-
PDF
- Split View
-
Views
-
Cite
Cite
Philippa Randall, Junior Mutsvangwa, Marriott Nliwasa, Lindsay Wilson, Beauty Makamure, Edson Makambwa, Richard Meldau, Keertan Dheda, Shungu Munyati, Omar Siddiqi, Elizabeth Corbett, Ali Esmail, Utility of Cerebrospinal Fluid Unstimulated Interferon-Gamma (IRISA-TB) as a Same-Day Test for Tuberculous Meningitis in a Tuberculosis-Endemic, Resource-Poor Setting, Open Forum Infectious Diseases, Volume 11, Issue 9, September 2024, ofae496, https://doi.org/10.1093/ofid/ofae496
Close - Share Icon Share
Abstract
Tuberculous meningitis (TBM) mortality is high and current diagnostics perform suboptimally. We evaluated the diagnostic performance of a DNA-based assay (GeneXpert Ultra) against a new same-day immunodiagnostic assay that detects unstimulated interferon-gamma (IRISA-TB).
In a stage 1 evaluation, IRISA-TB was evaluated in biobanked samples from Zambia (n = 82; tuberculosis [TB] and non-TBM), and specificity in a South African biobank (n = 291; non-TBM only). Given encouraging results, a stage 2 evaluation was performed in suspected TBM patients from Zimbabwe and Malawi (n = 668). Patients were classified as having definite, probable or possible TBM, or non-TBM based on their microbiological results, cerebrospinal fluid (CSF) chemistry, and whether they received treatment.
In the stage 1 evaluation, sensitivity and specificity of IRISA-TB were 75% and 87% in the Zambian samples, and specificity was 100% in the South African samples. In the stage 2 validation, IRISA-TB sensitivity (95% confidence interval [CI]) was significantly higher than Xpert Ultra (76.2% [55.0%–89.4%] vs 25% [8.9%–53.3%]; P = .0048) when trace readouts were considered negative. Specificity (95% CI) was similar for both assays (91.4% [88.8%–93.4%] vs 86.9% [83.4%–89.8%]). When the Xpert Ultra polymerase chain reaction product was verified by sequencing, the positive predictive value of trace readouts in CSF was 27.8%. Sensitivity of IRISA-TB was higher in human immunodeficiency virus (HIV)–infected versus uninfected participants (85.8% vs 66.7%).
As a same-day rule-in test, IRISA-TB had significantly better sensitivity than Xpert Ultra in a TB/HIV-endemic setting. An immunodiagnostic approach to TBM is promising, and further studies are warranted.
Globally, there are approximately 100 000 newly ill patients with tuberculous meningitis (TBM) annually [1, 2]. Although this makes up only approximately 7% of all individuals with tuberculosis (TB), TBM is a devastating disease with high morbidity and approximately 25% mortality, and is invariably fatal if untreated [2, 3]. Rapid and accurate diagnosis is important as diagnostic delay increases mortality [4]. However, current tools for the diagnosis of TBM are suboptimal [5].
Culture has a sensitivity of approximately 50%, but the result is delayed by several weeks and is thus not useful for diagnosis [5]. Cerebrospinal fluid (CSF) lipoarabinomannan TB antigen testing, in preliminary studies, had a sensitivity of approximately 30%–40% when used on CSF [6, 7]. Frontline nucleic acid amplification tests such as GeneXpert, which delivers same-day results, have been useful for the diagnosis of TBM [8], and the newer and more sensitive Xpert Ultra version of the assay, in an updated systematic review and meta-analysis (2021), had a sensitivity of 64% [9]. Three more recent large and well-conducted studies from TB-endemic countries (Vietnam, China, and India), enrolling several hundred patients with TBM, showed Xpert Ultra sensitivities of 45%, 47%, and 50%, respectively (39% in human-immunodeficiency virus [HIV]–uninfected persons in the Vietnam study) [10–12]. However, an important aspect to consider when interpreting these results is the inclusions of Xpert “trace readouts” signifying the detection of ultra-low levels of DNA (the assay has a semi-quantitative readout of mycobacterial burden, ie, high, medium, low, very low, and “trace”). However, approximately 40% of Xpert Ultra–positive TBM readouts are “trace,” which may be erroneously positive, and this may be falsely inflating sensitivity (see Discussion for detailed assessment of this issue) [5, 13, 14].
Unsurprisingly given the paucibacillary nature of TBM, and like in pleural TB [15–20], host biomarkers have been investigated for the diagnosis of TBM. Adenosine deaminase, an enzyme produced by mononuclear cells, has a sensitivity of approximately 80% but specificity, like in pleural TB [16–18], is suboptimal at approximately 80%, performance is highly variable, and cut points vary in different countries [5, 21]. Interferon-gamma release assays (IGRAs) on CSF showed a sensitivity of approximately 75% and a specificity of 91% for the diagnosis of TBM [22, 23], but drawbacks include a need for overnight sample incubation, antigen stimulation, the need for high volume of sample (∼4 to 5 mL), and high cost; a key problem is that a substantial proportion of samples have indeterminate readouts [22, 23]. However, IGRAs are unnecessary and functionally redundant in this context because TB antigen-specific interferon gamma (IFN-γ) production by mononuclear cells, after antigen stimulation, is already occurring within a confined compartmentalized space (analogous to a culture well), and thus logically unstimulated IFN-γ should perform as well. Indeed, several publications have shown (summarized in a systematic review and meta-analysis of 5 studies) that unstimulated IFN-γ had a pooled sensitivity and specificity of 86% and 92%, respectively, for the diagnosis of TBM [22]. Very recently a start-up associated with the University of Cape Town has produced a validated assay Inter-Gam Ultrasensitive Rapid Immuno-suspension Assay (IRISA-TB) for the detection of unprocessed IFN-γ in TB serositis and TB meningitis. IRISA-TB is not an IGRA but a clinically validated same-day low-cost assay (also accommodating single-patient use) that was evaluated in pleural and pericardial TB with excellent results, that is, sensitivity >90% and specificity greater than approximately 95% [16, 17, 24, 25].
Given IRISA-TB’s promise in pleural and pericardial TB diagnosis, the performance of IRISA-TB has yet to be validated in TBM and its performance compared to that of Xpert Ultra. To address this knowledge gap, we undertook diagnostic evaluation of IRISA-TB in patients with suspected TBM (Figure 1). This involved a promising stage 1 evaluation on biobanked samples (Zambia and South Africa) and a confirmatory follow-on stage 2 evaluation in a prospective diagnostic study (Malawi and Zimbabwe). The study also aimed to clarify the contribution of trace readouts, by performing sequencing of the Xpert Ultra polymerase chain reaction (PCR) product in a limited number of retained cartridges (thus, results are presented with and without trace readouts).
