-
PDF
- Split View
-
Views
-
Cite
Cite
Bin Cai, Glenn Tillotson, Darrin Benjumea, Patrick Callahan, Roger Echols, The Burden of Bloodstream Infections due to Stenotrophomonas Maltophilia in the United States: A Large, Retrospective Database Study, Open Forum Infectious Diseases, Volume 7, Issue 5, May 2020, ofaa141, https://doi.org/10.1093/ofid/ofaa141
Close - Share Icon Share
Abstract
Stenotrophomonas maltophilia is an opportunistic pathogen observed in both nosocomial and community-onset infections. S. maltophilia is intrinsically resistant to many currently available broad-spectrum antibiotics and is often not included in antimicrobial resistance surveillance studies or stewardship programs’ guidelines.
A retrospective cohort study of patients with S. maltophilia bloodstream infection (BSI) in the United States was conducted using the 2010–2015 US Premier Healthcare Database. This study described patient characteristics, infection characteristics, antibiotic treatment, and discharge status.
S. maltophilia was the most common carbapenem-resistant, gram-negative pathogen causing BSIs in this database. Of 486 unique patients with S. maltophilia BSI, 44.6% were assessed as community-onset, 95% of cultures were susceptible to trimethoprim-sulfamethoxazole (TMP-SMX), and 84% were susceptible to fluoroquinolones; 39.1% of patients received a potentially effective antibiotic (fluoroquinolone, doxycycline, ceftazidime, minocycline, or TMP-SMX) during the empiric treatment period (≤3 days post–index culture date), whereas 85.8% received a potential effective antibiotics during the definitive treatment period. The most common antibiotic received as definitive treatment was levofloxacin (48.9%). TMP-SMX was used infrequently empirically (10.5%) and in 38.3% during the definitive period. Compared with BSIs caused by other carbapenem-resistant gram-negative pathogens, S. maltophilia BSIs were more likely to be community-onset, and were more likely to be discharged to home and to have a lower mortality rate.
This study demonstrated that patients at risk for S. maltophilia BSI are highly variable and that standard of care is not clearly defined, leading to questions regarding the appropriateness of antibiotic treatment among patients. Further efforts are needed to better recognize and treat S. maltophilia BSI.
Stenotrophomonas maltophilia is a glucose-nonfermenting, gram-negative bacillus that has emerged as a serious opportunistic pathogen, particularly among critically ill and immunocompromised patients [1, 2]. Though historically identified as a cause of nosocomial infections, community-onset infections increasingly are being reported [3]. From 1996 to 2016, S. maltophilia was commonly isolated from patients hospitalized with pneumonia and bloodstream infection (BSI) [4], and the incidence of S. maltophilia infection is increasing [4–6].
S. maltophilia is intrinsically resistant to most currently available broad-spectrum antibiotics, including carbapenems and beta-lactams [7–9]. This intrinsic resistance is due to the presence of chromosomally expressed beta-lactamases, L1 (a metallo-carbapenemase), and L2 (an extended-spectrum beta-lactamases), which together can hydrolyze nearly all beta-lactam antibiotics including carbapenems. In vitro activity is observed with tetracyclines and fluoroquinolones, but resistance can be rapidly induced mainly due to efflux pumps [4, 10]. Trimethoprim/sulfamethoxazole (TMP-SMX) generally is considered the treatment of choice for S. maltophilia infection, although consideration of S. maltophilia in treatment guidelines is sparse [5, 11, 12]. Additionally, a recent study suggests that S. maltophilia susceptibility to TMP-SMX may be decreasing globally, due in part to acquired antibiotic resistence [4, 10]. Fluoroquinolones may be alternatives for TMP-SMX treatment in S. maltophilia infections due to their comparable effects on mortality [13].
Because S. maltophilia is intrinsically resistant to most antibiotics, it is not included in antimicrobial resistance surveillance studies because only the acquired resistance meets the consensus definition of multidrug-resistant (MDR) and extensively drug-resistant (XDR) bacteria [14–16]. Only a limited number of antibiotics are considered for in vitro susceptibility testing of S. maltophilia [17].
Assessing the pathogenicity of S. maltophilia is difficult. Clinical isolates from the respiratory tract sources are often mixed with other pathogenic bacteria, and distinguishing colonization from a true infection caused by S. maltophilia is problematic [18]. Much of the observational clinical reports of S. maltophilia infections have focused on bloodstream infections (BSIs) where convincing evidence of pathogenicity can be determined [19].
Despite rising global incidence and patterns of resistance among S. maltophilia BSIs, no broad, population-based studies of S. maltophilia in the United States have been conducted. Such information is necessary to understand associated risk factors, antibiotic treatment patterns, and outcomes of S. maltophilia BSIs. The purpose of this study was to identify patient characteristics, microbiology susceptibility, and treatment decisions for patients with S. maltophilia BSI in a large, geographically diverse sample of US hospitals.
METHODS
Study Design and Data Source
A retrospective analysis of characteristics and outcomes in patients with S. maltophilia–positive blood cultures was conducted using the Premier Healthcare Database (PHD; Premier Inc., Charlotte, NC, USA) [20]. PHD is a large, US-based, service-level, all-payer database that contains administrative, health care utilization, and financial data from patient encounters for >231 million unique patients sourced from >1000 contributing hospital/healthcare systems [20]. Hospitals included in PHD cover a range of geographically diverse facilities, including a mix of academic, community, and large hospital systems, representing ~25% of annual US inpatient admissions [20]. Patient hospital encounters, including diagnosis and procedures, are coded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) classification system [20].
