a physical therapist massaged the right arm and conducted a range of motion exercises. The following day, the patient developed progressive swelling of his entire right upper extremity, which ultimately led to hospital admission. He denied fevers or chills but endorsed night sweats for the past 1 month and a 40-pound weight loss over the preceding 3 months, which he attributed to decreased oral intake secondary to mucositis-associated pain.
On admission, he was afebrile with stable vital signs. Physical exam revealed extensive swelling of the right upper arm, forearm, and hand without overlying erythema. He had flexion contracture of the right hand and limited active range of motion of the right shoulder, elbow, wrist, and digits. Laboratory data were notable for white blood cell count of 15 700 mm3 with 91% neutrophils. Hemoglobin was 10.5 g/dL and platelet count was 779 000 mm3. Serum creatinine was 0.53 mg/dL. Liver function tests revealed alanine aminotransferase of 80 IU/L and alkaline phosphatase of 237 IU/L. Creatinine kinase level was normal at 36 IU/L.
Vascular studies excluded a venous clot in the right upper extremity. Magnetic resonance imaging (MRI) of the right upper extremity revealed skin thickening, complex appearing septated fluid collections around the biceps muscle and anterior compartment of the distal forearm, and extensive myositis and fasciitis (Figure 1). The patient underwent surgical exploration of the forearm. Intraoperatively, a white coating was noted over the biceps and forearm musculature and the muscle appeared to be viable. On pathology, there was evidence of extensive chronic and acute inflammation and focal myocyte necrosis (Figure 2). Gram stain revealed numerous polymorphonuclear leukocytes and no organisms. On day 5 postsurgical exploration, the patient was discharged to home. Arm swelling was mildly improved with wrapping of the extremity. Eight days after surgical exploration and 3 days after discharge, the microbiology laboratory reported that his muscle tissue was growing acid-fast bacilli (AFB) that was later identified as Mycobacterium avium complex (MAC) by deoxyribonucleic acid probe. This prompted rereview of pathology slides which were also found to have rare AFB (Figure 2). The AFB in muscle tissue were also identified as MAC by polymerase chain reaction. In addition, the anaerobic culture from the muscle tissue grew Propionibacterium acnes from the broth approximately 2 weeks postsurgery.
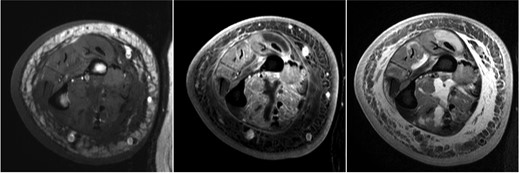
Magnetic resonance imaging on presentation showing skin thickening, subcutaneous reticulations, and microabscesses consistent with pyomyositis (left, T1; middle, T1F; right, T2FS).
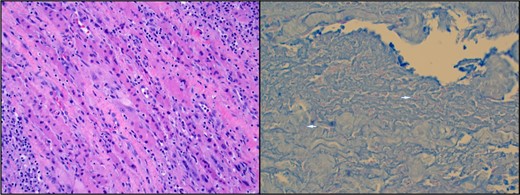
(Left) Hematoxylin and eosin stain of muscle biopsy revealing skeletal muscle cells with poorly defined borders, cellular swelling, pale cytoplasm, and associated white blood cells representing tissue in various stages of necrosis. (Right) Acid-fast bacilli muscle stain. Two bright pink filamentous shapes represent rare acid-fast bacilli within the muscle special stain (see arrows).
The patient was re-admitted for further evaluation and treatment. A computed tomography scan was performed and revealed an abnormal appearance of the proximal humerus with a sclerotic area surrounding a lytic region extending through the cortex of the anterior humerus concerning for possible osteomyelitis per our radiology colleague. The AFB blood cultures were obtained 2 weeks after muscle biopsy and ultimately grew MAC, which was identified via matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Human immunodeficiency virus (HIV) testing using a fourth-generation assay was negative. An orthopedic surgeon re-evaluated the patient and recommended medical management. The patient was started on treatment for disseminated MAC with oral azithromycin, ethambutol, rifabutin, and intravenous amikacin. Propionibacterium acnes was believed to be a contaminant.
Ten months later, the patient’s pain significantly improved, but he had continued right arm edema and limited right arm function despite ongoing physical therapy. He continued on (1) azithromycin, ethambutol, and rifabutin to treat MAC and (2) doxycycline for possible involvement by P acnes. A repeat MRI 4 months after starting treatment showed extensive chronic inflammation of the soft tissues of the right forearm with no abscess or septic arthritis identified.
DISCUSSION
Mycobacterium avium complex is a ubiquitous environmental pathogen found in soil, water, domesticated animals, and the human oral cavity [1]. Disseminated MAC was uncommonly reported before the HIV/acquired immune deficiency syndrome (AIDS) epidemic and now is seen almost exclusively as a pathogen in immunocompromised hosts [2, 3]. Nontuberuculous mycobacterial infections (NTMs) have been reported in patients who have undergone solid organ or hematopoietic stem cell transplants, which can cause cutaneous-, pulmonary-, or catheter- associated infections [4, 5]. We highlight this case because it demonstrates an unusual presentation of disseminated MAC with pyomyositis and bacteremia in an immunocompromised patient without HIV infection. We hypothesize that our patient acquired MAC via ingestion and subsequently developed bacteremia, possibly as a result of mucositis. Trauma to the right arm likely prompted hematogenous seeding of the arm and ultimately resulted in pyomyositis.