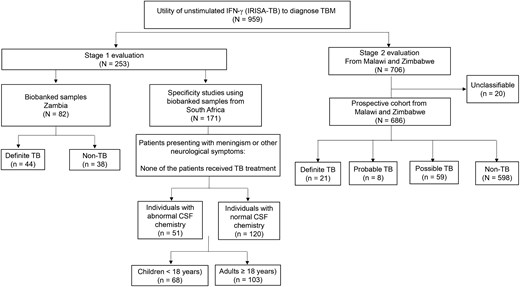
Study overview outlining stage 1 evaluation (n = 253) and stage 2 evaluation (n = 686). Abbreviations: CSF, cerebrospinal fluid; IFN-γ, interferon gamma; IRISA-TB, inter-gam ultrasensitive rapid immuno-suspension assay; TB, tuberculosis; TBM, tuberculous meningitis.
MATERIALS AND METHODS
Patient Recruitment, Categorization, and Routine Laboratory Testing
Stage 1 Evaluation
Biobanked samples were selected from a cohort of people with suspected TBM collected in Zambia (n = 82). The selected samples were categorized as definite TB, which was defined as those with a positive Xpert MTB/RIF and/or culture; and non-TB, which was defined as a negative on Xpert MTB/RIF and culture with positive cryptococcal latex agglutination test (CLAT) or Gram stain (alternative diagnosis). Biochemical and cytological analysis (protein and cell counts) and the HIV status of the patients were made available.
Patients were recruited from Groote Schuur Hospital, Khayelitsha Hospital, Victoria Hospital, Somerset Hospital, Mitchells Plain Hospital, and Red Cross Children’s Hospital in Cape Town, South Africa. The University of Cape Town Human Research Ethics Committee approved the study (HREC 235/2016). Individuals who presented with meningism or other neurological symptoms (ie, documented alternative diagnoses other than TBM), and were not initiated onto TB treatment, were enrolled in Cape Town. All patients (or guardians) provided informed consent. CSF was collected by lumbar puncture. CSF were subjected to routine biochemical and cytological analysis by the National Health Laboratory Services. This included protein, lymphocytes counts, and cytology. If viral or bacterial meningitis was suspected, a Gram stain and/or CLAT test was performed in those cases. The remaining fluid was placed in a biobank, frozen at −20°C, and subsequently used for IRISA-TB analyses. HIV testing was performed in consenting patients.
Stage 2 Evaluation
Patients were recruited in Zimbabwe at Harare Central Hospital, Parirenyatwa Hospital, Chitungwiza Hospital, and City Council hospitals (Beatrice Road and Wilkins Infectious diseases) and in Malawi at Queen Elizabeth Central Hospital and the Zomba Central Hospital. The University of Malawi Human Research Ethics Committee approved the study (P.09/17/2276), and in Zimbabwe the Medical Research Council Zimbabwe (MRCZ) and Research Council of Zimbabwe (RCZ) approved the study (MRCZ/A/2219). Participants were subjected to routine diagnostic procedures, including clinical and laboratory assessment of CSF collected by lumbar puncture. All patients (or guardians) provided informed consent. If available, HIV status was recorded. Laboratory tests included white cell counts, glucose levels, protein determination, culture (for Mycobacterium tuberculosis [Mtb] and other bacteria), microscopy (Gram stain and acid fast), tests for cryptococcal antigen, and/or molecular tests (GeneXpert MTB/RIF and GeneXpert Ultra). The remaining fluid was placed in a biobank, frozen at −20°C, and subsequently used for IRISA-TB analyses. In this large confirmatory prospective cohort (n = 686 patients with suspected TBM), definite TBM was defined as those with at least 1 microbiologically positive test (culture or Xpert MTB/RIF and/or Xpert Ultra), a negative Gram stain and CLAT, lumbar puncture findings consistent with TBM, initiated on TB treatment, but may or may not have improved (and alive or dead) at follow-up (Supplementary Table 1 outlines microbiological positivity by test type). Probable TB was defined as those with at least 1 confirmatory microbiologically positive test but from an alternative site (culture or Xpert MTB/RIF and/or Xpert Ultra), a negative Gram stain and CLAT, lumbar puncture findings consistent with TBM, initiated on TB treatment, and may or may not have improved and (and alive or dead) at follow-up. Possible TB was defined as those with a negative Gram stain and CLAT, lumbar puncture findings consistent with TBM, initiated on TB treatment, and may or may not have improved (and alive or dead) at follow-up. Non-TBM were those for whom all microbiological tests were negative (culture or Xpert MTB/RIF and/or Xpert Ultra), an alternative diagnosis may often have been made, lumbar puncture findings were normal or consistent with alternative cause of meningitis, and TB treatment was not initiated. Patients who did not fall into any of these categories remained unclassified (eg, negative microbiology on CSF but lost to follow-up after initial CSF sampling preventing classification into non-TB, possible TB, or probable TB).
Unstimulated IFN-γ Measurement (IRISA-TB)
IFN-γ concentrations were measured in CSF supernatants using the IRISA-TB assay (Antrum Biotech Pty Ltd, Cape Town, South Africa) according to the manufacturer's instructions. The assay was performed in duplicate and the average values reported. CSF supernatant was prepared by centrifuging 1 mL of CSF at 12 000 relative centrifugal force for 90 seconds as per the manufacturer's instructions.
Cepheid GeneXpert MTB/RIF and Gene Xpert Ultra Assays
Both the Xpert Ultra and Xpert MTB/RIF assays were performed using 1 mL of CSF diluted with 2 mL of Xpert sample buffer, followed by vigorous mixing. Xpert Ultra and Xpert MTB/RIF cartridges were run on a GeneXpert 4-module machine (Cepheid, Dx System version 4.7b) [17].
Sanger Sequencing of Xpert Ultra Cartridges
A total of 202 CSF samples from the Malawi prospective cohort with sufficient sample were subjected to Xpert Ultra for a second time. The PCR products were extracted from 42 of the remaining Xpert Ultra cartridges and stored at −80°C for downstream Sanger sequencing. The sample numbers according to Xpert Ultra results were 13 Mtb not detected (n = 10 confirmed non-TBM), 10 Mtb detected very low (n = 10 confirmed non-TBM), and 17 Mtb detected trace (n = 13 confirmed non-TBM). Xpert Ultra PCR products from sputum samples (suspected pulmonary TB) were extracted and included as controls for sample type bias. Sample numbers according to Xpert Ultra results for sputum-based were 5 Mtb not detected, 1 Mtb detected very low, 2 Mtb detected low, 1 Mtb detected, and 1 Mtb detected trace. All PCR product extracts were sent to Inqaba Biotech (Pty) Ltd for Sanger Sequencing. In brief, cartridge extract (Xpert Ultra PCR) products were purified, amplified, and sequenced. Sequencing was performed on neat and amplified PCR products. Successfully sequenced PCR products with identical matches to either IS6110 or IS1081 insertion sequences were considered a positive result. Sequencing was performed on the ABI 3730XL genetic analyzer with a 50 cm array and POP-7, and with Nimagen Brilliant Dye V3.1 sequencing kits. Sequencing products were purified with the Zymo DNA Sequencing Clean-up kit D4053 before injection. All kits were used according to manufacturer instructions.