This study sourced data from a subset of the PHD that includes 181 hospitals with microbiology laboratory data such as specimen source, pathogen identification, and antibiotic susceptibility test results. Patient, hospital, and microbiology characteristics, antibiotic treatments, infection-associated lengths of stay, and discharge status were assessed.
Study Population
Unique non–cystic fibrosis adult (≥18 years of age) inpatients with positive blood cultures for any gram-negative pathogen from October 2, 2010, to September 30, 2015, were included in this analysis. Specific focus was placed on patients with S. maltophilia BSI. If patients had multiple admissions with a positive blood culture for S. maltophilia, the first record was used as the index culture, and the remainder were excluded from analysis. The draw date of the first positive blood culture for S. maltophilia is considered the index date, and results in this analysis are based on index cultures.
Measures and Outcomes
Subject Demographics
The demographics of patients with S. maltophilia BSI, including age at the time of hospital admission, race, sex, admitting ICD-9 diagnosis codes, primary ICD-9 diagnosis codes at discharge, Charlson comorbidity Index (CCI) score and categories based on ICD-9 diagnoses codes, and source of admission were captured in this analysis.
Microbiology Characteristics
Each BSI was classified as hospital-acquired, health care–associated, or community-onset, using previously published definitions [21, 22]. Infections with an index date >3 days after admission were classified as hospital-acquired infections. Infections with an index date of ≤3 days following admission and evidence of recent contact with a health care setting (such as having transferred from health care facilities or having had a hospitalization in the same hospital within the last 30 days) were classified as health care–associated infections. Infections with an index date that was ≤3 days after hospital admission with no evidence of previous contact with a health care setting were classified as community-onset.
In vitro susceptibility testing of S. maltophilia isolates to carbapenems is not recommended due to intrinsic resistance, and therefore all S. maltophilia isolates were a priori defined as carbapenem-resistant (CR) [17].
Antibiotic Treatments
Based on the presumed availability of identification and susceptibility testing of the positive blood isolate, antibiotic use was divided into 3 time periods: before index date, empiric treatment (index date + 3 days), and definitive treatment (≥4 days post–index date). These treatment periods were used as a proxy for clinical decision-making regarding the choice of antibiotic therapy and antibiotic stewardship. The empiric treatment, often utilizing broad-spectrum antimicrobial agents, represents the initial antibiotic therapy to cover multiple possible pathogens. Once physicians have knowledge of the identified pathogen, definitive treatment may be more pathogen-targeted.
TMP-SMX, fluoroquinolones (levofloxacin or ciprofloxacin), doxycycline, ceftazidime, and minocycline were considered appropriate antibiotics because they are included in Clinical and Laboratory Standards Institute (CLSI) susceptibility testing for S. maltophilia or they have high in vitro activity against S. maltophilia. Use of these products in empiric and definitive treatment periods was tabulated to assess the proportion of patients likely receiving appropriate treatment.
Infection Course
The infection course was characterized by 3 components: (1) classification of infection onset (community-onset, health care–associated, or hospital-acquired), (2) infection-associated length of stay (LOS), and (3) discharge status. Infection-associated LOS was defined as the number of days between the index date and discharge date. Discharge status included in-hospital mortality, discharge to home or hospice, or transfer to another health care facility (eg, skilled nursing facility, long-term acute care hospital).
Statistical Analysis
Descriptive analyses summarizing the study population, antibiotic use, and infection course including discharge status were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). Continuous variables were summarized using mean, median, and range. Categorical variables were summarized as frequencies and percentages.
RESULTS
Subject Demographics and Hospital Characteristics
From October 2010 to September 2015, a total of 52 285 inpatient blood culture samples from 46 381 patients tested positive for gram-negative pathogens, and 1602 (3.06%) of the patients were CR. Among all patients with CR gram-negative BSIs, S. maltophilia was the most common (n = 486), followed by Pseudomonas aeruginosa (n = 379), Klebsiella pneumoniae (n = 253), and Acinetobacter baumannii (n = 235) (Figure 1).
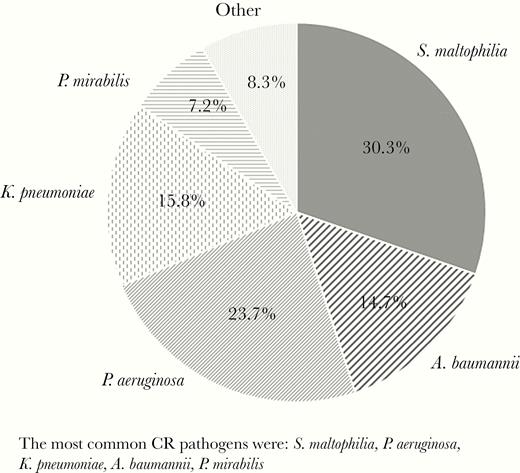
Carbapenem-resistant (CR), gram-negative pathogens causing bacteremia in the United States, based on the 1602 patients identified. The most common CR pathogens were Stenotrophomonas maltophilia, Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii, and Proteus mirabilis.
