-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth Christian, Alicia Johnston, CNS TB-IRIS Following Cessation of Adalimumab in an Adolescent With Crohn’s Disease, Open Forum Infectious Diseases, Volume 9, Issue 8, August 2022, ofac367, https://doi.org/10.1093/ofid/ofac367
Close - Share Icon Share
Abstract
Immune reconstitution inflammatory syndrome to tuberculosis (TB-IRIS) is an inflammatory response to M. tuberculosis infection that arises following restoration of the immune system and is increasingly recognized as a risk in patients treated with tumor necrosis factor α inhibitors who develop active tuberculosis infection. We present the case of a 19-year-old man treated with adalimumab for Crohn’s disease who presented with disseminated miliary tuberculosis. His treatment course was complicated by central nervous system TB-IRIS following cessation of his immunosuppression. We review the presentation and differential diagnosis of TB-IRIS, as well as risk factors for developing IRIS and the treatment of IRIS in this population.
Immune reconstitution inflammatory syndrome (IRIS) is an exuberant inflammatory response to preexisting infection following reconstitution of the immune system, leading to a clinical worsening of symptoms after an initial period of improvement. It is a reflection of increasing inflammatory burden, rather than progressive infection or treatment failure. The development of IRIS during the course of treatment for tuberculosis is well described in people living with HIV (PWHIV), and there are guidelines for appropriate timing of antiretroviral initiation to reduce the risk of developing this life-threatening complication [1]. IRIS to tuberculosis (TB-IRIS) has also been documented in patients with other etiologies of immunosuppression, for whom there are no clear recommendations around the management of immunosuppression following initiation of antituberculous therapy. We describe the case of a 19-year-old man who developed disseminated miliary tuberculosis while taking adalimumab, whose treatment course was complicated by the development of central nervous system (CNS) TB-IRIS.
CASE
A 19-year-old man with Crohn’s disease treated with adalimumab for 4 months presented to our institution’s emergency department in June 2020 with 5 days of fever, chills, abdominal pain, and diarrhea. There was concern for a Crohn’s flare and admission was recommended, but the patient declined and was discharged home without treatment. He presented again 1 week later with the same symptoms and a new symptom: lightheadedness. A computed tomography (CT) scan of the abdomen showed thickening and enhancement of the terminal ileum consistent with a Crohn’s flare, but he declined steroids and was discharged home. The following week, he was seen in the Gastroenterology clinic and prescribed prednisone 40 mg orally qd. His symptoms improved, and he began a slow prednisone taper. One month later, he developed severe throat pain and a large ulcerative lesion in his posterior pharynx. He was evaluated in the emergency department, where testing for Epstein-Barr virus and group A Streptococcus was negative. He followed up with his pediatrician, who prescribed 14 days of amoxicillin-clavulanate for suspected peritonsillar cellulitis. His symptoms did not resolve, and he presented to the emergency department after completing his antibiotic course, at which time he was found to be febrile to 41.2°C, rigoring, and hypotensive. He noted 3 months of previously unreported findings that included subjective fevers, night sweats, abdominal pain, and diarrhea, as well as several days of bilateral chest pain. He denied cough and shortness of breath.
A chest x-ray showed innumerable diffuse, tiny pulmonary nodules without lymphadenopathy (Figure 1). He was given IV fluids, IV epinephrine, empiric ceftriaxone, and clindamycin and admitted to the intensive care unit.
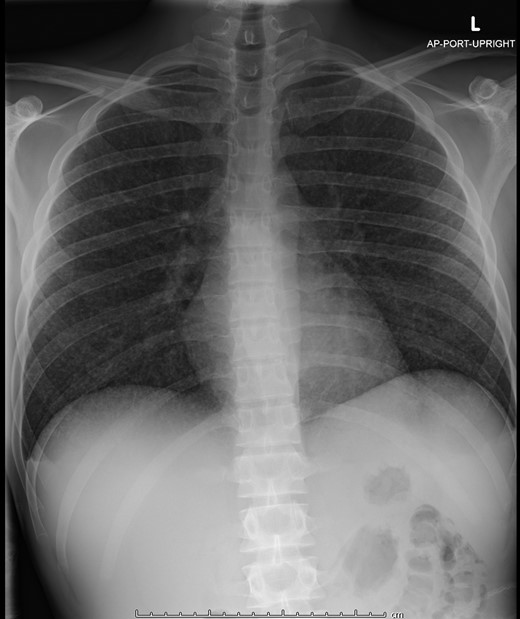
The patient’s initial chest x-ray showing innumerable diffuse, tiny pulmonary nodules bilaterally.