Statistical Analysis
Diagnostic accuracy, including 95% confidence intervals (CIs), was assessed using sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), area under the receiver operating characteristic curve, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio in definite TB and non-TB groups. Unpaired and paired categorical variables were compared using the χ2 and McNemar tests, respectively. Continuous variables were compared using Student t test where appropriate. The Mann-Whitney and Wilcoxon rank-sum test was used for unpaired and paired nonparametric continuous variables, respectively. Statistical analyses were performed using GraphPad Prism (version 6.0), Medcalc (version 18.6) and Microsoft Excel.
RESULTS
Performance of IRISA-TB in Stage 1 Evaluation (Retrospective or Case-Control Cohort [Zambia])
A Zambian case-control cohort from biobanked specimens (n = 82) was selected to evaluate the initial performance of IRISA-TB in diagnosing TBM. The definite TB group contained a significantly higher number of people living with HIV (PWH) (95.5%, P = .0114), whose CSF protein and unstimulated IFN-γ concentrations was elevated in comparison to the non-TBM group (P ≤ .0001) (Supplementary Table 2).
At a cutoff of 13 pg/mL, the sensitivity and specificity were 75% and 86.9%, respectively, with superior performance in PWH, although not significant (area under the curve [AUC], 0.92; Figure 2A and 2B). Overall, the IRISA-TB showed superior performance over current diagnostics, but a larger validation cohort was required to validate these observations.
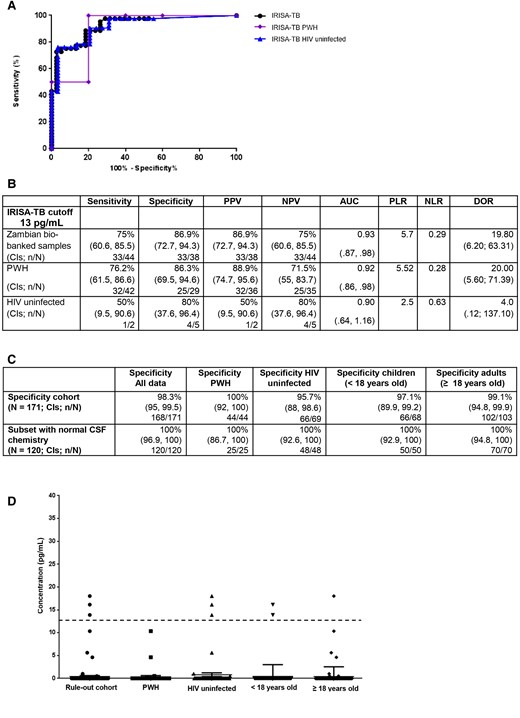
Performance data for stage 1 evaluation to assess specificity in biobanked South African samples. A, Receiver operating characteristic curve of IRISA-TB performance analyzed by human immunodeficiency virus (HIV) status. B, Performance data of IRISA-TB analyzed by HIV status. C, Specificity of IRISA-TB was assessed in those with clinical presentation of meningism or neurological symptoms (n = 171). An embedded study evaluated the specificity of IRISA-TB in those whose cerebrospinal fluid was normal (n = 120). D, Scatter plot depicting the performance of IRISA-TB in ruling out tuberculous meningitis (n = 171), analyzed by HIV status and age. Cutoff of 13 pg/mL is depicted by a dashed black line. Abbreviations: AUC, area under the curve; CI, confidence interval; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; IRISA-TB, inter-gam ultrasensitive rapid immuno-suspension assay; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; PWH, people with human immunodeficiency virus.
Specificity of IRISA-TB in Stage 1 Evaluation (Those Whose Pretest Probability of TBM Was Low [South Africa])
To interrogate IRISA-TB's specificity, individuals who presented with meningism or other neurological symptoms, but were not initiated onto TB treatment, were enrolled in Cape Town—that is, were suspected and diagnosed with either viral, bacterial, fungal, or aseptic meningitis (n = 171). An additional embedded evaluation on only those with normal CSF characteristics was included as this information will guide clinicians to explore additional possibilities should the IRISA-TB be negative (n = 120).
In this cohort, a larger majority of adults were PWH (41.7%, P ≤ .0001) and the CSF protein was significantly higher (P = .0349) in comparison to children (Supplementary Table 3). No significant differences were observed in levels of unstimulated IFN-γ when comparing HIV status and age (Supplementary Table 3). IRISA-TB showed superior rule-out value in those with other forms of meningitis and/or normal CSF results (Figure 2C). The performance was unaffected by HIV status or age (Figure 2C and 2D). IRISA-TB has the potential to be a significant rule-out test for TBM.
Performance of IRISA-TB and Xpert Ultra in Stage 2 Evaluation (Malawi and Zimbabwe)
In the large prospective cohort (n = 706 patients with suspected TBM), 20 patients were defined as unclassified and excluded from the downstream analysis. Mortality was higher in those with confirmed TBM (76.2%, P = .0222, Table 1). CSF characteristics were consistent for definite TB individuals with lower glucose (1.9, P ≤ .0001) and increased protein (2.7, P = .0002) (Table 1). Unstimulated IFN-γ was elevated in the definite TB group (44.3, P = .0031, Table 1). Similar significant demographic and clinical parameters were observed when definite and probable TB was compared to confirmed non-TB (Table 1).
