-
PDF
- Split View
-
Views
-
Cite
Cite
Nicholas W. Carris, Joe Pardo, Jose Montero, Kristy M. Shaeer, Minocycline as A Substitute for Doxycycline in Targeted Scenarios: A Systematic Review, Open Forum Infectious Diseases, Volume 2, Issue 4, Fall 2015, ofv178, https://doi.org/10.1093/ofid/ofv178
Close - Share Icon Share
Abstract
Doxycycline, a commonly prescribed tetracycline, remains on intermittent shortage. We systematically reviewed the literature to assess minocycline as an alternative to doxycycline in select conditions, given doxycycline's continued shortage. We identified 19 studies, 10 of which were published before 2000. Thirteen of the studies were prospective, but only 1 of these studies was randomized. Based on the available data, we found minocycline to be a reasonable substitute for doxycycline in the following scenarios: skin and soft-tissue infections and outpatient treatment of community-acquired pneumonia in young, otherwise healthy patients or in patients with macrolide-resistant Mycoplasma pneumoniae, as well as Lyme disease prophylaxis and select rickettsial disease should doxycycline be unavailable.
Antibacterial drug shortages are a growing interference in the clinical management of infectious diseases [1–4]. Close to 150 antibacterial agents experienced shortages between 2001 and 2013, with nearly one quarter of these drugs experiencing more than 1 shortage [2]. The impact of a drug shortage is felt at the patient-physician interface and on the institutional level. Seventy-eight percent of infectious diseases physicians surveyed reported that antimicrobial shortages have had a negative impact on their practice [5]. Furthermore, the difficulty of drug procurement during a shortage is often compounded by a substantial price increase for the products that remain on the market. Members of the US Congress have deliberated over how to assure the availability of clinically important generic drugs in the setting of shortage-driven market manipulations [4]. A centerpiece of congressional discussions was the example of oral doxycycline. Doxycycline, a tetracycline antibiotic with myriad labeled indications, experienced a 2000% increase in average retail price between 2012 and 2013 [6], and currently the list price for doxycycline is as high as $19 per capsule in our network. In the face of an inconsistent and steeply priced doxycycline supply, institutions have been forced to consider alternate therapies. Minocycline has emerged as a candidate to bridge therapeutic gaps and conserve financial resources, potentially serving as a simple substitute across a broad range of indications. The objective of this review was to summarize available study evidence and identify a clinical role for minocycline in light of the current or future shortages of doxycycline.
TETRACYCLINE OVERVIEW
Pharmacology and Pharmacokinetics
Tetracycline antibiotics exert their antibacterial action by disruption of protein synthesis. This is accomplished through reversible binding to the 30S subunit of the bacterial ribosome, which interferes with the interaction between aminoacyl transfer-RNA and messenger RNA [7, 8]. Doxycycline and minocycline are both second-generation tetracyclines with similar chemical structures (Figure 1) [7–9]. Minocycline is distinguished by minor structural differences at carbons 5 and 6 and the addition of a dimethylamino group at position 7 [8, 10]. These structural differences enhance the lipophilicity of minocycline compared with other tetracycline antibiotics. Both agents are between 90% and 100% bioavailable when taken orally, although absorption is impaired when coadministered with divalent and trivalent cations. Approximately 30%–65% of doxycycline is renally eliminated, with the remaining excretion occurring in the feces and bile [11–14]. In contrast, minocycline undergoes hepatic biotransformation. Metabolites and unchanged drug are eliminated in the urine and feces, with only 10% of the parent compound recovered unchanged in the urine.
![Molecular structures of doxycycline and minocycline [7, 9].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ofid/2/4/10.1093_ofid_ofv178/4/m_ofv17801.jpeg?Expires=1748868457&Signature=LMsrgswZHairdQawt9cEKjlrVTmusHcJ-4Y2Kxu072kNgTD5x57hxMKILME~uoOGTs0xr1YXbfsfYkNayPjjWBoE22W7bcHduKjsL15rxZaZ3o4x9Kgqha1J0-7Po8ZhGpx6pJX7~ZSGWKsJXjlF4UQOQbpYXufvwmz4npN4YTQCy2ArNGywQzKy9cO~Szv7dQ1IwyEdoYFK61zAOEb-hyHn~uydXt1KTxh0JvYw5dVxvE216u53TEVlQX7N9YCO4ocO8bI2~IaGP7WOGOHKNPl9CUk~XAmmqSpFobIGoO2iANojalEBpC58ZjwpyQd6NR7w~l5~YWCoM9ve1jOsgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Adverse Drug Reactions
The most common adverse drug reactions (ADRs) are characteristic of the tetracycline class: gastrointestinal disturbances, esophagitis, photosensitivity, pediatric tooth discoloration, and, rarely, hepatotoxicity, hypersensitivity, and idiopathic intracranial hypertension. Minocycline is more likely to cause other central nervous system effects (eg, dizziness, lack of concentration, ataxia, vertigo, tinnitus associated with weakness, nausea, and vomiting) and pigmentation of various body sites [8]. Overall, ADRs are reported more frequently for minocycline, but both drugs are generally well tolerated. The comparative safety of doxycycline and minocycline has been reviewed in detail previously [15].
Spectrum of Activity
Doxycycline and minocycline have comparable in vitro activity. Their antibacterial spectrum encompasses commonly isolated Gram-positive and Gram-negative bacteria (eg, staphylococci, streptococci, certain Enterobacteriaceae). In addition, activity is noted against atypical pathogens involved in pulmonary and sexually transmitted infections, Rickettsia, and the infectious agents of other less common syndromes (eg, brucellosis, melioidosis, leptospirosis, anthrax, plague, and Q fever). Minocycline has emerged as the tetracycline of choice for multidrug-resistant Acinetobacter baumannii infections, although doxycycline has also demonstrated activity. The use of tetracyclines for A baumannii infections was recently reviewed [16].
Clinical Efficacy
The clinical efficacy of minocycline should be critically evaluated before recommending direct therapeutic substitution for doxycycline. To our knowledge, this review is the first of its kind assessing the potential for minocycline use or substitution for doxycycline in targeted clinical scenarios. Because doxycycline can be used in the treatment of more than 30 different conditions and infections, we elected to review clinical data regarding minocycline in 4 specific scenarios, which were chosen a priori [9]. Skin and soft-tissue infections (SSTIs) and community-acquired pneumonia (CAP) were chosen for review because they are 2 of the most common infections that can be treated with tetracyclines [17, 18]. Lyme disease prophylaxis and the treatment of rickettsial disease were chosen for review because there is a limitation in the guidance previously provided by the Centers for Disease Control and Prevention (CDC) should doxycycline be entirely unavailable [1].
METHODS
Search Strategy and Selection Criteria
We searched Medline through PubMed and EMBASE via Ovid (up to August 20, 2015) for publications in English related to minocycline's use in SSTIs, CAP, tickborne rickettsial disease, and Lyme disease. We included publications if they reported original data from prospective clinical trials, prospective cohorts, or retrospective cohorts or cases series with ≥10 patients. We required clinical outcomes to be reported. The specific search terms we used were as follows: (minocycline [MeSH Terms] OR minocycline [Text Word]) AND (pneumonia [Text Word] OR pneumonia [MeSH Terms] OR soft tissue infection [MeSH Terms] OR soft tissue infection [Text Word] OR Rickettsia [Text Word] OR Rickettsia [MeSH Terms] OR Rocky Mountain Spotted Fever [Text Word] OR Rocky Mountain Spotted Fever [MeSH Terms] OR Erlichiosis [Text Word] OR Erlichiosis [MeSH Terms] OR Anaplasmosis [Text Word] OR Anaplasmosis [MeSH Terms] OR Lyme Disease [Text word] OR Lyme Disease [MeSH Terms]). Then, we reviewed the references of reports identified by this search for additional reports to include. Finally, all investigators critically reviewed and searched medical literature for additional pertinent reports. These details represent the full protocol for study identification. Studies for inclusion were identified by 1 investigator (N.W.C.) and were confirmed by all coinvestigators. We resolved discrepancies by consensus.