The mean age at admission for S. maltophilia BSI patients (SD) was 57.2 (17.0) years. Most patients were white (67.1%), with 19.1% black, and about half were female (52.2%). Over 77% of patients came from home before hospital admission, and 10.5% of patients were transferred from another health care facility (Table 1; Supplementary Table 1). The most common diagnosis at admission was an unspecified fever (12.3%); upon discharge, the most common diagnosis was BSI due to venous catheter (14.4%) (Supplementary Table 2). Nearly 89% were from urban hospitals, and 86.0% were in hospitals with ≥200 beds.
| Characteristic . | No. . | % . |
|---|---|---|
| Total number of patients with Stenotrophomonas maltophilia BSI . | 486 . | 100 . |
| Age at index, y | ||
| Mean (SD) | 52.2 (17.0) | |
| Median (min–max) | 53 (18–89) | |
| Gender | ||
| Male | 233 | 47.9 |
| Race | ||
| White | 326 | 67.1 |
| Black | 93 | 19.1 |
| Hispanic | 2 | 0.4 |
| Other | 64 | 13.2 |
| Unknown | 1 | 0.2 |
| Source of admission | ||
| Ambulatory | 409 | 84.2 |
| Transferred from another health care facility | 51 | 10.5 |
| Unknown | 26 | 5.3 |
| Classification of infection | ||
| Community-onset infection | 217 | 44.6 |
| Health care–associated infection | 32 | 6.6 |
| Hospital-acquired infection | 237 | 48.8 |
| Charlson comorbidity index | ||
| Mean (SD) | 3.05 (2.59) | |
| Median (min–max) | 3 (0–13) |
| Characteristic . | No. . | % . |
|---|---|---|
| Total number of patients with Stenotrophomonas maltophilia BSI . | 486 . | 100 . |
| Age at index, y | ||
| Mean (SD) | 52.2 (17.0) | |
| Median (min–max) | 53 (18–89) | |
| Gender | ||
| Male | 233 | 47.9 |
| Race | ||
| White | 326 | 67.1 |
| Black | 93 | 19.1 |
| Hispanic | 2 | 0.4 |
| Other | 64 | 13.2 |
| Unknown | 1 | 0.2 |
| Source of admission | ||
| Ambulatory | 409 | 84.2 |
| Transferred from another health care facility | 51 | 10.5 |
| Unknown | 26 | 5.3 |
| Classification of infection | ||
| Community-onset infection | 217 | 44.6 |
| Health care–associated infection | 32 | 6.6 |
| Hospital-acquired infection | 237 | 48.8 |
| Charlson comorbidity index | ||
| Mean (SD) | 3.05 (2.59) | |
| Median (min–max) | 3 (0–13) |
Abbreviation: BSI, bloodstream infection.
| Characteristic . | No. . | % . |
|---|---|---|
| Total number of patients with Stenotrophomonas maltophilia BSI . | 486 . | 100 . |
| Age at index, y | ||
| Mean (SD) | 52.2 (17.0) | |
| Median (min–max) | 53 (18–89) | |
| Gender | ||
| Male | 233 | 47.9 |
| Race | ||
| White | 326 | 67.1 |
| Black | 93 | 19.1 |
| Hispanic | 2 | 0.4 |
| Other | 64 | 13.2 |
| Unknown | 1 | 0.2 |
| Source of admission | ||
| Ambulatory | 409 | 84.2 |
| Transferred from another health care facility | 51 | 10.5 |
| Unknown | 26 | 5.3 |
| Classification of infection | ||
| Community-onset infection | 217 | 44.6 |
| Health care–associated infection | 32 | 6.6 |
| Hospital-acquired infection | 237 | 48.8 |
| Charlson comorbidity index | ||
| Mean (SD) | 3.05 (2.59) | |
| Median (min–max) | 3 (0–13) |
| Characteristic . | No. . | % . |
|---|---|---|
| Total number of patients with Stenotrophomonas maltophilia BSI . | 486 . | 100 . |
| Age at index, y | ||
| Mean (SD) | 52.2 (17.0) | |
| Median (min–max) | 53 (18–89) | |
| Gender | ||
| Male | 233 | 47.9 |
| Race | ||
| White | 326 | 67.1 |
| Black | 93 | 19.1 |
| Hispanic | 2 | 0.4 |
| Other | 64 | 13.2 |
| Unknown | 1 | 0.2 |
| Source of admission | ||
| Ambulatory | 409 | 84.2 |
| Transferred from another health care facility | 51 | 10.5 |
| Unknown | 26 | 5.3 |
| Classification of infection | ||
| Community-onset infection | 217 | 44.6 |
| Health care–associated infection | 32 | 6.6 |
| Hospital-acquired infection | 237 | 48.8 |
| Charlson comorbidity index | ||
| Mean (SD) | 3.05 (2.59) | |
| Median (min–max) | 3 (0–13) |
Abbreviation: BSI, bloodstream infection.
Microbiology Characteristics and Timing of Infection
Nearly 45% (n = 217) of S. maltophilia BSIs were assessed as community-onset, meaning that the index date was within 3 days of admission without previously identified health care–related contact (Table 1), and 161 (74.2%) community-onset infections were present upon admission (day 0 or 1). An additional 6.6% (n = 32) of patients were assessed as health care–associated infections, meaning that the index date was within 3 days of admission among those transferred from another health care facility or who had a prior hospitalization at the same hospital within the last 30 days. Less than half (n = 237, 48.8%) were considered to have a hospital-acquired infection. In most patients (n = 443, 91.2%), the original source of the S. maltophilia BSI was not identified, with the remainder (n = 43, 8.8%) having prior or concomitant S. maltophilia respiratory infection.