Demographic and Clinical Features in the Stage 2 Evaluation (Malawi and Zimbabwe) of Patients With Suspected Tuberculous Meningitis
| Characteristic . | Definite TB (n = 21) . | Non-TB (n = 598) . | Probable TB (n = 8) . | Possible TB (n = 59) . | P Value, Definite TB vs Non-TB . | Definite and Probable TB (n = 29) . | P Value, Definite TB and Probable TB vs Non-TB . |
|---|---|---|---|---|---|---|---|
| Male sex | 16 (76.2%) | 304 (50.8%) | 5 (62.5%) | 32 (54.2%) | .0222 | 21 (72.4%) | .0231 |
| Female sex | 5 (23.8%) | 294 (49.2%) | 3 (37.5%) | 27 (45.8%) | 8 (27.6%) | .0231 | |
| Age, y | 29.5 (24–44.5) | 36 (28–44) | 33 (29.8–39.5) | 38 (30.8–45.3) | ns | 31.5 (24–40) | ns |
| PWH | 14 (66.7%) | 390 (63.8%) | 7 (87.5%) | 48 (81.4%) | ns | 21 (72.4%) | ns |
| HIV uninfected | 6 (28.6%) | 155 (28.1%) | 0 | 6 (10.1%) | ns | 6 (20.7%) | ns |
| HIV status unknown | 1 (4.7%) | 53 (8.1%) | 1 (12.5%) | 5 (8.5%) | ns | 2 (6.9%) | ns |
| CD4 count, cells/mL | 57 (49–124.5) | 60 (16.8–141.8) | 38 (38–38) | 115 (73.25–430) | ns | 81 (43.5–279) | ns |
| CSF glucose, mmol/L | 1.9 (1.2–3.3) | 3.5 (2.9–4.4) | 3.7 (2.85–4.4) | 2.9 (1.55–4.08) | < .0001 | 2.8 (1.7–3.9) | .0005 |
| CSF protein, g/L | 2.7 (1.6–4.3) | 0.43 (0.3–0.9) | 0.56 (0.31–1.33) | 1.2 (0.57–3.33) | .0002 | 1.99 (0.51–3.46) | .0006 |
| CSF lymphocytes, cells/μL | 3 (2–15) | 1 (0–3) | 0 (0–4) | 2 (0–10) | ns | 3 (1.75–6.25) | ns |
| CSF IFN-γ, pg/mL | 44.3 (11.2–131.1) | 1.3 (0–5.4) | 1.22 (0–9.40) | 3.6 (0.27–17.32) | .0031 | 14.88 (4.86–93.3) | .0044 |
| Alive at follow-up | 10 (47.6%) | 473 (79.1%) | 5 (62.5%) | 40 (67.8%) | .0006 | 15 (51.7%) | .0005 |
| Deceased at follow-up | 11 (52.4%) | 110 (18.4%) | 3 (37.5%) | 19 (32.2%) | .0001 | 14 (48.3%) | .0001 |
| Lost to follow-up | 0 | 15 (2.5%) | 0 | 0 | ns | 0 | ns |
| Characteristic . | Definite TB (n = 21) . | Non-TB (n = 598) . | Probable TB (n = 8) . | Possible TB (n = 59) . | P Value, Definite TB vs Non-TB . | Definite and Probable TB (n = 29) . | P Value, Definite TB and Probable TB vs Non-TB . |
|---|---|---|---|---|---|---|---|
| Male sex | 16 (76.2%) | 304 (50.8%) | 5 (62.5%) | 32 (54.2%) | .0222 | 21 (72.4%) | .0231 |
| Female sex | 5 (23.8%) | 294 (49.2%) | 3 (37.5%) | 27 (45.8%) | 8 (27.6%) | .0231 | |
| Age, y | 29.5 (24–44.5) | 36 (28–44) | 33 (29.8–39.5) | 38 (30.8–45.3) | ns | 31.5 (24–40) | ns |
| PWH | 14 (66.7%) | 390 (63.8%) | 7 (87.5%) | 48 (81.4%) | ns | 21 (72.4%) | ns |
| HIV uninfected | 6 (28.6%) | 155 (28.1%) | 0 | 6 (10.1%) | ns | 6 (20.7%) | ns |
| HIV status unknown | 1 (4.7%) | 53 (8.1%) | 1 (12.5%) | 5 (8.5%) | ns | 2 (6.9%) | ns |
| CD4 count, cells/mL | 57 (49–124.5) | 60 (16.8–141.8) | 38 (38–38) | 115 (73.25–430) | ns | 81 (43.5–279) | ns |
| CSF glucose, mmol/L | 1.9 (1.2–3.3) | 3.5 (2.9–4.4) | 3.7 (2.85–4.4) | 2.9 (1.55–4.08) | < .0001 | 2.8 (1.7–3.9) | .0005 |
| CSF protein, g/L | 2.7 (1.6–4.3) | 0.43 (0.3–0.9) | 0.56 (0.31–1.33) | 1.2 (0.57–3.33) | .0002 | 1.99 (0.51–3.46) | .0006 |
| CSF lymphocytes, cells/μL | 3 (2–15) | 1 (0–3) | 0 (0–4) | 2 (0–10) | ns | 3 (1.75–6.25) | ns |
| CSF IFN-γ, pg/mL | 44.3 (11.2–131.1) | 1.3 (0–5.4) | 1.22 (0–9.40) | 3.6 (0.27–17.32) | .0031 | 14.88 (4.86–93.3) | .0044 |
| Alive at follow-up | 10 (47.6%) | 473 (79.1%) | 5 (62.5%) | 40 (67.8%) | .0006 | 15 (51.7%) | .0005 |
| Deceased at follow-up | 11 (52.4%) | 110 (18.4%) | 3 (37.5%) | 19 (32.2%) | .0001 | 14 (48.3%) | .0001 |
| Lost to follow-up | 0 | 15 (2.5%) | 0 | 0 | ns | 0 | ns |
Data are presented as No. (%) or median (interquartile range). Values in bold indicate significance (P ≤ .05).
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IFN-γ, interferon gamma; ns, not significant; PWH, people with human immunodeficiency virus; TB, tuberculosis.