Data Abstraction and Analysis
Data were abstracted after study eligibility was determined by the investigators. Data were abstracted using a standardized table (Table 1) and were corrected and confirmed by all investigators. The following information was retrieved: author, year, sample size, study design/setting, population, treatment, outcomes, and potential for bias. We elected not to perform a meta-analysis because inclusion was not limited based on study design and therefore included both retrospective and prospective studies, controlled and uncontrolled studies, and inpatient and outpatient studies over more than a 40-year period. We elected this systematic review method as a way to include all relevant clinical data rather than to significantly truncate an already small data set. A comparison to doxycycline was included if available; however, this was not required because the investigators sought to identify the potential utility of minocycline during doxycycline shortage rather than an alternative to doxycycline under circumstances of adequate supply. Therefore, our recommendations regarding the use of minocycline are placed into this context, based upon the data presented, developed in consideration of additional alternatives to doxycycline, and should be considered along with patient-specific factors, especially as they relate to potential ADRs.
Review of Literature on Patients Treated With Minocycline for Selected Diseases States
| Study/Sample Size . | Design/ Setting . | Population/Condition/Age; Mean or Median (Range), Years . | Reported Treatment(s) . | Outcome(s) N (%) . | Potential for Bias . |
|---|---|---|---|---|---|
| Skin and soft tissue infections | |||||
| Cappel and Klastersky [19] N = 20 | Retrospective/inpatient/ Belgium | Disseminated malignancy moderately severe (investigator reported) bacterial infection Age: 63 (25–77) SSTI, N = 11 | Minocycline (route not reported) DOT: 7 d | Cure: 10/11 (91) | Retrospective; small sample size Minocycline dose lower than current standard Compromised patient population |
| Phair et al [20] N = 10 | Prospective uncontrolled/ outpatient/United States | Purulent SSTI, identified Staphylococcus aureus Age: not reported | Minocycline (PO) DOT: 8–18 d Local soaks and debridement PRN | Cure: 8/10 (80) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Raff et al [21] N = 15 | Prospective uncontrolled/not reported/United States | Severe (investigator reported) S aureus SSTIs Age: 56 (22–83) | Minocycline (PO) DOT: 6–28 d | Cure: 15/15 (100) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Clumeck et al [22] N = 25 | Prospective uncontrolled/ inpatient/Belgium | Severe S aureus infection; Age: 62 (18–88) SSTI, N = 4 Other cohort indications: PNA, osteomyelitis septic thrombophlebitis, febrile urinary tract infection, endocarditis, and liver abscess | Minocycline + rifampin Entire cohort (N = 25) description DOT: 5–119 d (mean 22) (PO, N = 20; IV, N = 5) | SSTI Cure: 3/4 (75) | Uncontrolled study; small sample size Limited description of nonpharmacologic intervention 21 patients received minocycline 100 mg Q12H, and 4 patients received 200 mg every Q12H |
| Ruhe et al [23] N = 24 | Retrospective/inpatient/United States | Serious, tetracycline-susceptible MRSA infection Age: 51 (28–94) Complicated SSTI, N = 16 | Minocycline (PO); N = 5 Doxycycline (PO); N = 11 | Cure: 5/5 (100) 10/11 (91) | Retrospective; small sample size Allowed alternative initial antibiotic if ≤50% of appropriate treatment duration DOT not reported specific to SSTI |
| Barnes et al [24] N = 30 | Retrospective review with prospective observation/outpatient/United States | Nonserious; MRSA-SSTI; cellulitis, abscess, or both Age: 46 (18–83) | Minocycline (PO) Doxycycline (PO) Trim/sulfa (PO) Clindamycin (PO) Drainage only β-lactam (PO) + drainage Fluoroquinolone (PO) | Cure: 3/3 (100) 1/1 (100) 6/6 (100) 8/8 (100) 4/4 (100) 5/5 (100) 3/3 (100) | Retrospective/observational; small sample size Dosing not reported; DOT not reported 1 patient treated with drainage only, and 1 patient treated with β-lactam + drainage experience recurrence after 30 d |
| Ruhe and Menon [25] N = 282 | Retrospective/ outpatient/United States | Community-acquired purulent S aureus SSTI Age: 48 (18–85) | Minocycline (PO); N = 3 or Doxycycline (PO); N = 87 DOT: 3–20 d (median 10) Incision/drainage; 77% β-lactam (PO and/or IV); N = 192 DOT: not reported Incision/drainage; 81% | Cure: 86/90 (96) 168/192 (88) | Retrospective Few patients received minocycline Clinical outcomes of minocycline/doxycycline reported as aggregate 20 of 168 β-lactam treatment successes occurred in patients changed from β-lactam to targeted therapy based on antimicrobial susceptibility data |
| Rogers et al [26] N = 24 | Prospective uncontrolled/ inpatient/United States | Severe (investigator reported) infections SSTI, N = 6 Age: 56 (43–77) Mixed PNA, N = 14 Age: 48 (5–84) | Minocycline (IV) | Cure: SSTI; 4/6 (67) Mixed PNA; 14/14 (100) | Uncontrolled, small sample size Heterogeneous disease states DOT: Not reported |
| Community-acquired pneumonia | |||||
| Kawai et al [27] N = 30 | Prospective uncontrolled/inpatient or outpatient/Japan | Mycoplasma pneumoniae CAP Macrolide resistant, N = 21 Age: 8 (1–15) | Minocycline required for 15 patients with macrolide treatment failure DOT: 8–11 d (route not reported) | Defervesce within 48 H: 15/15 (100) | Uncontrolled study; only included confirmed M pneumonia; small sample size Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Okada et al [28] N = 202 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 176 Age: 8 (1–14) | Secondary agent used in macrolide resistance: Minocycline; N = 52 DOT: 2–7 d (mean 5) Doxycycline; N = 16 DOT: 3–7 d (mean 3) Macrolides; N = 13 DOT: 3–10 d (mean 6) Tosufloxacin; N = 13 DOT: 2–7 d (mean 5) (routes not reported) | Defervesce within 48 H: 47 (90) 14 (88) 6 (46) 9 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Kawai et al [29] N = 188 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP macrolide resistant, N = 150 Age: 8 (0–15) | Definitive treatment in macrolide resistance: Minocycline, N = 38 Azithromycin, N = 27 Clarithromycin, N = 23 Tosufloxacin, N = 62 (routes not reported) | Defervesce within 48 H: 33 (87) 11 (41) 11 (48) 43 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline (age ≥8)/tosufloxacin (age <8) used as definitive therapy Treatments determined by attending physician/not standardized DOT: Not reported |