The patient was born in the Dominican Republic and lived there until the age of 12 years, when he emigrated to the United States. A T-spot obtained before initiation of adalimumab was negative, so a Tuberculin Skin Test (TST) was deferred, and he had not traveled or had any known TB exposures since that time. A broad infectious workup looking for bacterial, viral, and fungal pathogens was undertaken. Given high suspicion for miliary tuberculosis, evaluation included 3 acid fast bacilli (AFB) smears and cultures of sputum, AFB blood and urine cultures, imaging of the chest, abdomen, pelvis, brain, and spine, and a dilated retinal exam. CT scans redemonstrated the presence of diffuse pulmonary nodules and showed lesions in the kidney and on the peritoneum, suggesting tuberculous deposits. Magnetic resonance imaging (MRI) of the brain revealed 3 areas of enhancement located at the gray-white matter junction, consistent with tuberculomas, but cerebrospinal fluid (CSF) studies were not suggestive of meningitis (total protein 12.2 mg/dL, glucose 62 mg/dL, white blood cell count 2 cells/mm3 with 75% polymorphonuclear neutrophils). The dilated retinal exam showed multiple subretinal nodules, consistent with tuberculous chorioretinal lesions. Sputum smear microscopy was positive for acid fast bacilli, and a nucleic acid amplification test (NAAT) was positive for Mycobacterium tuberculosis (MTB) complex. There was growth in liquid culture at 10 days, identified as MTB complex by MALDI-TOF. He was treated with rifampin, isoniazid, pyrazinamide, ethambutol, and pyridoxine (RIPE). Further doses of adalimumab were deferred. Because the patient did not have TB meningitis and there is a lack of evidence in the literature regarding the benefit of continued steroids for management of CNS tuberculomas, the patient completed his prednisone taper as previously planned. He received extensive counseling regarding the potential for IRIS, was educated about signs and symptoms that could signal the onset of IRIS, and was monitored closely in the inpatient setting following cessation of steroids. His fevers resolved, and he improved and was discharged on RIPE therapy.
Eighteen days later, he presented to the emergency department with new-onset fevers, vomiting, and diarrhea. A brain MRI showed increasing vasogenic edema around the largest tuberculoma (Figure 2) as well as an increase in the number and size of other lesions in the brain parenchyma and a new lesion on the left retina. The differential diagnosis for his worsening clinical status included TB-IRIS, poor enteral absorption of oral medications related to his Crohn’s disease, and drug-resistant tuberculosis. A full susceptibility panel of his MTB isolate was requested. His medication regimen was empirically transitioned to amikacin, linezolid, levofloxacin, rifampin, and isoniazid. Dexamethasone was initiated to treat possible IRIS.
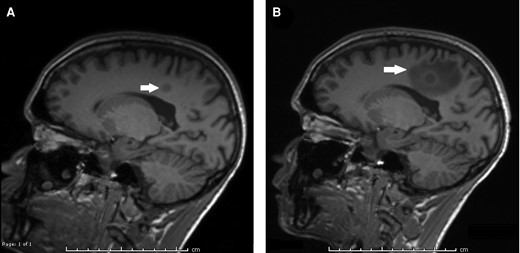
MRI of the brain with contrast, with T1 MP-RAGE sequences shown. A, MRI of the brain obtained at the time of initial TB diagnosis, showing a 1.0 × 1.2 cm area of enhancement in the right mesial parietal lobe. B, MRI of the brain obtained at the time of re-presentation and diagnosis with CNS TB-IRIS. The previously seen area of enhancement is slightly increased in size to 1.0 × 1.3 cm, and there is new surrounding vasogenic edema, measuring 4.5 × 2.3 cm. Abbreviations: CNS, central nervous system; MRI, magnetic resonance image; TB, tuberculosis; TB-IRIS, immune reconstitution inflammatory syndrome to tuberculosis.