Pathogen isolates were tested for antimicrobial susceptibility following the CLSI M100 guidelines [23]. In this sample, S. maltophilia isolates were most frequently tested for susceptibility to TMP-SMX and fluoroquinolones (95% and 83% of tested isolates, respectively). For isolates tested for TMP-SMX, 95% were susceptible, and in those tested for fluoroquinolone (either ciprofloxacin or levofloxacin), 84% were susceptible (Supplementary Table 3). Although tetracycline, including minocycline and tigecycline, was tested for susceptibility in only 15% of cultures, 91.8% of isolates were found to be susceptible. The antibiotic agents with the lowest rates of susceptibility were penicillin/beta-lactamase inhibitors (42%) and third-generation cephalosporin (44%). Additional therapies may have been tested but unreported.
Antibiotic Treatment Patterns
Antibiotic utilization over the study period is described in Table 2. The rate of oral or parenteral antibiotics prescribed is described for 2 different periods: in the empiric period of treatment and in the definitive period of treatment. Only treatments with activity against gram-negative pathogens are included in this report. In the empiric period, piperacillin/tazobactam was the most commonly used (32.3%), followed by cefepime (24.7%) and levofloxacin (19.3%) (Table 2). In the definitive period, levofloxacin (48.9%) and/or TMP-SMX (38.3%) were most commonly used. Patients receiving appropriate treatment (ie, any use of a fluoroquinolone, ceftazidime, doxycycline, minocycline, or TMP-SMX) increased from 39.1% during the empiric period to 85.8% in the definitive period (Table 2). Interestingly, a large percentage of patients continued to receive a carbapenem in the definitive period (5.0%–14.0%).
Gram-Negative Treatment Administration by Percentage of Patients Across 2 Time Intervals: Empiric Treatment (Index Culture + 3 Days) and Definitive Treatment (≥4 Days Post–Index Culture)
| . | . | Empiric Use (n = 486) . | Definitive Use (n = 444a) . | . | . | . |
|---|---|---|---|---|---|---|
| Drug Class . | Gram-Negative Anti-infective . | % of Patients . | % of Patients . | Directional Change in Use From Empiric Period to Definitive Period by >1%b . | ||
| Fluoroquinolone | Levofloxacin | 19.3 | 48.9 | ↑ | ||
| Ciprofloxacin | 8.02 | 14.2 | ↑ | |||
| Moxifloxacin | 1.4 | 2.0 | - | |||
| Cephalosporin | Cefepime | 24.7 | 19.8 | ↓ | ||
| Ceftriaxone | 14.4 | 9.5 | ↓ | |||
| Cefazolin | 3.9 | 7.7 | ↑ | |||
| Ceftazidime | 4.9 | 10.8 | ↑ | |||
| Other cephalosporins | 1.7 | 1.6 | - | |||
| Penicillin/beta-lactamase inhibitor | Piperacillin/tazobactam | 32.3 | 27.0 | ↓ | ||
| Ampicillin/sulbactam | 2.3 | 2.5 | - | |||
| Ticarcillin/clavulanate | 0.6 | 2.5 | ↑ | |||
| Amoxicillin/clavulanate | 0.2 | 0.7 | - | |||
| Carbapenem | Meropenem | 15.0 | 14.0 | ↓ | ||
| Ertapenem | 5.4 | 6.3 | - | |||
| Imipenem | 7.2 | 6.5 | - | |||
| Doripenem | 4.9 | 5.0 | - | |||
| Folic acid inhibitor/sulfonamide | TMP-SMX | 10.5 | 38.3 | ↑ | ||
| Aminoglycoside | Gentamicin | 9.3 | 7.0 | ↓ | ||
| Tobramycin | 7.4 | 5.6 | ↓ | |||
| Amikacin | 1.0 | 2.0 | ↑ | |||
| Tetracycline | Tigecycline | 2.9 | 5.4 | ↑ | ||
| Doxycycline | 2.3 | 2.9 | - | |||
| Minocycline | 0.4 | 1.6 | ↑ | |||
| Demeclocycline | 0.2 | 0.7 | - | |||
| Tetracycline | 0.0 | 0.2 | - | |||
| Monobactam | Aztreonam | 3.5 | 3.8 | - | ||
| % of patients who received at least 1 of appropriate treatmentsc (any fluoroquinolone, TMP-SMX, doxycycline, ceftazidime, or minocycline) | 39.1 | 85.8 | ↑ | |||
| . | . | Empiric Use (n = 486) . | Definitive Use (n = 444a) . | . | . | . |
|---|---|---|---|---|---|---|
| Drug Class . | Gram-Negative Anti-infective . | % of Patients . | % of Patients . | Directional Change in Use From Empiric Period to Definitive Period by >1%b . | ||
| Fluoroquinolone | Levofloxacin | 19.3 | 48.9 | ↑ | ||
| Ciprofloxacin | 8.02 | 14.2 | ↑ | |||
| Moxifloxacin | 1.4 | 2.0 | - | |||
| Cephalosporin | Cefepime | 24.7 | 19.8 | ↓ | ||
| Ceftriaxone | 14.4 | 9.5 | ↓ | |||
| Cefazolin | 3.9 | 7.7 | ↑ | |||
| Ceftazidime | 4.9 | 10.8 | ↑ | |||
| Other cephalosporins | 1.7 | 1.6 | - | |||
| Penicillin/beta-lactamase inhibitor | Piperacillin/tazobactam | 32.3 | 27.0 | ↓ | ||
| Ampicillin/sulbactam | 2.3 | 2.5 | - | |||