| Miyashita et al [30] N = 73 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 30 Age: 23 (16–45) | Initial treatment in macrolide resistance: Minocycline, N = 7 Macrolides, N = 14 Quinolones, N = 9 (All admitted patients received IV minocycline, other specifics of route not reported) | Defervesce within 48 H: 6 (85) 4 (28) 7 (77) | Observational study; only included confirmed M pneumoniae; small sample size Clinical outcome assessed as presence of fever DOT: Not reported |
| Lyme disease | |||||
| Weber et al [31] N = 107 | Prospective, nonrandomized intervention/not reported/Germany | Early erythema migrans, N = 97 Age: Not adequately reported | Minocycline, N = 11 DOT: 10–15 d Doxy/tetracycline, N = 8 DOT: 10–14 d Penicillin (PO), N = 65 DOT: 10–14 d Penicillin (parenteral), N = 7 DOT: 10–14 d Erythromycin, N = 6 DOT: 10 d (other routes not specifically reported) | Time to cure (weeks): mean/median 7/2 19/2 22/2 7/2 2/9 | Nonrandomized; small sample size Limited differentiation in outcomes between minocycline and doxycycline |
| Muellegger et al [32] N = 14 | Prospective uncontrolled/not reported/Austria | Erythema migrans, Borrelia burgdorferi DNA confirmed (57%) Age: 53 (34–79) | Minocycline (PO); N = 14 DOT: 14 d | Good clinical response: 14/14 (100) | Uncontrolled; small sample size |
| Breier et al [33] N = 60 | Prospective open label randomized/outpatient/Austria | Erythema migrans Age: 43 (19–80) | Minocycline, N = 30 DOT: 21 d Penicillin V, N = 30 DOT: 21 d (route not reported) | Both groups: Complete recovery; no late disease at 1 y | Withdraw due to side effects (minocycline, 12; penicillin V, 9) excluded from analysis Open label design; small sample size |
| Schmidt et al [34] N = 26 | Prospective uncontrolled/not reported/Austria | Erythema migrans Age: 56 (20–84) | Minocycline (PO) DOT: 14 d | 8 wks post therapy: 20/22 (91) Erythema Migrans clear; 0/22 (0) Additional symptoms present | Uncontrolled; small sample size 4 patients lost to follow up at 8 wks Limited report of clinical outcomes |
| Stanek et al [35] N = 99 | Prospective uncontrolled/outpatient/Austria | Erythema migrans Age: 50 (10–80) | Minocycline (PO) Amoxicillin (PO) Azithromycin (PO) Doxycycline (PO) Penicillin V (PO) DOT: 2–3 wks (all treatments) | Complete resolution in all patients within 3 wks | Uncontrolled; limited report of clinical outcomes Number of patients treated with each antibiotic not reported |
| Glatz et al [36] N = 113 | Retrospective/not reported/ Austria | Erythema migrans Age: 51 (5–78) | Minocycline (PO), N = 61* Doxycycline (PO), N = 13* β-lactam (PO/IV), N = 36 DOT: Not reported | Complete symptom resolution within 1 mo: 109/113 (96) | Retrospective; variable antibiotic treatment Limited report of clinical outcomes Clinical outcome reported as aggregate |
| Rickettsial infections | |||||
| Kodama et al [37] N = 28 | Retrospective/inpatient/Japan | Japanese spotted fever Age: 60 (12–78) Died before treatment, N = 1 | Minocycline; N = 25 Minocycline + steroids; N = 2 (route not reported) | Cure: 25/25 (100%) 2/2 (100%) | Retrospective, small sample size Minocycline dosing not reported DOT: Not reported |
| Study/Sample Size . | Design/ Setting . | Population/Condition/Age; Mean or Median (Range), Years . | Reported Treatment(s) . | Outcome(s) N (%) . | Potential for Bias . |
|---|---|---|---|---|---|
| Skin and soft tissue infections | |||||
| Cappel and Klastersky [19] N = 20 | Retrospective/inpatient/ Belgium | Disseminated malignancy moderately severe (investigator reported) bacterial infection Age: 63 (25–77) SSTI, N = 11 | Minocycline (route not reported) DOT: 7 d | Cure: 10/11 (91) | Retrospective; small sample size Minocycline dose lower than current standard Compromised patient population |
| Phair et al [20] N = 10 | Prospective uncontrolled/ outpatient/United States | Purulent SSTI, identified Staphylococcus aureus Age: not reported | Minocycline (PO) DOT: 8–18 d Local soaks and debridement PRN | Cure: 8/10 (80) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Raff et al [21] N = 15 | Prospective uncontrolled/not reported/United States | Severe (investigator reported) S aureus SSTIs Age: 56 (22–83) | Minocycline (PO) DOT: 6–28 d | Cure: 15/15 (100) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Clumeck et al [22] N = 25 | Prospective uncontrolled/ inpatient/Belgium | Severe S aureus infection; Age: 62 (18–88) SSTI, N = 4 Other cohort indications: PNA, osteomyelitis septic thrombophlebitis, febrile urinary tract infection, endocarditis, and liver abscess | Minocycline + rifampin Entire cohort (N = 25) description DOT: 5–119 d (mean 22) (PO, N = 20; IV, N = 5) | SSTI Cure: 3/4 (75) | Uncontrolled study; small sample size Limited description of nonpharmacologic intervention 21 patients received minocycline 100 mg Q12H, and 4 patients received 200 mg every Q12H |
| Ruhe et al [23] N = 24 | Retrospective/inpatient/United States | Serious, tetracycline-susceptible MRSA infection Age: 51 (28–94) Complicated SSTI, N = 16 | Minocycline (PO); N = 5 Doxycycline (PO); N = 11 | Cure: 5/5 (100) 10/11 (91) | Retrospective; small sample size Allowed alternative initial antibiotic if ≤50% of appropriate treatment duration DOT not reported specific to SSTI |
| Barnes et al [24] N = 30 | Retrospective review with prospective observation/outpatient/United States | Nonserious; MRSA-SSTI; cellulitis, abscess, or both Age: 46 (18–83) | Minocycline (PO) Doxycycline (PO) Trim/sulfa (PO) Clindamycin (PO) Drainage only β-lactam (PO) + drainage Fluoroquinolone (PO) | Cure: 3/3 (100) 1/1 (100) 6/6 (100) 8/8 (100) 4/4 (100) 5/5 (100) 3/3 (100) | Retrospective/observational; small sample size Dosing not reported; DOT not reported 1 patient treated with drainage only, and 1 patient treated with β-lactam + drainage experience recurrence after 30 d |