Demographic and Clinical Features in the Stage 2 Evaluation (Malawi and Zimbabwe) of Patients With Suspected Tuberculous Meningitis
| Characteristic . | Definite TB (n = 21) . | Non-TB (n = 598) . | Probable TB (n = 8) . | Possible TB (n = 59) . | P Value, Definite TB vs Non-TB . | Definite and Probable TB (n = 29) . | P Value, Definite TB and Probable TB vs Non-TB . |
|---|---|---|---|---|---|---|---|
| Male sex | 16 (76.2%) | 304 (50.8%) | 5 (62.5%) | 32 (54.2%) | .0222 | 21 (72.4%) | .0231 |
| Female sex | 5 (23.8%) | 294 (49.2%) | 3 (37.5%) | 27 (45.8%) | 8 (27.6%) | .0231 | |
| Age, y | 29.5 (24–44.5) | 36 (28–44) | 33 (29.8–39.5) | 38 (30.8–45.3) | ns | 31.5 (24–40) | ns |
| PWH | 14 (66.7%) | 390 (63.8%) | 7 (87.5%) | 48 (81.4%) | ns | 21 (72.4%) | ns |
| HIV uninfected | 6 (28.6%) | 155 (28.1%) | 0 | 6 (10.1%) | ns | 6 (20.7%) | ns |
| HIV status unknown | 1 (4.7%) | 53 (8.1%) | 1 (12.5%) | 5 (8.5%) | ns | 2 (6.9%) | ns |
| CD4 count, cells/mL | 57 (49–124.5) | 60 (16.8–141.8) | 38 (38–38) | 115 (73.25–430) | ns | 81 (43.5–279) | ns |
| CSF glucose, mmol/L | 1.9 (1.2–3.3) | 3.5 (2.9–4.4) | 3.7 (2.85–4.4) | 2.9 (1.55–4.08) | < .0001 | 2.8 (1.7–3.9) | .0005 |
| CSF protein, g/L | 2.7 (1.6–4.3) | 0.43 (0.3–0.9) | 0.56 (0.31–1.33) | 1.2 (0.57–3.33) | .0002 | 1.99 (0.51–3.46) | .0006 |
| CSF lymphocytes, cells/μL | 3 (2–15) | 1 (0–3) | 0 (0–4) | 2 (0–10) | ns | 3 (1.75–6.25) | ns |
| CSF IFN-γ, pg/mL | 44.3 (11.2–131.1) | 1.3 (0–5.4) | 1.22 (0–9.40) | 3.6 (0.27–17.32) | .0031 | 14.88 (4.86–93.3) | .0044 |
| Alive at follow-up | 10 (47.6%) | 473 (79.1%) | 5 (62.5%) | 40 (67.8%) | .0006 | 15 (51.7%) | .0005 |
| Deceased at follow-up | 11 (52.4%) | 110 (18.4%) | 3 (37.5%) | 19 (32.2%) | .0001 | 14 (48.3%) | .0001 |
| Lost to follow-up | 0 | 15 (2.5%) | 0 | 0 | ns | 0 | ns |
| Characteristic . | Definite TB (n = 21) . | Non-TB (n = 598) . | Probable TB (n = 8) . | Possible TB (n = 59) . | P Value, Definite TB vs Non-TB . | Definite and Probable TB (n = 29) . | P Value, Definite TB and Probable TB vs Non-TB . |
|---|---|---|---|---|---|---|---|
| Male sex | 16 (76.2%) | 304 (50.8%) | 5 (62.5%) | 32 (54.2%) | .0222 | 21 (72.4%) | .0231 |
| Female sex | 5 (23.8%) | 294 (49.2%) | 3 (37.5%) | 27 (45.8%) | 8 (27.6%) | .0231 | |
| Age, y | 29.5 (24–44.5) | 36 (28–44) | 33 (29.8–39.5) | 38 (30.8–45.3) | ns | 31.5 (24–40) | ns |
| PWH | 14 (66.7%) | 390 (63.8%) | 7 (87.5%) | 48 (81.4%) | ns | 21 (72.4%) | ns |
| HIV uninfected | 6 (28.6%) | 155 (28.1%) | 0 | 6 (10.1%) | ns | 6 (20.7%) | ns |
| HIV status unknown | 1 (4.7%) | 53 (8.1%) | 1 (12.5%) | 5 (8.5%) | ns | 2 (6.9%) | ns |
| CD4 count, cells/mL | 57 (49–124.5) | 60 (16.8–141.8) | 38 (38–38) | 115 (73.25–430) | ns | 81 (43.5–279) | ns |
| CSF glucose, mmol/L | 1.9 (1.2–3.3) | 3.5 (2.9–4.4) | 3.7 (2.85–4.4) | 2.9 (1.55–4.08) | < .0001 | 2.8 (1.7–3.9) | .0005 |
| CSF protein, g/L | 2.7 (1.6–4.3) | 0.43 (0.3–0.9) | 0.56 (0.31–1.33) | 1.2 (0.57–3.33) | .0002 | 1.99 (0.51–3.46) | .0006 |
| CSF lymphocytes, cells/μL | 3 (2–15) | 1 (0–3) | 0 (0–4) | 2 (0–10) | ns | 3 (1.75–6.25) | ns |
| CSF IFN-γ, pg/mL | 44.3 (11.2–131.1) | 1.3 (0–5.4) | 1.22 (0–9.40) | 3.6 (0.27–17.32) | .0031 | 14.88 (4.86–93.3) | .0044 |
| Alive at follow-up | 10 (47.6%) | 473 (79.1%) | 5 (62.5%) | 40 (67.8%) | .0006 | 15 (51.7%) | .0005 |
| Deceased at follow-up | 11 (52.4%) | 110 (18.4%) | 3 (37.5%) | 19 (32.2%) | .0001 | 14 (48.3%) | .0001 |
| Lost to follow-up | 0 | 15 (2.5%) | 0 | 0 | ns | 0 | ns |
Data are presented as No. (%) or median (interquartile range). Values in bold indicate significance (P ≤ .05).
Abbreviations: CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IFN-γ, interferon gamma; ns, not significant; PWH, people with human immunodeficiency virus; TB, tuberculosis.
At a cut-off point of 13 pg/mL, the sensitivity, specificity, and NPV of IRISA-TB was 76.2%, 91.4%, and 99%, respectively (Figure 3A). The test sensitivity, although not significant, improved in PWH (Figure 3B; AUC = 0.93). A 76% sensitivity from a same-day diagnostic test represents a major advance in TBM diagnosis.
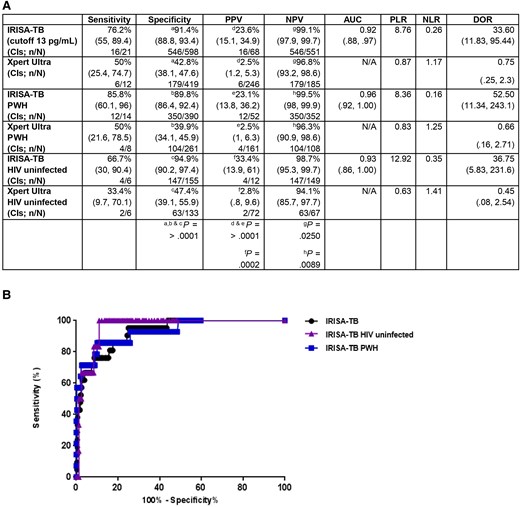
IRISA-TB performance (definite and non-tuberculosis) with trace readouts included (considered positive). A, Performance of IRISA-TB and Xpert Ultra in stage 2 evaluation, analyzed by human immunodeficiency virus (HIV) status (P < .05). B, Receiver operating characteristic curve of IRISA-TB performance in the stage 2 cohort analyzed by HIV status. Trace results were considered a positive Xpert Ultra in both A and B. Abbreviations: AUC, area under the curve; CI, confidence interval; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; IRISA-TB, inter-gam ultrasensitive rapid immuno-suspension assay; N/A, not applicable; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; PWH, people with human immunodeficiency virus.