| Ticarcillin/clavulanate | 0.6 | 2.5 | ↑ | |||
| Amoxicillin/clavulanate | 0.2 | 0.7 | - | |||
| Carbapenem | Meropenem | 15.0 | 14.0 | ↓ | ||
| Ertapenem | 5.4 | 6.3 | - | |||
| Imipenem | 7.2 | 6.5 | - | |||
| Doripenem | 4.9 | 5.0 | - | |||
| Folic acid inhibitor/sulfonamide | TMP-SMX | 10.5 | 38.3 | ↑ | ||
| Aminoglycoside | Gentamicin | 9.3 | 7.0 | ↓ | ||
| Tobramycin | 7.4 | 5.6 | ↓ | |||
| Amikacin | 1.0 | 2.0 | ↑ | |||
| Tetracycline | Tigecycline | 2.9 | 5.4 | ↑ | ||
| Doxycycline | 2.3 | 2.9 | - | |||
| Minocycline | 0.4 | 1.6 | ↑ | |||
| Demeclocycline | 0.2 | 0.7 | - | |||
| Tetracycline | 0.0 | 0.2 | - | |||
| Monobactam | Aztreonam | 3.5 | 3.8 | - | ||
| % of patients who received at least 1 of appropriate treatmentsc (any fluoroquinolone, TMP-SMX, doxycycline, ceftazidime, or minocycline) | 39.1 | 85.8 | ↑ | |||
Patients may have been managed by multiple drug classes and multiple drugs within each class during the hospitalization. The percentages here reflect the percentage of patients who ever used any anti-infective. For initiation of gram-negative treatments in the empiric and definitive periods, refer to Supplementary Table 4.
Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
aForty-two patients were lost to follow-up between the empiric and definitive phases of treatment: 19 died during the empiric period, and 23 were discharged during the empiric period.
bDirectional arrows for changes are limited to changes of >1%. All other changes are considered within margins of error.
cAppropriate treatments are defined as the drugs included in CLSI susceptibility testing for S. maltophilia.
Gram-Negative Treatment Administration by Percentage of Patients Across 2 Time Intervals: Empiric Treatment (Index Culture + 3 Days) and Definitive Treatment (≥4 Days Post–Index Culture)
| . | . | Empiric Use (n = 486) . | Definitive Use (n = 444a) . | . | . | . |
|---|---|---|---|---|---|---|
| Drug Class . | Gram-Negative Anti-infective . | % of Patients . | % of Patients . | Directional Change in Use From Empiric Period to Definitive Period by >1%b . | ||
| Fluoroquinolone | Levofloxacin | 19.3 | 48.9 | ↑ | ||
| Ciprofloxacin | 8.02 | 14.2 | ↑ | |||
| Moxifloxacin | 1.4 | 2.0 | - | |||
| Cephalosporin | Cefepime | 24.7 | 19.8 | ↓ | ||
| Ceftriaxone | 14.4 | 9.5 | ↓ | |||
| Cefazolin | 3.9 | 7.7 | ↑ | |||
| Ceftazidime | 4.9 | 10.8 | ↑ | |||
| Other cephalosporins | 1.7 | 1.6 | - | |||
| Penicillin/beta-lactamase inhibitor | Piperacillin/tazobactam | 32.3 | 27.0 | ↓ | ||
| Ampicillin/sulbactam | 2.3 | 2.5 | - | |||
| Ticarcillin/clavulanate | 0.6 | 2.5 | ↑ | |||
| Amoxicillin/clavulanate | 0.2 | 0.7 | - | |||
| Carbapenem | Meropenem | 15.0 | 14.0 | ↓ | ||
| Ertapenem | 5.4 | 6.3 | - | |||
| Imipenem | 7.2 | 6.5 | - | |||
| Doripenem | 4.9 | 5.0 | - | |||
| Folic acid inhibitor/sulfonamide | TMP-SMX | 10.5 | 38.3 | ↑ | ||
| Aminoglycoside | Gentamicin | 9.3 | 7.0 | ↓ | ||
| Tobramycin | 7.4 | 5.6 | ↓ | |||
| Amikacin | 1.0 | 2.0 | ↑ | |||
| Tetracycline | Tigecycline | 2.9 | 5.4 | ↑ | ||
| Doxycycline | 2.3 | 2.9 | - | |||
| Minocycline | 0.4 | 1.6 | ↑ | |||
| Demeclocycline | 0.2 | 0.7 | - | |||
| Tetracycline | 0.0 | 0.2 | - | |||
| Monobactam | Aztreonam | 3.5 | 3.8 | - | ||
| % of patients who received at least 1 of appropriate treatmentsc (any fluoroquinolone, TMP-SMX, doxycycline, ceftazidime, or minocycline) | 39.1 | 85.8 | ↑ | |||
| . | . | Empiric Use (n = 486) . | Definitive Use (n = 444a) . | . | . | . |
|---|---|---|---|---|---|---|
| Drug Class . | Gram-Negative Anti-infective . | % of Patients . | % of Patients . | Directional Change in Use From Empiric Period to Definitive Period by >1%b . | ||
| Fluoroquinolone | Levofloxacin | 19.3 | 48.9 | ↑ | ||
| Ciprofloxacin | 8.02 | 14.2 | ↑ | |||
| Moxifloxacin | 1.4 | 2.0 | - | |||
| Cephalosporin | Cefepime | 24.7 | 19.8 | ↓ | ||
| Ceftriaxone | 14.4 | 9.5 | ↓ | |||
| Cefazolin | 3.9 | 7.7 | ↑ | |||
| Ceftazidime | 4.9 | 10.8 | ↑ | |||
| Other cephalosporins | 1.7 | 1.6 | - | |||
| Penicillin/beta-lactamase inhibitor | Piperacillin/tazobactam | 32.3 | 27.0 | ↓ | ||
| Ampicillin/sulbactam | 2.3 | 2.5 | - | |||
| Ticarcillin/clavulanate | 0.6 | 2.5 | ↑ | |||
| Amoxicillin/clavulanate | 0.2 | 0.7 | - | |||
| Carbapenem | Meropenem | 15.0 | 14.0 | ↓ | ||