| Ruhe and Menon [25] N = 282 | Retrospective/ outpatient/United States | Community-acquired purulent S aureus SSTI Age: 48 (18–85) | Minocycline (PO); N = 3 or Doxycycline (PO); N = 87 DOT: 3–20 d (median 10) Incision/drainage; 77% β-lactam (PO and/or IV); N = 192 DOT: not reported Incision/drainage; 81% | Cure: 86/90 (96) 168/192 (88) | Retrospective Few patients received minocycline Clinical outcomes of minocycline/doxycycline reported as aggregate 20 of 168 β-lactam treatment successes occurred in patients changed from β-lactam to targeted therapy based on antimicrobial susceptibility data |
| Rogers et al [26] N = 24 | Prospective uncontrolled/ inpatient/United States | Severe (investigator reported) infections SSTI, N = 6 Age: 56 (43–77) Mixed PNA, N = 14 Age: 48 (5–84) | Minocycline (IV) | Cure: SSTI; 4/6 (67) Mixed PNA; 14/14 (100) | Uncontrolled, small sample size Heterogeneous disease states DOT: Not reported |
| Community-acquired pneumonia | |||||
| Kawai et al [27] N = 30 | Prospective uncontrolled/inpatient or outpatient/Japan | Mycoplasma pneumoniae CAP Macrolide resistant, N = 21 Age: 8 (1–15) | Minocycline required for 15 patients with macrolide treatment failure DOT: 8–11 d (route not reported) | Defervesce within 48 H: 15/15 (100) | Uncontrolled study; only included confirmed M pneumonia; small sample size Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Okada et al [28] N = 202 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 176 Age: 8 (1–14) | Secondary agent used in macrolide resistance: Minocycline; N = 52 DOT: 2–7 d (mean 5) Doxycycline; N = 16 DOT: 3–7 d (mean 3) Macrolides; N = 13 DOT: 3–10 d (mean 6) Tosufloxacin; N = 13 DOT: 2–7 d (mean 5) (routes not reported) | Defervesce within 48 H: 47 (90) 14 (88) 6 (46) 9 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Kawai et al [29] N = 188 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP macrolide resistant, N = 150 Age: 8 (0–15) | Definitive treatment in macrolide resistance: Minocycline, N = 38 Azithromycin, N = 27 Clarithromycin, N = 23 Tosufloxacin, N = 62 (routes not reported) | Defervesce within 48 H: 33 (87) 11 (41) 11 (48) 43 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline (age ≥8)/tosufloxacin (age <8) used as definitive therapy Treatments determined by attending physician/not standardized DOT: Not reported |
| Miyashita et al [30] N = 73 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 30 Age: 23 (16–45) | Initial treatment in macrolide resistance: Minocycline, N = 7 Macrolides, N = 14 Quinolones, N = 9 (All admitted patients received IV minocycline, other specifics of route not reported) | Defervesce within 48 H: 6 (85) 4 (28) 7 (77) | Observational study; only included confirmed M pneumoniae; small sample size Clinical outcome assessed as presence of fever DOT: Not reported |
| Lyme disease | |||||
| Weber et al [31] N = 107 | Prospective, nonrandomized intervention/not reported/Germany | Early erythema migrans, N = 97 Age: Not adequately reported | Minocycline, N = 11 DOT: 10–15 d Doxy/tetracycline, N = 8 DOT: 10–14 d Penicillin (PO), N = 65 DOT: 10–14 d Penicillin (parenteral), N = 7 DOT: 10–14 d Erythromycin, N = 6 DOT: 10 d (other routes not specifically reported) | Time to cure (weeks): mean/median 7/2 19/2 22/2 7/2 2/9 | Nonrandomized; small sample size Limited differentiation in outcomes between minocycline and doxycycline |
| Muellegger et al [32] N = 14 | Prospective uncontrolled/not reported/Austria | Erythema migrans, Borrelia burgdorferi DNA confirmed (57%) Age: 53 (34–79) | Minocycline (PO); N = 14 DOT: 14 d | Good clinical response: 14/14 (100) | Uncontrolled; small sample size |
| Breier et al [33] N = 60 | Prospective open label randomized/outpatient/Austria | Erythema migrans Age: 43 (19–80) | Minocycline, N = 30 DOT: 21 d Penicillin V, N = 30 DOT: 21 d (route not reported) | Both groups: Complete recovery; no late disease at 1 y | Withdraw due to side effects (minocycline, 12; penicillin V, 9) excluded from analysis Open label design; small sample size |
| Schmidt et al [34] N = 26 | Prospective uncontrolled/not reported/Austria | Erythema migrans Age: 56 (20–84) | Minocycline (PO) DOT: 14 d | 8 wks post therapy: 20/22 (91) Erythema Migrans clear; 0/22 (0) Additional symptoms present | Uncontrolled; small sample size 4 patients lost to follow up at 8 wks Limited report of clinical outcomes |
| Stanek et al [35] N = 99 | Prospective uncontrolled/outpatient/Austria | Erythema migrans Age: 50 (10–80) | Minocycline (PO) Amoxicillin (PO) Azithromycin (PO) Doxycycline (PO) Penicillin V (PO) DOT: 2–3 wks (all treatments) | Complete resolution in all patients within 3 wks | Uncontrolled; limited report of clinical outcomes Number of patients treated with each antibiotic not reported |
| Glatz et al [36] N = 113 | Retrospective/not reported/ Austria | Erythema migrans Age: 51 (5–78) | Minocycline (PO), N = 61* Doxycycline (PO), N = 13* β-lactam (PO/IV), N = 36 DOT: Not reported | Complete symptom resolution within 1 mo: 109/113 (96) | Retrospective; variable antibiotic treatment Limited report of clinical outcomes Clinical outcome reported as aggregate |
| Rickettsial infections | |||||
| Kodama et al [37] N = 28 | Retrospective/inpatient/Japan | Japanese spotted fever Age: 60 (12–78) Died before treatment, N = 1 | Minocycline; N = 25 Minocycline + steroids; N = 2 (route not reported) | Cure: 25/25 (100%) 2/2 (100%) | Retrospective, small sample size Minocycline dosing not reported DOT: Not reported |
Abbreviations: CAP, community-acquired pneumonia; DOT, duration of therapy; H, hours; IV, intravenous; MRSA, methicillin-resistant S aureus; PNA, pneumonia; PO, per oral; PRN, as needed; Q, every; SSTI, skin and soft-tissue infections; Trim/sulfa, trimethoprim/sulfamethoxazole.
a Values calculated from data presented in Table 1 of Arch Dermatol 2006;142(7):862–868.