In the prospective cohort, IRISA-TB showed superior performance in PWH (Supplementary Table 4).
In this cohort, Xpert Ultra specificity was low (91.4% vs 42.8%, P > .0001; Figure 3B). Many of the Xpert Ultra trace results were “false positives” (verified using Sanger sequencing of the cartridge-generated amplicon) (Figure 4A). GeneXpert Ultra cartridge results are enumerated along a semi-quantitative scale (high, medium, low, and very low based on cycle threshold values). A “trace readout” is also possible, implying a borderline signal at the threshold of detection (implying likely very low levels of DNA). These results show that the performance of Xpert Ultra, at least in TBM, has been overestimated; the PPV of the trace result in CSF was 27.8% (Figure 4A). As a control, sputum samples subjected to Xpert Ultra were also validated by Sanger sequencing and the predictive value was 100% (Figure 4B). Therefore, the trace results were considered a negative result, and performance of IRISA-TB was compared to Xpert Ultra (Table 2). IRISA-TB outperformed Xpert Ultra in sensitivity, specificity, and PPV (76.2% vs 25%, P = .0048; 91.4% vs 86.9%, P = .0210; 23.6% vs 5.2%, P = .0042, respectively) (Table 2).

Clarification of Xpert Ultra Trace readouts using Sanger Sequencing. A, The positive predictive value of the Xpert Trace result in cerebrospinal fluid, using available retained cartridges, was 27.8%. B, As a control and to validate the value of the sequencing approach, sputum samples subjected to Xpert Ultra were also validated by Sanger sequencing. The predictive value of Xpert Ultra was 100%. Abbreviations: Mtb, Mycobacterium tuberculosis; TBM, tuberculous meningitis.
IRISA-TB Performance (Definite and Non-Tuberculosis) With Trace Readouts Excluded (Considered Negative)
| Assay . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | PLR . | NLR . | DOR (95% CI) . |
|---|---|---|---|---|---|---|---|
| IRISA-TB (cutoff 13 pg/mL) | 76.2 (55–89.4)a n = 16/21 | 91.4 (88.8–93.4)b n = 546/598 | 23.6 (15.1–34.9)c n = 16/68 | 99.1 (97.9–99.7) n = 546/551 | 8.76 | 0.26 | 33.60 (11.83–95.44) |
| Xpert Ultra | 25 (8.9–53.3)a n = 3/12 | 86.9 (83.4–89.8)b n = 364/419 | 5.2 (1.8–14.2)c n = 3/58 | 97.6 (95.5–98.8) n = 364/373 | 1.95 | 0.86 | 2.206 (.58–7.7) |
| Among people with HIV | |||||||
| IRISA-TB (cutoff 13 pg/mL) | 85.8 (60.1–96)d n = 12/14 | 89.8 (86.4–92.4) n = 350/390 | 23.1 (13.8–36.2)e n = 12/52 | 99.5 (98–99.9)f n = 350/352 | 8.36 | 0.16 | 52.50 (11.34–243.1) |
| Xpert Ultra | 25 (7.2–59.1)d n = 2/8 | 87.8 (83.3–91.2) n = 229/261 | 5.9 (1.7–19.1)e n = 2/34 | 97.5 (94.6–98.9)f n = 229/235 | 2.04 | 0.85 | 2.385 (.46–12.33) |
| Among HIV uninfected | |||||||
| IRISA-TB (cutoff 13 pg/m) | 66.7 (3090.4) 4/6 | 94.9 (90.297.4)g 147/155 | 33.4 (13.961)h 4/12 | 98.7 (95.399.7) 147/149 | 12.92 | 0.35 | 36.75 (5.83231.6) |
| Xpert Ultra | 16.7 (3.1–56.4) n = 1/6 | 83.5 (76.3–88.9)g n = 111/133 | 4.4 (.8–21)h n = 1/23 | 95.7 (90.4–98.2) n = 111/116 | 1.008 | 0.998 | 1.009 (.11–9.07) |
| Assay . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | PLR . | NLR . | DOR (95% CI) . |
|---|---|---|---|---|---|---|---|
| IRISA-TB (cutoff 13 pg/mL) | 76.2 (55–89.4)a n = 16/21 | 91.4 (88.8–93.4)b n = 546/598 | 23.6 (15.1–34.9)c n = 16/68 | 99.1 (97.9–99.7) n = 546/551 | 8.76 | 0.26 | 33.60 (11.83–95.44) |
| Xpert Ultra | 25 (8.9–53.3)a n = 3/12 | 86.9 (83.4–89.8)b n = 364/419 | 5.2 (1.8–14.2)c n = 3/58 | 97.6 (95.5–98.8) n = 364/373 | 1.95 | 0.86 | 2.206 (.58–7.7) |
| Among people with HIV | |||||||
| IRISA-TB (cutoff 13 pg/mL) | 85.8 (60.1–96)d n = 12/14 | 89.8 (86.4–92.4) n = 350/390 | 23.1 (13.8–36.2)e n = 12/52 | 99.5 (98–99.9)f n = 350/352 | 8.36 | 0.16 | 52.50 (11.34–243.1) |
| Xpert Ultra | 25 (7.2–59.1)d n = 2/8 | 87.8 (83.3–91.2) n = 229/261 | 5.9 (1.7–19.1)e n = 2/34 | 97.5 (94.6–98.9)f n = 229/235 | 2.04 | 0.85 | 2.385 (.46–12.33) |
| Among HIV uninfected | |||||||
| IRISA-TB (cutoff 13 pg/m) | 66.7 (3090.4) 4/6 | 94.9 (90.297.4)g 147/155 | 33.4 (13.961)h 4/12 | 98.7 (95.399.7) 147/149 | 12.92 | 0.35 | 36.75 (5.83231.6) |
| Xpert Ultra | 16.7 (3.1–56.4) n = 1/6 | 83.5 (76.3–88.9)g n = 111/133 | 4.4 (.8–21)h n = 1/23 | 95.7 (90.4–98.2) n = 111/116 | 1.008 | 0.998 | 1.009 (.11–9.07) |
Performance of IRISA-TB and Xpert Ultra in stage-2 cohort, analyzed by HIV status (P < .05). Trace results were considered a negative Xpert Ultra.