Since the late 1980s, most cases of disseminated MAC have been reported in persons with HIV, with few published cases in patients with other types of immunocompromise [6–8]. A review of 29 patients with nontuberculous mycobacterial skin infections over a 14-year period included 2 cases of MAC cutaneous infection complicated by deeper tissue invasion [9]. One patient with diabetes developed an ulcerated abscess due to MAC after acupuncture, which was complicated by myositis [9]. The second case occurred in a patient with AIDS who had multiple ulcerated abscesses of the right leg and foot that were complicated by synovitis [9]. Both of these cases describe MAC deep tissue invasion occurring as a result of skin trauma or exogenous spread. However, our case of MAC pyomyositis likely occurred as a result of endogenous infection and hematogenous spread. A retrospective case series performed between 1940 and 1984 identified only 37 cases of disseminated MAC in patients without known HIV/AIDS [1]. Review of these 37 cases identified that MAC most commonly was isolated in culture from sputum or lung, bone marrow, bone, liver, and lymph nodes. Mycobacterium avium complex grew in the psoas muscle in one patient with osteomyelitis [10].
A review of pyomyositis cases in the United States from 2002 to 2014 revealed that HIV infection, diabetes, and hematologic malignancy were common risk factors, and Staphylococcus aureus was the most commonly identified bacterial pathogen [11]. Pyomyositis due to mycobacteria was rare but more common in persons with HIV infection than persons without [11]. The pathogenesis of pyomyositis is not well defined but could involve local trauma or injury to muscle resulting in transient bacteremia and subsequent infection [12]. Infections of skeletal muscles are rare, and in the case of mycobacterium-associated myositis, larger muscles are usually involved [13]. Lawn et al [14] described a woman with AIDS who developed MAC myositis of the gastrocnemius muscle with associated cutaneous abscesses as an immune reconstitution phenomenon 2 weeks after initiation of combination antiretroviral therapy. Although no inciting trauma to the area was noted, the patient identified pain in this area for over 1 year before initiation of antiviral therapy [14]. Diego Miralles et al [15] describe a case of MAC necrotizing myositis after sustained trauma to the affected area in a patient with AIDS. The authors hypothesized that the patient was transiently bacteremic and trauma allowed for the formation of a hematoma where organisms could replicate [15]. We believe our patient had a similar pathogenesis of primary bacteremia leading to pyomyositis.
Our patient was receiving an aggressive immunosuppressive regimen given severe graft-versus-host disease. It is notable that 3 weeks before his presentation, he was restarted on a steroid burst given concern for worsening acute rejection. In 1 case study, a woman without HIV developed MAC osteomyelitis in the setting of chronic steroid use for systemic lupus erythematosus, which was thought to be an important risk factor for this opportunistic infection [6].
In addition to steroid use, he restarted ruxolitinib, a Janus-associated kinase inhibitor, which suppresses both innate and adaptive immunity through a variety of mechanisms [16, 17]. In an immunocompetent host, after macrophages engulf mycobacterium, they release the cytokine interleukin (IL)-12 to recruit T lymphocytes and natural killer cells. These cells release interferon-γ, which activates tumor necrosis factor-α and IL-12, leading to a positive feedback loop and the targeting of these intracellular pathogens [18]. Mutations in genes encoding for portions of this feedback loop have been associated with disseminated mycobacterium infection in the absence of HIV [19, 20]. Ruxolitinib has been shown to inhibit cytokine release from macrophages, thereby reducing the recruitment of natural killer cells, which are critical components of the immune response to intracellular pathogens such as MAC [21]. There have been multiple documented cases of disseminated MAC in patients receiving ruxolitinib [22–24]. In a 2019 case series of 65 patients receiving ruxolitinib for either polycythemia vera or myelofibrosis, there were 2 cases of mycobacterial infections, including 1 case of disseminated MAC infection that was ultimately fatal [23]. In addition, a pharmacovigilance study of patients on ruxolitinib using the US FDA Adverse Events Reporting System (FAERS) identified 91 cases of Mycobacterium tuberculosis (MTB) and 23 cases of atypical mycobacterial infections (NTM), with case-fatality rates of 13.2% and 34.8%, respectively [24]. Both MTB and NTM were reported more commonly with ruxolitinib compared to other medications with reporting odds ratios of 9.2 for MTB and 8.3 for NTM [24]. In addition to his immunosuppressive regimen, chronic graft-versus-host disease may also pose an increased risk for mycobacterial infection [25].
CONCLUSIONS
This case illustrates a rare presentation of disseminated MAC, manifesting with pyomyositis and bacteremia, in an immunocompromised patient receiving ruxolitinib. A high index of suspicion is needed to diagnose MAC, and special stains and culture media are often required to identify the organism [18]. In a case study of 37 patients with disseminated MAC, patients went an average of 5.3 months before a diagnosis was made [1]. Treatment of complicated MAC infections is often prolonged and requires multiple drugs guided by susceptibility testing. Duration of treatment is often guided by tolerance of complex medical regimens as well as clinical response. Given the increasing number of persons on immunosuppressive therapies, specifically ruxolitinib, MAC should often be included on the infectious differential.
Acknowledgments
This case report and literature review were performed by medical trainees with the mentorship of their attendings.
Author contributions. All authors have made substantial contributions to the case report, drafted and revised this case report, and approved the final version for publication.
References
Jakafi (ruxolitinib) [package insert].
Author notes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
- hiv
- acquired immunodeficiency syndrome
- bone marrow transplantation, allogeneic
- bacteremia
- graft-versus-host disease
- chronic lymphocytic leukemia
- chronic b-cell leukemias
- mycobacterium avium complex
- mycobacterium avium-intracellulare infections
- myositis
- steroids
- diagnosis
- pathogenic organism
- pyomyositis
- epidemics
- ruxolitinib





Comments