Review of Literature on Patients Treated With Minocycline for Selected Diseases States
| Study/Sample Size . | Design/ Setting . | Population/Condition/Age; Mean or Median (Range), Years . | Reported Treatment(s) . | Outcome(s) N (%) . | Potential for Bias . |
|---|---|---|---|---|---|
| Skin and soft tissue infections | |||||
| Cappel and Klastersky [19] N = 20 | Retrospective/inpatient/ Belgium | Disseminated malignancy moderately severe (investigator reported) bacterial infection Age: 63 (25–77) SSTI, N = 11 | Minocycline (route not reported) DOT: 7 d | Cure: 10/11 (91) | Retrospective; small sample size Minocycline dose lower than current standard Compromised patient population |
| Phair et al [20] N = 10 | Prospective uncontrolled/ outpatient/United States | Purulent SSTI, identified Staphylococcus aureus Age: not reported | Minocycline (PO) DOT: 8–18 d Local soaks and debridement PRN | Cure: 8/10 (80) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Raff et al [21] N = 15 | Prospective uncontrolled/not reported/United States | Severe (investigator reported) S aureus SSTIs Age: 56 (22–83) | Minocycline (PO) DOT: 6–28 d | Cure: 15/15 (100) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Clumeck et al [22] N = 25 | Prospective uncontrolled/ inpatient/Belgium | Severe S aureus infection; Age: 62 (18–88) SSTI, N = 4 Other cohort indications: PNA, osteomyelitis septic thrombophlebitis, febrile urinary tract infection, endocarditis, and liver abscess | Minocycline + rifampin Entire cohort (N = 25) description DOT: 5–119 d (mean 22) (PO, N = 20; IV, N = 5) | SSTI Cure: 3/4 (75) | Uncontrolled study; small sample size Limited description of nonpharmacologic intervention 21 patients received minocycline 100 mg Q12H, and 4 patients received 200 mg every Q12H |
| Ruhe et al [23] N = 24 | Retrospective/inpatient/United States | Serious, tetracycline-susceptible MRSA infection Age: 51 (28–94) Complicated SSTI, N = 16 | Minocycline (PO); N = 5 Doxycycline (PO); N = 11 | Cure: 5/5 (100) 10/11 (91) | Retrospective; small sample size Allowed alternative initial antibiotic if ≤50% of appropriate treatment duration DOT not reported specific to SSTI |
| Barnes et al [24] N = 30 | Retrospective review with prospective observation/outpatient/United States | Nonserious; MRSA-SSTI; cellulitis, abscess, or both Age: 46 (18–83) | Minocycline (PO) Doxycycline (PO) Trim/sulfa (PO) Clindamycin (PO) Drainage only β-lactam (PO) + drainage Fluoroquinolone (PO) | Cure: 3/3 (100) 1/1 (100) 6/6 (100) 8/8 (100) 4/4 (100) 5/5 (100) 3/3 (100) | Retrospective/observational; small sample size Dosing not reported; DOT not reported 1 patient treated with drainage only, and 1 patient treated with β-lactam + drainage experience recurrence after 30 d |
| Ruhe and Menon [25] N = 282 | Retrospective/ outpatient/United States | Community-acquired purulent S aureus SSTI Age: 48 (18–85) | Minocycline (PO); N = 3 or Doxycycline (PO); N = 87 DOT: 3–20 d (median 10) Incision/drainage; 77% β-lactam (PO and/or IV); N = 192 DOT: not reported Incision/drainage; 81% | Cure: 86/90 (96) 168/192 (88) | Retrospective Few patients received minocycline Clinical outcomes of minocycline/doxycycline reported as aggregate 20 of 168 β-lactam treatment successes occurred in patients changed from β-lactam to targeted therapy based on antimicrobial susceptibility data |
| Rogers et al [26] N = 24 | Prospective uncontrolled/ inpatient/United States | Severe (investigator reported) infections SSTI, N = 6 Age: 56 (43–77) Mixed PNA, N = 14 Age: 48 (5–84) | Minocycline (IV) | Cure: SSTI; 4/6 (67) Mixed PNA; 14/14 (100) | Uncontrolled, small sample size Heterogeneous disease states DOT: Not reported |
| Community-acquired pneumonia | |||||
| Kawai et al [27] N = 30 | Prospective uncontrolled/inpatient or outpatient/Japan | Mycoplasma pneumoniae CAP Macrolide resistant, N = 21 Age: 8 (1–15) | Minocycline required for 15 patients with macrolide treatment failure DOT: 8–11 d (route not reported) | Defervesce within 48 H: 15/15 (100) | Uncontrolled study; only included confirmed M pneumonia; small sample size Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Okada et al [28] N = 202 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 176 Age: 8 (1–14) | Secondary agent used in macrolide resistance: Minocycline; N = 52 DOT: 2–7 d (mean 5) Doxycycline; N = 16 DOT: 3–7 d (mean 3) Macrolides; N = 13 DOT: 3–10 d (mean 6) Tosufloxacin; N = 13 DOT: 2–7 d (mean 5) (routes not reported) | Defervesce within 48 H: 47 (90) 14 (88) 6 (46) 9 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Kawai et al [29] N = 188 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP macrolide resistant, N = 150 Age: 8 (0–15) | Definitive treatment in macrolide resistance: Minocycline, N = 38 Azithromycin, N = 27 Clarithromycin, N = 23 Tosufloxacin, N = 62 (routes not reported) | Defervesce within 48 H: 33 (87) 11 (41) 11 (48) 43 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline (age ≥8)/tosufloxacin (age <8) used as definitive therapy Treatments determined by attending physician/not standardized DOT: Not reported |
| Miyashita et al [30] N = 73 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 30 Age: 23 (16–45) | Initial treatment in macrolide resistance: Minocycline, N = 7 Macrolides, N = 14 Quinolones, N = 9 (All admitted patients received IV minocycline, other specifics of route not reported) | Defervesce within 48 H: 6 (85) 4 (28) 7 (77) | Observational study; only included confirmed M pneumoniae; small sample size Clinical outcome assessed as presence of fever DOT: Not reported |
| Lyme disease | |||||
| Weber et al [31] N = 107 | Prospective, nonrandomized intervention/not reported/Germany | Early erythema migrans, N = 97 Age: Not adequately reported | Minocycline, N = 11 DOT: 10–15 d Doxy/tetracycline, N = 8 DOT: 10–14 d Penicillin (PO), N = 65 DOT: 10–14 d Penicillin (parenteral), N = 7 DOT: 10–14 d Erythromycin, N = 6 DOT: 10 d (other routes not specifically reported) | Time to cure (weeks): mean/median 7/2 19/2 22/2 7/2 2/9 | Nonrandomized; small sample size Limited differentiation in outcomes between minocycline and doxycycline |
| Muellegger et al [32] N = 14 | Prospective uncontrolled/not reported/Austria | Erythema migrans, Borrelia burgdorferi DNA confirmed (57%) Age: 53 (34–79) | Minocycline (PO); N = 14 DOT: 14 d | Good clinical response: 14/14 (100) | Uncontrolled; small sample size |
| Breier et al [33] N = 60 | Prospective open label randomized/outpatient/Austria | Erythema migrans Age: 43 (19–80) | Minocycline, N = 30 DOT: 21 d Penicillin V, N = 30 DOT: 21 d (route not reported) | Both groups: Complete recovery; no late disease at 1 y | Withdraw due to side effects (minocycline, 12; penicillin V, 9) excluded from analysis Open label design; small sample size |
| Schmidt et al [34] N = 26 | Prospective uncontrolled/not reported/Austria | Erythema migrans Age: 56 (20–84) | Minocycline (PO) DOT: 14 d | 8 wks post therapy: 20/22 (91) Erythema Migrans clear; 0/22 (0) Additional symptoms present | Uncontrolled; small sample size 4 patients lost to follow up at 8 wks Limited report of clinical outcomes |
| Stanek et al [35] N = 99 | Prospective uncontrolled/outpatient/Austria | Erythema migrans Age: 50 (10–80) | Minocycline (PO) Amoxicillin (PO) Azithromycin (PO) Doxycycline (PO) Penicillin V (PO) DOT: 2–3 wks (all treatments) | Complete resolution in all patients within 3 wks | Uncontrolled; limited report of clinical outcomes Number of patients treated with each antibiotic not reported |
| Glatz et al [36] N = 113 | Retrospective/not reported/ Austria | Erythema migrans Age: 51 (5–78) | Minocycline (PO), N = 61* Doxycycline (PO), N = 13* β-lactam (PO/IV), N = 36 DOT: Not reported | Complete symptom resolution within 1 mo: 109/113 (96) | Retrospective; variable antibiotic treatment Limited report of clinical outcomes Clinical outcome reported as aggregate |
| Rickettsial infections | |||||
| Kodama et al [37] N = 28 | Retrospective/inpatient/Japan | Japanese spotted fever Age: 60 (12–78) Died before treatment, N = 1 | Minocycline; N = 25 Minocycline + steroids; N = 2 (route not reported) | Cure: 25/25 (100%) 2/2 (100%) | Retrospective, small sample size Minocycline dosing not reported DOT: Not reported |
| Study/Sample Size . | Design/ Setting . | Population/Condition/Age; Mean or Median (Range), Years . | Reported Treatment(s) . | Outcome(s) N (%) . | Potential for Bias . |
|---|---|---|---|---|---|