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
aP = .0048.
bP = .0210.
cP = .0042.
dP = .0053.
eP = .0358.
fP = .0363.
gP = .0016.
hP = .0220.
IRISA-TB Performance (Definite and Non-Tuberculosis) With Trace Readouts Excluded (Considered Negative)
| Assay . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | PLR . | NLR . | DOR (95% CI) . |
|---|---|---|---|---|---|---|---|
| IRISA-TB (cutoff 13 pg/mL) | 76.2 (55–89.4)a n = 16/21 | 91.4 (88.8–93.4)b n = 546/598 | 23.6 (15.1–34.9)c n = 16/68 | 99.1 (97.9–99.7) n = 546/551 | 8.76 | 0.26 | 33.60 (11.83–95.44) |
| Xpert Ultra | 25 (8.9–53.3)a n = 3/12 | 86.9 (83.4–89.8)b n = 364/419 | 5.2 (1.8–14.2)c n = 3/58 | 97.6 (95.5–98.8) n = 364/373 | 1.95 | 0.86 | 2.206 (.58–7.7) |
| Among people with HIV | |||||||
| IRISA-TB (cutoff 13 pg/mL) | 85.8 (60.1–96)d n = 12/14 | 89.8 (86.4–92.4) n = 350/390 | 23.1 (13.8–36.2)e n = 12/52 | 99.5 (98–99.9)f n = 350/352 | 8.36 | 0.16 | 52.50 (11.34–243.1) |
| Xpert Ultra | 25 (7.2–59.1)d n = 2/8 | 87.8 (83.3–91.2) n = 229/261 | 5.9 (1.7–19.1)e n = 2/34 | 97.5 (94.6–98.9)f n = 229/235 | 2.04 | 0.85 | 2.385 (.46–12.33) |
| Among HIV uninfected | |||||||
| IRISA-TB (cutoff 13 pg/m) | 66.7 (3090.4) 4/6 | 94.9 (90.297.4)g 147/155 | 33.4 (13.961)h 4/12 | 98.7 (95.399.7) 147/149 | 12.92 | 0.35 | 36.75 (5.83231.6) |
| Xpert Ultra | 16.7 (3.1–56.4) n = 1/6 | 83.5 (76.3–88.9)g n = 111/133 | 4.4 (.8–21)h n = 1/23 | 95.7 (90.4–98.2) n = 111/116 | 1.008 | 0.998 | 1.009 (.11–9.07) |
| Assay . | Sensitivity, % (95% CI) . | Specificity, % (95% CI) . | PPV, % (95% CI) . | NPV, % (95% CI) . | PLR . | NLR . | DOR (95% CI) . |
|---|---|---|---|---|---|---|---|
| IRISA-TB (cutoff 13 pg/mL) | 76.2 (55–89.4)a n = 16/21 | 91.4 (88.8–93.4)b n = 546/598 | 23.6 (15.1–34.9)c n = 16/68 | 99.1 (97.9–99.7) n = 546/551 | 8.76 | 0.26 | 33.60 (11.83–95.44) |
| Xpert Ultra | 25 (8.9–53.3)a n = 3/12 | 86.9 (83.4–89.8)b n = 364/419 | 5.2 (1.8–14.2)c n = 3/58 | 97.6 (95.5–98.8) n = 364/373 | 1.95 | 0.86 | 2.206 (.58–7.7) |
| Among people with HIV | |||||||
| IRISA-TB (cutoff 13 pg/mL) | 85.8 (60.1–96)d n = 12/14 | 89.8 (86.4–92.4) n = 350/390 | 23.1 (13.8–36.2)e n = 12/52 | 99.5 (98–99.9)f n = 350/352 | 8.36 | 0.16 | 52.50 (11.34–243.1) |
| Xpert Ultra | 25 (7.2–59.1)d n = 2/8 | 87.8 (83.3–91.2) n = 229/261 | 5.9 (1.7–19.1)e n = 2/34 | 97.5 (94.6–98.9)f n = 229/235 | 2.04 | 0.85 | 2.385 (.46–12.33) |
| Among HIV uninfected | |||||||
| IRISA-TB (cutoff 13 pg/m) | 66.7 (3090.4) 4/6 | 94.9 (90.297.4)g 147/155 | 33.4 (13.961)h 4/12 | 98.7 (95.399.7) 147/149 | 12.92 | 0.35 | 36.75 (5.83231.6) |
| Xpert Ultra | 16.7 (3.1–56.4) n = 1/6 | 83.5 (76.3–88.9)g n = 111/133 | 4.4 (.8–21)h n = 1/23 | 95.7 (90.4–98.2) n = 111/116 | 1.008 | 0.998 | 1.009 (.11–9.07) |
Performance of IRISA-TB and Xpert Ultra in stage-2 cohort, analyzed by HIV status (P < .05). Trace results were considered a negative Xpert Ultra.
Abbreviations: CI, confidence interval; DOR, diagnostic odds ratio; HIV, human immunodeficiency virus; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
aP = .0048.
bP = .0210.
cP = .0042.
dP = .0053.
eP = .0358.
fP = .0363.
gP = .0016.
hP = .0220.
DISCUSSION
We evaluated the diagnostic performance of a DNA-based assay (GeneXpert Ultra) compared to a new same-day immunodiagnostic assay that detects unstimulated IFN-γ in CSF (IRISATB). In the stage 1 evaluation, IRISATB sensitivity and specificity were promising. The key findings in the stage 2 evaluation were that (1) IRISATB sensitivity for diagnosis of definite TBM was 76% and was significantly higher than that of Xpert Ultra (25% when trace readouts were excluded), but specificity was similar for both assays (∼90%); (2) the PPV of the trace readout for TB was approximately 28% and Xpert Ultra specificity dropped substantially to approximately 40% when trace results were included in the analysis; and (3) sensitivity of IRISATB was higher in PWH and uninfected persons (86% vs 67%).