| Ertapenem | 5.4 | 6.3 | - | |||
| Imipenem | 7.2 | 6.5 | - | |||
| Doripenem | 4.9 | 5.0 | - | |||
| Folic acid inhibitor/sulfonamide | TMP-SMX | 10.5 | 38.3 | ↑ | ||
| Aminoglycoside | Gentamicin | 9.3 | 7.0 | ↓ | ||
| Tobramycin | 7.4 | 5.6 | ↓ | |||
| Amikacin | 1.0 | 2.0 | ↑ | |||
| Tetracycline | Tigecycline | 2.9 | 5.4 | ↑ | ||
| Doxycycline | 2.3 | 2.9 | - | |||
| Minocycline | 0.4 | 1.6 | ↑ | |||
| Demeclocycline | 0.2 | 0.7 | - | |||
| Tetracycline | 0.0 | 0.2 | - | |||
| Monobactam | Aztreonam | 3.5 | 3.8 | - | ||
| % of patients who received at least 1 of appropriate treatmentsc (any fluoroquinolone, TMP-SMX, doxycycline, ceftazidime, or minocycline) | 39.1 | 85.8 | ↑ | |||
Patients may have been managed by multiple drug classes and multiple drugs within each class during the hospitalization. The percentages here reflect the percentage of patients who ever used any anti-infective. For initiation of gram-negative treatments in the empiric and definitive periods, refer to Supplementary Table 4.
Abbreviation: TMP-SMX, trimethoprim-sulfamethoxazole.
aForty-two patients were lost to follow-up between the empiric and definitive phases of treatment: 19 died during the empiric period, and 23 were discharged during the empiric period.
bDirectional arrows for changes are limited to changes of >1%. All other changes are considered within margins of error.
cAppropriate treatments are defined as the drugs included in CLSI susceptibility testing for S. maltophilia.
Infection Course (Onset, LOS, and Outcome)
Nearly 45% of patients had positive culture within 3 days of admission without documentation of prior health care association, which we defined as community-onset. The median infection-associated LOS was 9 days, with an average (SD) of 13.2 (19.8) days. Upon discharge, most S. maltophilia patients (60.9%) were discharged to home. Approximately one-fourth (24.1%) of patients were discharged to another health care facility, and a small percentage of them (2.3%) were discharged to hospice. In this data set, 12.8% of S. maltophilia BSI patients died before discharge (Table 3).
| . | Carbapenem-Resistant Pathogensa . | ||||
|---|---|---|---|---|---|
| . | Stenotrophomonas maltophilia . | Acinetobacter baumannii . | Pseudomonas aeruginosa . | Klebsiella pneumoniae . | Proteus mirabilis . |
| Classification of infection, % | |||||
| Community-onset infection | 44.6 | 0 | 0.5 | 0.4 | 0 |
| Health care–associated infection | 6.6 | 40.4 | 42.5 | 44.3 | 73.9 |
| Hospital-acquired infection | 48.8 | 59.6 | 57.0 | 55.3 | 26.1 |
| Infection-associated length of stay, d | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Mean (SD) | 13.2 (19.8) | 12.9 (16.3) | 16.3 (22.6) | 15.0 (20.7) | 9.9 (8.9) |
| Median (min–max) | 9 (0–353) | 9 (1–143) | 10 (1–197) | 10 (1–187) | 7 (2–50) |
| Discharge status, % | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Discharge to home | 60.9 | 7.7 | 23.2 | 15.8 | 26.1 |
| Discharge to hospice | 2.3 | 2.1 | 6.3 | 10.7 | 6.1 |
| Discharge to other facilities (eg, skilled nursing facility) | 24.1 | 47.7 | 46.2 | 53.0 | 60.9 |
| In-hospital mortality | 12.8 | 47.7 | 24.3 | 20.6 | 7.0 |
| . | Carbapenem-Resistant Pathogensa . | ||||
|---|---|---|---|---|---|
| . | Stenotrophomonas maltophilia . | Acinetobacter baumannii . | Pseudomonas aeruginosa . | Klebsiella pneumoniae . | Proteus mirabilis . |
| Classification of infection, % | |||||
| Community-onset infection | 44.6 | 0 | 0.5 | 0.4 | 0 |
| Health care–associated infection | 6.6 | 40.4 | 42.5 | 44.3 | 73.9 |
| Hospital-acquired infection | 48.8 | 59.6 | 57.0 | 55.3 | 26.1 |
| Infection-associated length of stay, d | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Mean (SD) | 13.2 (19.8) | 12.9 (16.3) | 16.3 (22.6) | 15.0 (20.7) | 9.9 (8.9) |
| Median (min–max) | 9 (0–353) | 9 (1–143) | 10 (1–197) | 10 (1–187) | 7 (2–50) |
| Discharge status, % | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Discharge to home | 60.9 | 7.7 | 23.2 | 15.8 | 26.1 |
| Discharge to hospice | 2.3 | 2.1 | 6.3 | 10.7 | 6.1 |
| Discharge to other facilities (eg, skilled nursing facility) | 24.1 | 47.7 | 46.2 | 53.0 | 60.9 |
| In-hospital mortality | 12.8 | 47.7 | 24.3 | 20.6 | 7.0 |
Abbreviation: LOS, length of stay.
aPathogens were considered carbapenem-resistant if the interpretation for any carbapenem was listed as resistant or intermediate, excluding ertapenem for A. baumannii and P. aeruginosa.
| . | Carbapenem-Resistant Pathogensa . | ||||
|---|---|---|---|---|---|
| . | Stenotrophomonas maltophilia . | Acinetobacter baumannii . | Pseudomonas aeruginosa . | Klebsiella pneumoniae . | Proteus mirabilis . |
| Classification of infection, % | |||||
| Community-onset infection | 44.6 | 0 | 0.5 | 0.4 | 0 |
| Health care–associated infection | 6.6 | 40.4 | 42.5 | 44.3 | 73.9 |
| Hospital-acquired infection | 48.8 | 59.6 | 57.0 | 55.3 | 26.1 |
| Infection-associated length of stay, d | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Mean (SD) | 13.2 (19.8) | 12.9 (16.3) | 16.3 (22.6) | 15.0 (20.7) | 9.9 (8.9) |
| Median (min–max) | 9 (0–353) | 9 (1–143) | 10 (1–197) | 10 (1–187) | 7 (2–50) |
| Discharge status, % | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Discharge to home | 60.9 | 7.7 | 23.2 | 15.8 | 26.1 |
| Discharge to hospice | 2.3 | 2.1 | 6.3 | 10.7 | 6.1 |
| Discharge to other facilities (eg, skilled nursing facility) | 24.1 | 47.7 | 46.2 | 53.0 | 60.9 |
| In-hospital mortality | 12.8 | 47.7 | 24.3 | 20.6 | 7.0 |
| . | Carbapenem-Resistant Pathogensa . | ||||
|---|---|---|---|---|---|
| . | Stenotrophomonas maltophilia . | Acinetobacter baumannii . | Pseudomonas aeruginosa . | Klebsiella pneumoniae . | Proteus mirabilis . |
| Classification of infection, % | |||||
| Community-onset infection | 44.6 | 0 | 0.5 | 0.4 | 0 |
| Health care–associated infection | 6.6 | 40.4 | 42.5 | 44.3 | 73.9 |
| Hospital-acquired infection | 48.8 | 59.6 | 57.0 | 55.3 | 26.1 |
| Infection-associated length of stay, d | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Mean (SD) | 13.2 (19.8) | 12.9 (16.3) | 16.3 (22.6) | 15.0 (20.7) | 9.9 (8.9) |
| Median (min–max) | 9 (0–353) | 9 (1–143) | 10 (1–197) | 10 (1–187) | 7 (2–50) |
| Discharge status, % | |||||
| No. | 486 | 235 | 379 | 253 | 115 |
| Discharge to home | 60.9 | 7.7 | 23.2 | 15.8 | 26.1 |
| Discharge to hospice | 2.3 | 2.1 | 6.3 | 10.7 | 6.1 |
| Discharge to other facilities (eg, skilled nursing facility) | 24.1 | 47.7 | 46.2 | 53.0 | 60.9 |
| In-hospital mortality | 12.8 | 47.7 | 24.3 | 20.6 | 7.0 |
Abbreviation: LOS, length of stay.
aPathogens were considered carbapenem-resistant if the interpretation for any carbapenem was listed as resistant or intermediate, excluding ertapenem for A. baumannii and P. aeruginosa.
The comparison with other CR gram-negative BSIs showed some substantial differences. Patients with S. maltophilia BSI had a much higher rate of community-onset than observed in the other 4 most common CR pathogens (range, 0%–0.5%), although their median infection-related LOS were similar. Crude in-hospital mortality was lower for patients with S. maltophilia BSI compared with patients with CR A. baumannii, CR P. aeruginosa, and CR K. pneumoniae BSIs (42.6%, 24.3%, and 20.6%, respectively) (Table 3).
DISCUSSION
In this large, retrospective, multicenter cohort study of S. maltophilia BSI patients in the United States, we found that S. maltophilia is the most common cause of CR gram-negative BSI in the United States, with >40% of the infections identified as community-onset (44.6%). Because S. maltophilia is not included in most surveillance studies for antimicrobial resistance, this is an important epidemiologic fact for physicians considering empiric treatment in septic patients. A recent report identifying difficult-to-treat resistant (DTR) gram-negative bacteremia utilizing the same Premier database failed to include S. maltophilia as a contributing pathogen because S. maltophilia did not meet the consensus definitions of MDR and XDR [24, 25].
This study identified 486 unique patients with S. maltophilia BSI. The majority of the patients were >50 years old, white, and female. Diagnoses at admission were often nonspecific, whereas on discharge diagnoses were more frequently related to medical device–associated bacteremia, including venous catheter (14.4%) and vascular devices (6.6%). A previously published, single-center patient chart review study in Greece found that catheter-related S. maltophilia BSIs were approximately one-third (36.4%) of S. maltophilia BSIs, a much higher percentage than found in our study [26]. The difference might relate to fact that a catheter-related infection may be more thoroughly identified in a chart review than when using reported diagnosis codes in a secondary electronic health care database such as the PHD.
This study found that most isolates of S. maltophilia were susceptible to TMP-SMX (94.6%) and tetracycline (92%). More interestingly, despite these high susceptibility rates, TMP-SMX in oral or parenteral form was only used in 38.3% of patients during the definitive treatment period. Other studies have found similar susceptibility rates for TMP-SMX, ranging from 86.8% to 97.1% [26–29]. Samonis et al. in their 2012 publication found that most isolates were susceptible or intermediately susceptible to TMP-SMX (86.8%), yet <10% of patients (9.1%) received it at any time and only 5.5% of patients received it as empirical therapy [26]. Similarly, our study found relatively low usage of TMP-SMX, especially during empiric treatment (10.5%), although a higher percentage of patients (38.3%) received this treatment during the definitive treatment period. This demonstrates that TMP-SMX is not often included in empiric regimens for patients with suspected gram-negative sepsis, even when there is concern for carbapenem resistance. Furthermore, fluoroquinolones such as levofloxacin and ciprofloxacin were more likely to be used than TMP-SMX even after S. maltophilia had been definitively identified from blood culture. This is consistent with the conclusions of Ko et al. that levofloxacin may be an alternative to TMP-SMX [13]. When considering all appropriate treatments for S. maltophilia BSI, only 39.1% received 1 of these treatments during the empiric period. This suggests that S. maltophilia is not often considered a likely cause of BSI. It was not until blood culture results identified the pathogen and susceptibility that 85.8% of patients received a treatment appropriate for S. maltophilia.
In this patient population, the average infection-associated LOS with S. maltophilia (SD) was 13.2 (19.8) days, similar to patients with BSIs from other CR gram-negative pathogens. In contrast to LOS, crude mortality in these patients did not appear to be as high as BSIs from other CR pathogens such as A. baumannii, P. aeruginosa, or K. pneumoniae.
This study has several strengths and limitations. This study utilized a large data set representative of US hospitals to identify patient characteristics and the burden of S. maltophilia BSI. It is the first study to identify the importance of S. maltophilia as the most common CR gram-negative bacterial pathogen causing bacteremia in the United States. Although the geographic regional prevalence may differ, S. maltophilia is a cause of BSI across the United States in a variety of hospital settings. In addition, the available microbiology data allow for proxy assessment of temporal patterns between timing of culture relative to hospital admission and initiation or adjustment of antibiotic treatment. It should be noted, however, that while PHD is largely representative of US hospitals, the southern portion of the United States is overrepresented in the database [20]. This may have an impact on the generalizability of our results to the entire US population, as physicians prescribing antibiotics vary from state to state, with southern states showing increased prescription trends [30]. A further limitation is that the PHD does not capture medical history and medical treatment before hospitalization, which could impact the characterization of health care–associated infections. Our figure of 6.6% representing health care–associated infection is likely an underestimate of health care–associated admissions due to limitations in reporting health care–related activities before admission. Consistent with the previous literature, the source of gram-negative BSI is often not identified, although many BSIs appear to be device related.
CONCLUSIONS
In this representative multicenter US study, S. maltophilia was found to be the most common cause of carbapenem-resistant gram-negative bacteremia, over half of which were present upon admission, representing community onset. Ironically, S. maltophilia is not included in most surveillance studies for antimicrobial resistance. This study demonstrated that patients at risk for S. maltophilia BSI are highly variable and that empiric treatment for possible CR gram-negative BSI does not include appropriate antibiotics. Standard of treatment is not clearly defined, and only a third of patients receive the recommended treatment with TMP-SMX during the definitive treatment period. Further efforts are needed to adequately describe patients with S. maltophilia infections in hospital settings across the United States to more adequately characterize treatment decisions in real-world practice and the resulting effectiveness.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors are grateful for analytical support from Hemanth Kanakamedala and Yun (Anna) Zhou at Genesis Research LLC.
Financial support. This study was funded by Shionogi, Inc.
Potential conflicts of interest. B.C. is an employee of Shionogi, Inc. G.T. is a consultant for Shionogi, Inc., and has received consulting fees. D.B. is an employee of Genesis Research, LLC (Hoboken, NJ, USA), and has received consulting fees from Shionogi, Inc. P.C. is an employee of Genesis Research, LLC (Hoboken, NJ, USA), and been contracted by Shionogi, Inc., during the conduct of the study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Centers for Disease Control and Prevention.





Comments