| Skin and soft tissue infections | |||||
| Cappel and Klastersky [19] N = 20 | Retrospective/inpatient/ Belgium | Disseminated malignancy moderately severe (investigator reported) bacterial infection Age: 63 (25–77) SSTI, N = 11 | Minocycline (route not reported) DOT: 7 d | Cure: 10/11 (91) | Retrospective; small sample size Minocycline dose lower than current standard Compromised patient population |
| Phair et al [20] N = 10 | Prospective uncontrolled/ outpatient/United States | Purulent SSTI, identified Staphylococcus aureus Age: not reported | Minocycline (PO) DOT: 8–18 d Local soaks and debridement PRN | Cure: 8/10 (80) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Raff et al [21] N = 15 | Prospective uncontrolled/not reported/United States | Severe (investigator reported) S aureus SSTIs Age: 56 (22–83) | Minocycline (PO) DOT: 6–28 d | Cure: 15/15 (100) | Uncontrolled study Small sample size Limited description of nonpharmacologic intervention |
| Clumeck et al [22] N = 25 | Prospective uncontrolled/ inpatient/Belgium | Severe S aureus infection; Age: 62 (18–88) SSTI, N = 4 Other cohort indications: PNA, osteomyelitis septic thrombophlebitis, febrile urinary tract infection, endocarditis, and liver abscess | Minocycline + rifampin Entire cohort (N = 25) description DOT: 5–119 d (mean 22) (PO, N = 20; IV, N = 5) | SSTI Cure: 3/4 (75) | Uncontrolled study; small sample size Limited description of nonpharmacologic intervention 21 patients received minocycline 100 mg Q12H, and 4 patients received 200 mg every Q12H |
| Ruhe et al [23] N = 24 | Retrospective/inpatient/United States | Serious, tetracycline-susceptible MRSA infection Age: 51 (28–94) Complicated SSTI, N = 16 | Minocycline (PO); N = 5 Doxycycline (PO); N = 11 | Cure: 5/5 (100) 10/11 (91) | Retrospective; small sample size Allowed alternative initial antibiotic if ≤50% of appropriate treatment duration DOT not reported specific to SSTI |
| Barnes et al [24] N = 30 | Retrospective review with prospective observation/outpatient/United States | Nonserious; MRSA-SSTI; cellulitis, abscess, or both Age: 46 (18–83) | Minocycline (PO) Doxycycline (PO) Trim/sulfa (PO) Clindamycin (PO) Drainage only β-lactam (PO) + drainage Fluoroquinolone (PO) | Cure: 3/3 (100) 1/1 (100) 6/6 (100) 8/8 (100) 4/4 (100) 5/5 (100) 3/3 (100) | Retrospective/observational; small sample size Dosing not reported; DOT not reported 1 patient treated with drainage only, and 1 patient treated with β-lactam + drainage experience recurrence after 30 d |
| Ruhe and Menon [25] N = 282 | Retrospective/ outpatient/United States | Community-acquired purulent S aureus SSTI Age: 48 (18–85) | Minocycline (PO); N = 3 or Doxycycline (PO); N = 87 DOT: 3–20 d (median 10) Incision/drainage; 77% β-lactam (PO and/or IV); N = 192 DOT: not reported Incision/drainage; 81% | Cure: 86/90 (96) 168/192 (88) | Retrospective Few patients received minocycline Clinical outcomes of minocycline/doxycycline reported as aggregate 20 of 168 β-lactam treatment successes occurred in patients changed from β-lactam to targeted therapy based on antimicrobial susceptibility data |
| Rogers et al [26] N = 24 | Prospective uncontrolled/ inpatient/United States | Severe (investigator reported) infections SSTI, N = 6 Age: 56 (43–77) Mixed PNA, N = 14 Age: 48 (5–84) | Minocycline (IV) | Cure: SSTI; 4/6 (67) Mixed PNA; 14/14 (100) | Uncontrolled, small sample size Heterogeneous disease states DOT: Not reported |
| Community-acquired pneumonia | |||||
| Kawai et al [27] N = 30 | Prospective uncontrolled/inpatient or outpatient/Japan | Mycoplasma pneumoniae CAP Macrolide resistant, N = 21 Age: 8 (1–15) | Minocycline required for 15 patients with macrolide treatment failure DOT: 8–11 d (route not reported) | Defervesce within 48 H: 15/15 (100) | Uncontrolled study; only included confirmed M pneumonia; small sample size Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Okada et al [28] N = 202 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 176 Age: 8 (1–14) | Secondary agent used in macrolide resistance: Minocycline; N = 52 DOT: 2–7 d (mean 5) Doxycycline; N = 16 DOT: 3–7 d (mean 3) Macrolides; N = 13 DOT: 3–10 d (mean 6) Tosufloxacin; N = 13 DOT: 2–7 d (mean 5) (routes not reported) | Defervesce within 48 H: 47 (90) 14 (88) 6 (46) 9 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline used as secondary agent |
| Kawai et al [29] N = 188 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP macrolide resistant, N = 150 Age: 8 (0–15) | Definitive treatment in macrolide resistance: Minocycline, N = 38 Azithromycin, N = 27 Clarithromycin, N = 23 Tosufloxacin, N = 62 (routes not reported) | Defervesce within 48 H: 33 (87) 11 (41) 11 (48) 43 (69) | Observational study; only included confirmed M pneumoniae Clinical outcome assessed as presence of fever Minocycline (age ≥8)/tosufloxacin (age <8) used as definitive therapy Treatments determined by attending physician/not standardized DOT: Not reported |
| Miyashita et al [30] N = 73 | Prospective observational/ inpatient or outpatient/Japan | M pneumoniae CAP Macrolide resistant, N = 30 Age: 23 (16–45) | Initial treatment in macrolide resistance: Minocycline, N = 7 Macrolides, N = 14 Quinolones, N = 9 (All admitted patients received IV minocycline, other specifics of route not reported) | Defervesce within 48 H: 6 (85) 4 (28) 7 (77) | Observational study; only included confirmed M pneumoniae; small sample size Clinical outcome assessed as presence of fever DOT: Not reported |
| Lyme disease | |||||
| Weber et al [31] N = 107 | Prospective, nonrandomized intervention/not reported/Germany | Early erythema migrans, N = 97 Age: Not adequately reported | Minocycline, N = 11 DOT: 10–15 d Doxy/tetracycline, N = 8 DOT: 10–14 d Penicillin (PO), N = 65 DOT: 10–14 d Penicillin (parenteral), N = 7 DOT: 10–14 d Erythromycin, N = 6 DOT: 10 d (other routes not specifically reported) | Time to cure (weeks): mean/median 7/2 19/2 22/2 7/2 2/9 | Nonrandomized; small sample size Limited differentiation in outcomes between minocycline and doxycycline |
| Muellegger et al [32] N = 14 | Prospective uncontrolled/not reported/Austria | Erythema migrans, Borrelia burgdorferi DNA confirmed (57%) Age: 53 (34–79) | Minocycline (PO); N = 14 DOT: 14 d | Good clinical response: 14/14 (100) | Uncontrolled; small sample size |
| Breier et al [33] N = 60 | Prospective open label randomized/outpatient/Austria | Erythema migrans Age: 43 (19–80) | Minocycline, N = 30 DOT: 21 d Penicillin V, N = 30 DOT: 21 d (route not reported) | Both groups: Complete recovery; no late disease at 1 y | Withdraw due to side effects (minocycline, 12; penicillin V, 9) excluded from analysis Open label design; small sample size |
| Schmidt et al [34] N = 26 | Prospective uncontrolled/not reported/Austria | Erythema migrans Age: 56 (20–84) | Minocycline (PO) DOT: 14 d | 8 wks post therapy: 20/22 (91) Erythema Migrans clear; 0/22 (0) Additional symptoms present | Uncontrolled; small sample size 4 patients lost to follow up at 8 wks Limited report of clinical outcomes |
| Stanek et al [35] N = 99 | Prospective uncontrolled/outpatient/Austria | Erythema migrans Age: 50 (10–80) | Minocycline (PO) Amoxicillin (PO) Azithromycin (PO) Doxycycline (PO) Penicillin V (PO) DOT: 2–3 wks (all treatments) | Complete resolution in all patients within 3 wks | Uncontrolled; limited report of clinical outcomes Number of patients treated with each antibiotic not reported |
| Glatz et al [36] N = 113 | Retrospective/not reported/ Austria | Erythema migrans Age: 51 (5–78) | Minocycline (PO), N = 61* Doxycycline (PO), N = 13* β-lactam (PO/IV), N = 36 DOT: Not reported | Complete symptom resolution within 1 mo: 109/113 (96) | Retrospective; variable antibiotic treatment Limited report of clinical outcomes Clinical outcome reported as aggregate |
| Rickettsial infections | |||||
| Kodama et al [37] N = 28 | Retrospective/inpatient/Japan | Japanese spotted fever Age: 60 (12–78) Died before treatment, N = 1 | Minocycline; N = 25 Minocycline + steroids; N = 2 (route not reported) | Cure: 25/25 (100%) 2/2 (100%) | Retrospective, small sample size Minocycline dosing not reported DOT: Not reported |
Abbreviations: CAP, community-acquired pneumonia; DOT, duration of therapy; H, hours; IV, intravenous; MRSA, methicillin-resistant S aureus; PNA, pneumonia; PO, per oral; PRN, as needed; Q, every; SSTI, skin and soft-tissue infections; Trim/sulfa, trimethoprim/sulfamethoxazole.