Plasma antibiotic drug concentrations had been obtained during his initial presentation due to concerns about malabsorption in the setting of Crohn’s disease, and these results returned during his second admission, showing concentrations of rifampin, isoniazid, and ethambutol slightly under target. Susceptibility testing revealed resistance only to pyrazinamide, suggesting that the patient’s isolate was M. bovis, rather than M. tuberculosis; specific testing to confirm the isolate as M. bovis was not pursued. He improved following initiation of dexamethasone, and his clinical decompensation was felt to be due to IRIS. He was treated with 2 months of rifampin, isoniazid, levofloxacin, and linezolid, followed by 16 months of rifampin and isoniazid. Steroids were slowly tapered over a period of 8 weeks. Serial brain MRIs showed decreasing size of the tuberculomas, with complete resolution by the time he had completed 18 months of TB therapy. His Crohn’s disease was managed with vedolizumab due to its gut selective anti-inflammatory activity with limited systemic impact.
DISCUSSION
IRIS is an excessive inflammatory response to preexisting infection in an immunosuppressed host following immune reconstitution, and this response may be aimed at antigens arising from live or dead pathogens [2]. Though initially recognized as a risk in people living with HIV who developed immune reconstitution after initiating antiretroviral therapy, IRIS is now increasingly recognized in other clinical scenarios of immune reconstitution, including postpartum women, solid organ transplant recipients, patients with hematologic malignancies, and following the withdrawal of tumor necrosis factor alpha (TNF-α) inhibitors [3].
There are 2 forms of IRIS. Unmasking IRIS describes a scenario in which an opportunistic infection is not recognized before immune system reconstitution but becomes clinically apparent once the patient is able to mount an immune response. By contrast, paradoxical IRIS, which our patient had, refers to a scenario in which an infection is recognized, treated, and initially improves, followed by a clinical worsening after the immune system recovers. The diagnosis of paradoxical IRIS is one of exclusion (Table 1), and an evaluation for other etiologies that could be the cause of clinical worsening is required. The differential diagnosis includes poor medication adherence, suboptimal drug absorption, adverse drug reaction, infection with a drug-resistant microorganism, or a new secondary infection [2, 4]. In our patient, a Crohn’s disease flare was also considered, as he presented with fevers, vomiting, and diarrhea, all of which could have been due to his underlying disease.
|
| AND |
|
| AND |
|
|
| AND |
|
| AND |
|
Abbreviations: TB, tuberculosis; TB-IRIS, immune reconstitution inflammatory syndrome to tuberculosis.
|
| AND |
|
| AND |
|
|
| AND |
|
| AND |
|
Abbreviations: TB, tuberculosis; TB-IRIS, immune reconstitution inflammatory syndrome to tuberculosis.
TB-IRIS may present with a variety of nonspecific symptoms. Malaise, fever, worsening lymphadenopathy, new pulmonary symptoms, and radiographic lung infiltrates are the most commonly reported [2, 4]. Lymph nodes are the most commonly involved site of IRIS, with 68% of patients developing lymphadenitis in 1 case series [5]. Our patient was unusual in that the predominant areas of worsening inflammation were his CNS tuberculomas.
The time to onset of IRIS varies by risk factor, and for patients with TNF-α inhibition, expected onset is related to the half-life of their medication. Infliximab is dosed every 18 weeks, and cases of TB-IRIS are reported with onset of symptoms between 5 and 16 weeks after infliximab discontinuation. By contrast, adalimumab is dosed every 14 days, and etanercept is dosed 1 or 2 times per week; thus the reported times to onset of IRIS have been shorter for patients treated with these medications [4].
Risk factors for the development of IRIS in HIV-uninfected patients are still being defined (Table 2). One case–control study using a French registry looked specifically at patients who developed TB-IRIS following TNF-α inhibitor withdrawal and found that disseminated TB, previous TB exposure, and treatment with steroids after discontinuation of TNF-α inhibitor therapy at the time of TB diagnosis were risk factors specific to this population [6]. They did not find a correlation between lymphocyte count at the time of TB diagnosis and risk for IRIS, though this was reported in another study [6, 7]. Retrospective cohort studies including HIV-negative immunosuppressed and nonimmunosuppressed patients who developed TB-IRIS have identified extrapulmonary disease or lymph node involvement at the time of diagnosis, anemia (Hb < 10.5 g/dL), and marked lymphocytosis during immune reconstitution as risk factors for the development of paradoxical IRIS [5, 7].