Xpert Ultra sensitivity for the diagnosis of TBM in our study, with inclusion of the trace readout, was approximately 50% and in keeping with other large recent studies like those performed in Vietnam, China, and India [10–12]. Trace readouts suggest the finding of extremely low levels of DNA around the detection threshold of the assay, but trace readouts may also be falsely positive due to technical reasons including stochastic fluorophore emission around limit of detection. However, when trace readouts were excluded (ie, considered negative) in our study, sensitivity dropped to 27%. This may not be surprising as other studies in TBM have shown that approximately 40% of samples where DNA was detected have a trace readout [5, 13, 14]. In our primary analysis, we excluded trace readouts as, based on our sequencing results, the PPV of the trace readout was only approximately 25%. This implies that most trace readouts were likely false positives (though it should be emphasized that the sequencing sample size was small). This is in keeping with other studies in pulmonary TB. In a large South African study of >2500 participants, there were >700 samples with a trace readout, and only 10% of these were culture positive [13]. Similarly, in a Zambian study of patients with pulmonary TB, of 33 trace readouts in 144 Xpert-positive individuals, only 8% were culture positive [26]. Trace results may also be falsely positive in those with previous TB [14]. There was also some DNA detected in the non-TBM group (trace results and/or positive sequencing), and we are unclear if these were cases of laboratory contamination or self-resolving TBM. Thus, in countries like South Africa, given the uncertainty of what trace results mean and that they may be falsely positive, results in this category are reported as “trace” by the National Health Laboratory Service rather than a positive result, leaving it up to the clinician to initiate treatment depending on the pretest probability of disease. Only 1 study, to our knowledge, sequenced PCR product in individuals with trace readouts. Bahr and colleagues, the first to investigate Xpert Ultra in those with suspected TBM, found that of only 4 cartridge-specific PCR products that were sequenced, no DNA was detected in 1 sample (in this study, 9 of 21 [43%] of TBM samples where DNA was detected by Xpert Ultra were trace) [27]. However, this, like in our study, could simply reflect a small sample size. In another study, 14 of 39 (36%) of Xpert Ultra results where DNA was detected in CSF were trace readouts (and only 6 of the 14 trace readouts were positive by culture) [28]. Unfortunately, in our study a limited number of cartridges were retained from 1 study site for sequencing and the number of samples sequenced were constrained by study budgets. Thus, the significance of trace readouts in the broader context of TBM remains unclear and future studies should address this issue. In our own study, inclusion of trace readouts substantially reduced specificity, whereas in other published studies, specificity when trace readouts were included was approximately 91% (95%CI, 83%–95%) based on a systematic review and meta-analysis incorporating several studies [8]. Reasons for the surprisingly more marked drop in specificity in our study, when trace readouts were included, remain unclear. However, to verify the robustness of our findings, we checked the sequencing specificity using sputum samples and repeat tested a large batch of CSF samples, and the results remained unchanged. It is possible (we speculate) that in our population with a high proportion of HIV-infected participants, where there is a high rate of previous TB and/or subclinical forms of TB, this may have influenced the high rates of trace readouts. However, this aspect needs verification in future studies.
IFN-γ had a sensitivity of 76% with a reasonably high specificity of 91%. These findings are in keeping with 5 studies included in the systematic review and meta-analysis by Shi et al, which found a combined sensitivity and specificity of 86% and 92%, respectively [22]. However, until now, there has been no low-cost standardized same-day single-use assay to measure unstimulated IFN-γ and CSF. The IRISATB assay has shown good performance for the diagnosis of pericardial TB and pleural TB when using pleural biopsy as a reference standard (sensitivity of 90%–95% and specificity of ≥95%) [16, 17, 24, 25]. Mycobacterial antigens drive a potent T-helper 1 (Th1) response [20, 29, 30], and for reasons not completely understood, IFN-γ and Th1 cytokines remain “trapped” within compartments, in contradistinction to other infectious and inflammatory diseases. IRISATB uses specific antibody pairs that detect IFN-γ in compartmentalized fluid with improved accuracy compared to whole blood, where pH, chemistry, and the concentration heterophile molecules (which interfere with antigen antibody binding and hence detection capability) are vastly different [31]. The very typical host response seen in TB (characterized by high levels of Th1 cytokines and chemokines, and type 1 interferon responses) has been exploited for the immune diagnosis of TB in many different contexts including TB serositis, that is, pericardial and pleural and peritoneal TB [16, 17, 19, 20, 24], TB meningitis [22], active TB (blood transcriptomic signature) [32], and incipient TB (blood transcriptomic signatures) [33]. Indeed, Bahr and colleagues have elegantly outlined that an immunodiagnostic approach, using host biomarkers, might be the optimal one for the diagnosis of paucibacillary compartmentalized disease like in TB meningitis and TB serositis [34].
Our study had several strengths including the fact that patients in our study were carefully categorized using strict definitions and that it was multicentric and involving different population subgroups including PWH. The latter form a vulnerable group where TBM mortality is approximately 50%; therefore, rapid same-day diagnosis in this group is particularly important, especially because the performance of other diagnostic markers is often suboptimal. Interestingly, an as seen in other studies involving TB serositis [16, 17, 24], IRISATB has a higher sensitivity in PWH with TBM (86%). We speculate that the latter may be due to a higher mycobacterial load in such persons and that there are still high levels of antigen-specific lymphocytes at the site of disease despite low CD4 counts in peripheral blood [20, 29, 30].
There are also several limitations of our study. First, the sample size of those with definite TBM was small, resulting in wide CIs of our estimates. However, the number of participants recruited (73 patients with definite or probable TB and approximately 760 with non-TB) is one of the largest published studies on TBM. Second, we were unable to determine the predictive value of trace readouts with a great amount of certainty given the small number of samples sequenced. However, we have demonstrated that the predictive value of trace readouts may be low and highly variable, and we are currently in the process of undertaking a large study sequencing the product of Ultra cartridges so that a better determination can be made. An additional consideration is that in some trace Ultra cartridge, extracts may have been below the detection limit of the sequencing assay despite having contained amplified TB-specific DNA. Indeed, we know from newer targeted sequencing platforms like AmPORE TB (Oxford Nanopore) that although detection of amplified DNA in smear-negative sputum samples can be as high as 70% and hence 90% in all samples (data unpublished at the time of writing), there are approximately 10% of samples in which the amount of TB DNA is below the detection limit of the assay despite the presequencing amplification step. Nevertheless, that overall effect of this consideration is likely to be small. Finally, the clinical impact of using an immunodiagnostic assay such IRISATB in clinical practice remains unclear. To address this question, a large prospective clinical trial is currently underway [35].
In conclusion, measurement of unstimulated IFN-γ in CSF is a promising immunodiagnostic approach for the diagnosis of TBM. These findings require validation in larger multicentric studies.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. Although the IRISA-TB kits were donated by Antrum Biotech, Antrum Biotech, or its associates or employees, had no role in recruitment of the patients, classification of the patient subgroups, or final analysis and presentation of the data.
Financial support. This study was funded by the South African Network of Biosciences (BioFISA II Programme Flagship Grant: V6LFISA/2016/SFG001) awarded to Antrum Biotech, a University of Cape Town associated start-up, with a subaward to the University of Cape Town Lung Institute, Biomedical Research and Training Institute in Zimbabwe, and College of Medicine, University of Malawi.
References
Author notes
Potential conflicts of interest. All authors: No reported conflicts.





Comments