a Values calculated from data presented in Table 1 of Arch Dermatol 2006;142(7):862–868.
RESULTS
Literature Review
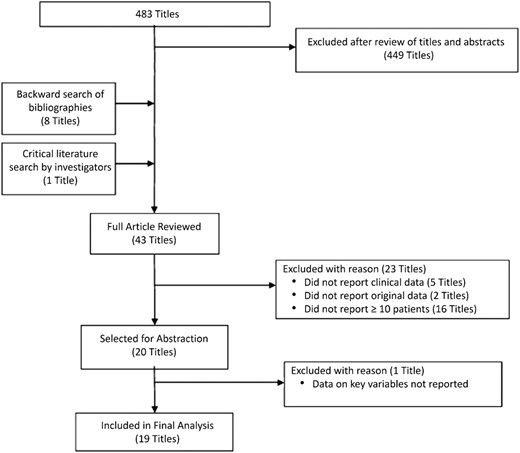
The electronic search of Medline through PubMed identified 483 titles. Of the 43 reports [19–61] selected for full review, 23 reports [38–60] were excluded for reason before abstraction (Figure 2). One additional title [61] was excluded during record abstraction because key data were missing regarding the use of minocycline. The remaining 19 reports [19–37] are described in Table 1. No additional titles were identified through EMBASE. We identified 8 reports related to SSTIs [19–26], 5 related to CAP [26–30], 6 related to Lyme disease [31–36], and 1 related to rickettsial disease [37]. Six of the published reports were retrospective in nature, whereas 13 were prospective. Of the prospective studies, only 1 was randomized—however open-label—whereas an additional 6 included clinical outcomes on treatments in addition to minocycline. Published reports were from 1971 to 2013 and included outcome data on 336 patients treated with minocycline.
Skin and Soft Tissue Infections
In 3 of the studies regarding SSTIs, minocycline was dosed with an initial 200 mg loading dose, and 2 of those studies continued therapy with minocycline 100 mg twice daily [20, 21], and the third study continued minocycline 100 mg once daily [19]. Three studies dosed minocycline 100 mg twice daily without a loading dose [23, 25, 26], 1 study dosed minocycline 100 mg twice daily or 200 mg twice daily [22], and the final study did not report specific dosing [24]. Cure rate was high (89%) in the 7 studies that reported cure specific to minocycline use [19–24, 26]. However, in 1 report [26], 2 of 6 (33%) patients with severe infection failed minocycline therapy. One patient was infected with an organism resistant to minocycline, and the other patient suffered from concurrent bacteremia. In contrast, the largest single report of minocycline use in SSTIs demonstrated cure in all 15 patients treated for severe Staphylococcus aureus infections [21]. Limitations of the data must be recognized. Only 4 studies were prospective, and none were randomized. The largest single report of minocycline use in SSTIs included only 15 patients. In addition, reports were generally limited in their description of nonpharmacologic treatments or in details regarding infection purulence. Therefore, the potential exists that some patients included in these reports may be treated differently based on current guidelines, that is, incision and drainage without systemic antibiotics. However, it should be noted that several of the studies did include patients with severe or complicated infections that would most likely require systemic antibiotics under current guidelines [19, 21, 22, 26].
Community-Acquired Pneumonia
All but 1 identified report focused on the treatment of Mycoplasma pneumoniae [26]. In addition, these reports were published recently, all from Japan, and primarily related to macrolide-resistant M pneumoniae [27–30]. All studies were prospective, although none were randomized. Three of the reports are exclusively related to pediatrics, whereas 1 included adults [30]. In all studies, the clinical outcome was defervescence, which occurred in a large proportion of patients (85%–100%). Three studies included treatments other than minocycline—eg, doxycycline, quinolones, and macrolides—and in each study, minocycline had the highest proportion of responders. However, comparisons should be made cautiously due to the observational nature of these studies. An additional limitation is that the majority of the studies focused on confirmed M pneumoniae rather than empiric treatment of CAP. The remaining report included patients with mixed pneumonia presentations, not exclusively CAP [26]. Although there was great heterogeneity in patient presentation, all were considered “severe”. All 14 patients achieved a satisfactory clinical response after intravenous minocycline therapy.
Lyme Disease Prophylaxis
No reports were identified that assessed Lyme disease prophylaxis with minocycline. The 6 included studies (all completed in Europe) assessed minocycline in the treatment of early erythema migrans [31–36]. Each study—including 1 prospective open-label randomized study—demonstrated high cure rates with minocycline therapy. In the randomized study, minocycline was compared with penicillin V. The dropout rate was high, with 30% (penicillin V) and 40% (minocycline) of patients stopping treatment before completing the planned 21-day course [33]. All patients that completed the course of minocycline achieved cure at the end of therapy and were symptom free at 1 year. In the most recent report, 109 of 113 (96.5%) patients treated for early erythema migrans with minocycline, doxycycline, or a β-lactam achieved complete symptom resolution by 1 month [36]. However, this report was primarily focused upon serum antibody responses and did not report cure per treatment strategy. However, >50% of the patients were treated with minocycline, and only 4 patients overall had any remaining symptoms at 1 month.
Rickettsial Disease
The 1 identified report regarding rickettsial disease was in 28 Japanese patients treated for Japanese spotted fever, the causative organism being Rickettsia japonica [37]. Twenty-seven (96%) patients achieved cure with minocycline therapy. One patient with fulminant disease died before administration of minocycline. The study included 5 other patients with severe and potentially life-threatening diseases that were successfully treated with minocycline.
DISCUSSION
Skin and Soft-Tissue Infection
The data reported herein support the effectiveness of minocycline in a broad range of SSTIs. Patients that are immunocompromised or presenting with severe disease will likely be treated with nontetracycline intravenous antibiotics, in line with the current standard of care [17, 26, 62]. However, in circumstances amenable to the use of doxycycline, it appears that minocycline is more than a reasonable alternative. In addition, there are no pharmacokinetic properties, in vitro data, or in vivo data that we are aware of that portend minocycline to be inferior to doxycycline in the treatment of SSTIs. The 2011 Infectious Disease Society of America (IDSA) MRSA (methicillin-resistant S aureus) guideline [17] includes “doxycycline or minocycline” as A-II treatment options for empirical coverage of community-acquired MRSA SSTIs in outpatients. Furthermore, minocycline may be advantageous over doxycycline because it does not appear to be prone to inducing its own resistance [63]. These final points are absent from the most recent SSTI guidelines from the IDSA [62], potentially omitted to aid concision because the MRSA guidelines are still considered up-to-date. Therefore, we recommend minocycline substitution for doxycycline in the treatment of SSTIs during doxycycline shortage. Considering the frequency of SSTIs [62], the substitution of minocycline is a reasonable initiative to conserve doxycycline supplies for indications with less desirable alternatives.
Community-Acquired Pneumonia
The decision to use minocycline in CAP must be considered in context of the overarching treatment recommendations for CAP [18, 64–66]. Current guidelines recommend doxycycline monotherapy as an alternative treatment only for otherwise healthy patients with low risk for drug-resistant Streptococcus pneumoniae (weak recommendation, level III evidence) [18]. Likewise, we found limited evidence to support minocycline monotherapy in severe infections or in confirmed S pneumoniae infection. The reviewed data for minocycline in CAP were positive but mostly limited to M pneumoniae infection. In considering the outpatient treatment of young otherwise healthy patients—where atypical organisms (primarily M pneumoniae) may be of more concern—minocycline could be used within the same framework in which doxycycline is recommended. Our recommendation also takes into consideration the increased vaccination for pneumococcus and the typically severe presentation of Legionnaires' disease [66]. Overall, empirical tetracycline monotherapy should be reserved for uncomplicated cases of CAP [18].
Contrary to this narrow recommendation, there is compelling data to support minocycline's use in CAP specifically caused by macrolide-resistant M pneumoniae [27–30]. Considering the increased incidence of macrolide-resistance M pneumoniae in the United States and globally [67], there will likely be an increased need for tetracycline therapy in CAP. Furthermore, there is increased attention to the cardiovascular risk profile of macrolides and fluoroquinolones, both of which are commonly used for empirical coverage of atypical pathogens in CAP [68, 69]. For patients with underlying cardiac comorbidities, tetracyclines, including minocycline, may be preferred as adjuncts to antipneumococcal β-lactams. In circumstances in which there is concern for treating macrolide-resistant M pneumoniae or when doxycycline would be preferred for empirical atypical coverage, we recommend substituting minocycline for doxycycline.
Lyme Disease Prophylaxis
At this time, the CDC only recommends doxycycline as an option for Lyme disease prophylaxis due to lack of evidence with other medications. The CDC specifically recommends against single-dose amoxicillin due to its short half-life (eg, approximately 1 hour) [1, 70]. However, minocycline has an extended half-life similar to doxycycline (eg, >12 hours) [7, 9]. Minocycline appears effective in the treatment of early Lyme disease [31–36]. Unfortunately, we found no data regarding minocycline for Lyme disease prophylaxis following tick bite. However, most studies regarding prophylaxis are equivocal due to the low event rate [71–75]. The most recent guideline from the IDSA includes an option for prophylaxis with single-dose doxycycline in patients meeting strict criteria [76]. Therefore, when prophylaxis is indicated in the absence of doxycycline, we recommend clinicians consider minocycline and exercise shared decision making [77]. We recommend that this conversation include at least 5 key points: (1) the overall risk of developing Lyme disease is low and forgoing prophylaxis may be reasonable; (2) there are effective treatments for early Lyme disease; (3) the benefit of antibiotic prophylaxis is small and may be offset by the risk for adverse effects with antibiotic treatment; (4) doxycycline is usually recommended, however, if unavailable, minocycline is a similar but less studied antibiotic; and (5) although minocycline may work better, as well, or not as well other antibiotics, it is suggested as a potential alternative because it works similarly to doxycycline, and it has been shown to be effective in patients who have developed Lyme disease.
Rickettsial Diseases
The literature to support the use of minocycline in rickettsial diseases is severely limited. Although the included report of Japanese spotted fever had a rate of life-threatening disease on par with Rocky Mountain spotted fever—the most common rickettsial disease in the United States—it is difficult to use these data as a surrogate. Rocky Mountain spotted fever is a circumstance that warrants retaining a supply of doxycycline in endemic areas. However, the CDC did not definitively recommend alternative therapy should doxycycline be entirely unavailable [1]. Therefore, we pose that it is worthy to consider minocycline's place in therapy for Rocky Mountain spotted fever—given its lethality—in the scenario of complete doxycycline unavailability.
Older and in vitro data demonstrate that tetracycline may be a potential alternative to doxycycline [78–80]. To our knowledge, there are no reports of increasing resistance of Rickettsia rickettsii to any tetracycline antibiotic. Considering minocycline and doxycycline's similar pharmacokinetic and susceptibility profiles, we portend minocycline to be a potential alternative, despite the dearth of clinical data. Important in this consideration is that further alternatives may be considerably less desirable. The CDC mentions the potential use of chloramphenicol as an alternative to doxycycline [1]. However, the CDC rightly notes that chloramphenicol is associated with a greater mortality risk compared with treatment regimens that include a tetracycline. This fact is supported by 2 large surveillance studies that demonstrate chloramphenicol monotherapy to be statistically associated with fatal disease compared with treatment with any tetracycline as monotherapy or with any tetracycline in combination with chloramphenicol [81, 82]. Unfortunately, these studies did not delineate the different tetracycline agents used. Considering the lethality of Rocky Mountain spotted fever and the potential inferiority of chloramphenicol monotherapy, we recommend treatment with either minocycline or tetracycline in combination with chloramphenicol over chloramphenicol monotherapy in the absence of doxycycline. The limitations of these data are significant and more research—including observational and retrospective reports—is greatly needed. We make this tenuous recommendation because there is the potential for this circumstance to occur and no direct guidance from the CDC or other authorities.
CONCLUSIONS
In conclusion, drug shortages interfere with the management of infectious diseases and necessitate the use of less familiar alternatives. This systematic review supports the use of minocycline as a substitute for doxycycline in SSTIs, an alternative in the outpatient treatment of CAP in young otherwise healthy patients (with more evidence in macrolide-resistant M pneumoniae), an alternative to doxycycline for Lyme disease prophylaxis (should prophylaxis be strongly desired and doxycycline is unavailable), and a last alternative in select rickettsial diseases should doxycycline be entirely unavailable. Given the myriad of indications for which doxycycline can be used, further research, review, and guidance are needed to prepare practitioners, institutions, and health systems to provide adequate care in the face of tetracycline shortages and antibiotic shortages in general.
Acknowledgments
Disclaimer. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References





Comments