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: ALC, absolute lymphocyte count; Hb, hemoglobin; IRIS, immune reconstitution inflammatory syndrome; MTB, Mycobacterium tuberculosis; TB, tuberculosis; TB-IRIS, immune reconstitution inflammatory syndrome to tuberculosis.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviations: ALC, absolute lymphocyte count; Hb, hemoglobin; IRIS, immune reconstitution inflammatory syndrome; MTB, Mycobacterium tuberculosis; TB, tuberculosis; TB-IRIS, immune reconstitution inflammatory syndrome to tuberculosis.
Disseminated disease is a significant risk factor for the development of TB-IRIS, and the risk of IRIS is strongly associated with the burden of residual disease present at the time of immune recovery; one study found that patients with extrapulmonary and disseminated disease had an odds ratio of 8.2 for developing IRIS [3]. Our patient had a significant delay in diagnosis and was treated with prednisone before diagnosis, which likely facilitated widespread dissemination and a high bacterial burden as his immune system recovered following exposure to adalimumab and steroids.
Some cases of TB-IRIS resolve without directed therapy, while others—CNS-IRIS in particular—can be life-threatening without intervention [2]. There is no consensus on the optimal treatment and few data on which to base recommendations. A single randomized controlled trial assessed the effect of prednisone on PWHIV treated for TB-IRIS and found an overall decrease in hospital days and requirement for therapeutic procedures, as well as greater improvement in symptoms, functionality, and quality of life at 2 and 4 weeks of treatment in the intervention group, although no difference was observed at later time points. Notably, patients with potentially life-threatening manifestations of IRIS, including CNS involvement, were excluded from the trial [8].
For patients with steroid-refractory TB-IRIS, the use of TNF-α inhibitors for adjunctive therapy has been reported. The largest body of evidence exists for patients who had not previously been treated with TNF-α inhibition [9, 10]. A review of the literature by Santin et al. found 43 patients (13 adults and 30 children) with steroid-refractory CNS TB-IRIS who were treated with infliximab (6 patients), adalimumab (2 patients), or thalidomide. Nine patients had concurrent HIV infection as a risk factor for IRIS, and 16 patients were not immunocompromised; the other patients were immunocompromised due to an underlying autoimmune condition, a hematologic malignancy, or malnutrition. There was an overall survival rate of 83.5%, with approximately half of surviving patients experiencing full recovery without neurologic sequelae [9].
There are a few case reports of reintroducing a TNF-α inhibitor for management of steroid-refractory IRIS in patients previously treated with TNF-α blockade. Wallis et al. reported the case of a 29-year-old woman with rheumatoid arthritis treated with adalimumab who presented with pulmonary TB complicated by IRIS that did not respond to methylprednisolone. She was restarted on adalimumab after 17 days of antituberculous treatment and improved [11]. Hess et al. reported the case of a 17-year-old woman with SAPHO syndrome treated with adalimumab who presented with disseminated TB, including TB meningitis. Her initial TB regimen included dexamethasone, but she developed IRIS despite this, so thalidomide was added and she had clinical improvement [12]. Jorge et al. reported the case of a 20-year-old man with variant juvenile idiopathic arthritis treated with infliximab who presented with miliary TB, including meningitis and multiple CNS tuberculomas. He developed IRIS that did not respond to steroids or methotrexate but clinically improved following a dose of infliximab [13].
Our case illustrates that risk for TB-IRIS should be considered in patients treated with TNF-α inhibitors who develop active tuberculosis infection. The timing of TB-IRIS onset will vary depending on the half-life of the TNF-α inhibitor used. The optimal management of TB-IRIS in patients previously treated with TNF-α inhibitors is not known. Though a small randomized controlled trial of prednisone for treating IRIS in PWHIV showed a morbidity benefit, patients with life-threatening IRIS disease were excluded from that trial. There may be a role for re-introduction of TNF-α inhibition for management of steroid-refractory IRIS, although the current evidence base consists only of case reports at this time.
Acknowledgments
The authors gratefully acknowledge Thomas Fleisher, MD, for his insightful comments and feedback during manuscript editing, and Ethan Bremner for his assistance in formatting the figures.
Financial support. No funding was used to support the writing of this case report.
Author contributions. E.C. performed the literature review and wrote the first draft. A.J. edited the manuscript for content and wording.
Patient consent. The patient described in this case has consented to the use of his history and clinical images.
References
Centers for Disease Control and Prevention, National Institutes for Health, the HIV Medicine Association, and the Infectious Disease Society of America,
Author notes
Potential conflicts of interest. Neither Dr. Christian nor Dr. Johnston has any conflicts of interest to declare. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments