-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Butler-Laporte, Elizabeth Smyth, Alexandre Amar-Zifkin, Matthew P Cheng, Emily G McDonald, Todd C Lee, Low-Dose TMP-SMX in the Treatment of Pneumocystis jirovecii Pneumonia: A Systematic Review and Meta-analysis, Open Forum Infectious Diseases, Volume 7, Issue 5, May 2020, ofaa112, https://doi.org/10.1093/ofid/ofaa112
Close - Share Icon Share
Abstract
Pneumocystis jirovecii pneumonia (PJP) remains a common and highly morbid infection for immunocompromised patients. Trimethoprim-sulfamethoxazole (TMP-SMX) is the antimicrobial treatment of choice. However, treatment with TMP-SMX can lead to significant dose-dependent renal and hematologic adverse events. Although TMP-SMX is conventionally dosed at 15–20 mg/kg/d of trimethoprim for the treatment of PJP, reduced doses may be effective and carry an improved safety profile.
We conducted a systematic search in the Medline, Embase, and Cochrane Library databases from inception through March 2019 for peer-reviewed studies reporting on reduced doses of TMP-SMX (15 mg/kg/d of trimethoprim or less) for the treatment of PJP. PRISMA, MOOSE, and Cochrane guidelines were followed. Gray literature was excluded.
Ten studies were identified, and 6 were included in the meta-analysis. When comparing standard doses with reduced doses of TMP-SMX, there was no statistically significant difference in mortality (absolute risk difference, –9% in favor of reduced dose; 95% confidence interval [CI], –27% to 8%). When compared with standard doses, reduced doses of TMP-SMX were associated with an 18% (95% CI, –31% to –5%) absolute risk reduction of grade ≥3 adverse events.
In this systematic review, treatment of PJP with doses of ≤10 mg/kg/d of trimethoprim was associated with similar rates of mortality when compared with standard doses and with significantly fewer treatment-emergent severe adverse events. Although limited by the observational nature of the studies included, this review provides the most current available evidence for the optimal dosing of TMP-SMX in the treatment of PJP.
Pneumocystis jirovecii pneumonia (PJP) is an opportunistic infection that is associated with significant morbidity and mortality, particularly among non-HIV immunocompromised patient populations [1]. The overall mortality from PJP is ~6%–11% [2], but can reach as high as 50% depending on the degree of immune suppression, the number and complexity of comorbidities, access to social support, and living conditions [3].
For more than 4 decades, the treatment of choice for PJP has been weight-based trimethoprim-sulfamethoxazole (TMP-SMX) at a dose of 15–20 mg/kg/d of trimethoprim component. This recommendation was based on a seminal study [4] of 20 children with leukemia, which determined that a dose of 20 mg/kg/d of TMP-SMX led to numerically improved outcomes compared with 4–7 mg/kg/d. Ultimately, this study was underpowered to compare the 2 doses, and the small difference between groups was not statistically different. Nonetheless, the higher dose was adopted into practice and extrapolated to adult patients, becoming the standard of care for PJP therapy.
Although highly effective for the treatment of PJP, TMP-SMX is associated with serious adverse events in up to 57% of HIV-infected patients [5]. This includes hypersensitivity reactions, drug-induced liver injury, cytopenias, hyperkalemia, and renal failure [6]. The frequency of adverse hematologic and renal events increases in a dose-dependent manner [6], which commonly limits the use of TMP-SMX in patients with underlying hematologic diagnoses or solid organ transplants, who represent up to a third of PJP cases [1]. Two strategies have been proposed to mitigate treatment-emergent adverse events due to TMP-SMX, including (1) sequential step-down to a reduced dose of TMP-SMX to complete the full course of treatment or (2) initiating treatment with a reduced total daily dose from the outset. To better inform future discussions on optimal PJP therapy, we conducted a systematic review and meta-analysis of reduced dose regimens of TMP-SMX, including step-down therapy, in the treatment of PJP in immunocompromised adult patients, with and without HIV.
METHODS
Search Strategy
This article was prepared according to PRISMA and MOOSE reporting guidelines [7, 8], and followed recommendations from the Cochrane Handbook on Systematic Reviews of Interventions [9]. We searched the MEDLINE, Embase, and Cochrane Library databases for studies reporting treatment outcomes when using different TMP-SMX treatment regimens for PJP. We also mined the selected studies’ reference lists to further identify relevant articles for inclusion in our review. The search strategy was designed for use on the OvidSP platform with the help of an experienced hospital librarian (A.A.-Z.) and included variations and synonyms of TMP-SMX and PJP. The full search strategy can be found in the Supplementary Data.
Study Selection
Selected studies were separated into 3 categories for analysis. The first category included studies that reported on mortality and adverse events comparing standard and reduced (defined as ≤15 mg/kg/d) doses of TMP-SMX. The second category included studies that reported on mortality rates in cohorts that only contained a reduced-dose TMP-SMX treatment arm, in the absence of a standard-dose comparator. The third category included studies that examined mortality following “step-down treatment,” whereby treatment was started at a higher dose and later lowered to a reduced dose (timing of step-down varied between studies). To compare cohorts that only reported on reduced-dose TMP-SMX, we estimated the mortality rate for standard-dose TMP-SMX from a historical cohort of patients treated with higher doses of TMP-SMX using data extracted from PJP randomized controlled trials obtained from our search strategy.
To limit selection bias, we excluded studies that had a high likelihood of poor external validity. We specifically excluded studies that only involved children, studies with <20 patients, conference abstracts, and other gray literature. There were no language exclusion criteria. Given the evolving nature of microbiological methods used to diagnose PJP, we did not exclude studies based on the criteria used to diagnose the infection. Three independent reviewers screened the study database (G.B.L., E.S., M.P.C.), first by title and abstract, and then assessed their eligibility and quality by full-text review. Disagreements were resolved by consensus.
Data Extraction and Quality Assessment
Once the studies were selected and categorized, we extracted the data using an extraction form and protocol. Specifically, we abstracted data on mortality and adverse events. The duration of follow-up and the definition of adverse events that were included in the meta-analysis were based on the included studies’ methodologies (see the “Results” section). We also abstracted basic epidemiological data to allow better study comparison (patient age and sex, underlying immunosuppressive condition(s), inclusion and exclusion criteria, microbiological methods, and adjunctive corticosteroid usage). The Robins-1 quality assessment tool [10] was used for studies with 2 or more treatment arms. Observational cohort studies with data availability limited to reduced-dose TMP-SMX and randomized controlled trials used for estimating standard-dose TMP-SMX mortality were assessed qualitatively only.
Data Synthesis and Analysis
After data extraction, we performed a random-effects meta-analysis to pool mortality and adverse events where appropriate. All analyses and graphs were performed with R statistical software (version 3.5.1) using the meta package (version 4.9–5).
RESULTS
Included Studies
The original search was performed on December 6, 2018, and updated March 29, 2019. Figure 1 outlines the review process. After duplicate removal, 6544 articles were screened based on title and abstract, leaving 63 for full-text review. Most studies that were excluded had no reduced-dose TMP-SMX component (15/63), had <20 patients (6/63), or did not clearly specify the drug dose (5/63). For the estimation of standard-dose mortality, the major reason to exclude was an unclear specification of dose (4/63). Screening references of the included papers yielded no additional articles, as all potentially relevant titles had previously been included or excluded based on abstract or full-text review. Overall study quality and the included populations varied between studies, with detailed study characteristics and the Robins-1 outcome summary included in the Supplementary Data.
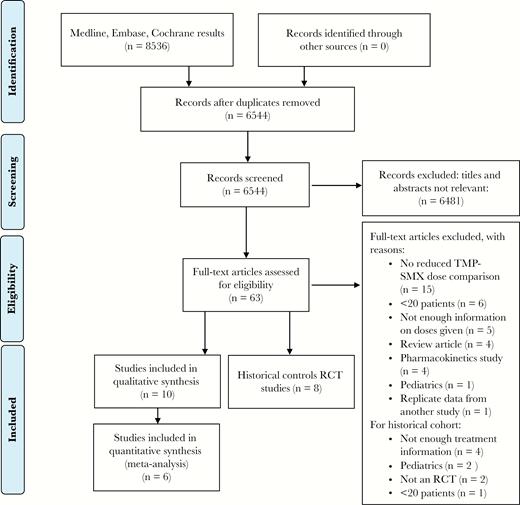
Prisma flow diagram. Abbreviations: RCT, randomized controlled trial; TMP-SMX, trimethoprim-sulfamethoxazole.
Standard-Dose vs Reduced-Dose TMP-SMX
We found 4 studies comparing the use of at least 2 different TMP-SMX doses [11–14]. All studies were retrospective. Three of the studies [11–13] were performed in Japan in the last 5 years and included immunosuppressed patients without HIV who mainly had rheumatologic diseases or solid/hematologic malignancies. In 1 of these 3 studies, patients with creatinine clearance <30 mL/min were excluded. In the other 2, the dose of TMP-SMX given to patients was adjusted for renal dysfunction using the Japanese TMP-SMX nomogram [13], but patients were analyzed according to the projected TMP-SMX dose (standard-dose vs reduced-dose group) as though their renal function was normal. For example, patients with creatinine clearance of 15–30 mL/min and receiving 7.5 mg/kg/d of TMP-SMX would be analyzed as though they received 15 mg/kg/d of TMP-SMX. Using the Robins-1 tool, these 3 studies were considered at moderate risk of bias, mainly due to the retrospective nature.
The fourth study [14] was not included in the meta-analysis as it was deemed to be at critical risk of bias using the Robins-1 tool. While it found improved outcomes in the standard–TMP-SMX dose group in the unadjusted analysis, there was no statistical difference between the 2 groups in the multivariate analysis, which controlled for renal function. This suggests that the reduced-dose group may have contained more patients with renal dysfunction and a worse prognosis. However, we did not have access to sufficient data to adjust for patients’ renal function and had to exclude it from our analysis.
The meta-analysis was therefore performed on 3 Japanese studies with mortality at 30 days (90 days for Kosaka et al. [11]) as the treatment outcome. The 3 studies that were included differed based on TMP-SMX dosing strategy. Kosaka et al. [11] had 2 arms: 15–20 mg/kg/d and <15 mg/kg/d. Nakashima et al. [12] also had 2 arms: 10–20 mg/kg/d and 4–10 mg/kg/d, whereas Ohmura et al. [13] had 3 dosing regimens: 15–20 mg/kg/d, 10–15 mg/kg/d, and <10 mg/kg/d. For the meta-analysis, we pooled the 2 lowest-dose treatment arms in Ohmura et al. [13] and compared the 2 TMP-SMX dose groups obtained in each study (Table 1). The meta-analysis showed no statistically significant difference in mortality between standard-dose and reduced-dose treatment (absolute risk difference, –9%; 95% confidence interval [CI], –27% to 8%) (Figure 2).
| . | Comparisons . | |
|---|---|---|
| Analysis . | Reduced-Dose TMP-SMX, mg/kg/d | Standard-Dose TMP-SMX, mg/kg/d |
| Comparative cohorts: mortality | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Comparative cohorts: adverse events | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Reduced-dose cohorts only: mortality | Cohorts reporting on TMP-SMX for PJP at a dose less than 15 mg/kg/d: Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: 4–10 Sattler et al. [17]: mean 12 Thomas et al. [18]: median 9.7 Yang et al. [19]: median 14.4 | Historical cohorts of RCTs reporting on TMP-SMX therapy for PJP: Gagnon et al. [20]: 15 Klein et al. [21]: 20 Medina et al. [5]: 20 Saffrin et al. [22]: 12–20 Sattler et al. [23]: 20 Toma et al. [24]: >16 Toma et al. [25]: >16 Wharton et al. [26]: 20 |
| . | Comparisons . | |
|---|---|---|
| Analysis . | Reduced-Dose TMP-SMX, mg/kg/d | Standard-Dose TMP-SMX, mg/kg/d |
| Comparative cohorts: mortality | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Comparative cohorts: adverse events | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Reduced-dose cohorts only: mortality | Cohorts reporting on TMP-SMX for PJP at a dose less than 15 mg/kg/d: Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: 4–10 Sattler et al. [17]: mean 12 Thomas et al. [18]: median 9.7 Yang et al. [19]: median 14.4 | Historical cohorts of RCTs reporting on TMP-SMX therapy for PJP: Gagnon et al. [20]: 15 Klein et al. [21]: 20 Medina et al. [5]: 20 Saffrin et al. [22]: 12–20 Sattler et al. [23]: 20 Toma et al. [24]: >16 Toma et al. [25]: >16 Wharton et al. [26]: 20 |
Abbreviations: PJP, Pneumocystis carinii pneumonia; RCT, randomized controlled trial; TMP-SMX, trimethoprim-sulfamethoxazole.
| . | Comparisons . | |
|---|---|---|
| Analysis . | Reduced-Dose TMP-SMX, mg/kg/d | Standard-Dose TMP-SMX, mg/kg/d |
| Comparative cohorts: mortality | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Comparative cohorts: adverse events | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Reduced-dose cohorts only: mortality | Cohorts reporting on TMP-SMX for PJP at a dose less than 15 mg/kg/d: Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: 4–10 Sattler et al. [17]: mean 12 Thomas et al. [18]: median 9.7 Yang et al. [19]: median 14.4 | Historical cohorts of RCTs reporting on TMP-SMX therapy for PJP: Gagnon et al. [20]: 15 Klein et al. [21]: 20 Medina et al. [5]: 20 Saffrin et al. [22]: 12–20 Sattler et al. [23]: 20 Toma et al. [24]: >16 Toma et al. [25]: >16 Wharton et al. [26]: 20 |
| . | Comparisons . | |
|---|---|---|
| Analysis . | Reduced-Dose TMP-SMX, mg/kg/d | Standard-Dose TMP-SMX, mg/kg/d |
| Comparative cohorts: mortality | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Comparative cohorts: adverse events | Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: <15 | Kosaka et al. [11]: 15–20 Nakashima et al. [12]: 10–20 Ohmura et al. [13]: 15–20 |
| Reduced-dose cohorts only: mortality | Cohorts reporting on TMP-SMX for PJP at a dose less than 15 mg/kg/d: Kosaka et al. [11]: <15 Nakashima et al. [12]: 4–10 Ohmura et al. [13]: 4–10 Sattler et al. [17]: mean 12 Thomas et al. [18]: median 9.7 Yang et al. [19]: median 14.4 | Historical cohorts of RCTs reporting on TMP-SMX therapy for PJP: Gagnon et al. [20]: 15 Klein et al. [21]: 20 Medina et al. [5]: 20 Saffrin et al. [22]: 12–20 Sattler et al. [23]: 20 Toma et al. [24]: >16 Toma et al. [25]: >16 Wharton et al. [26]: 20 |
Abbreviations: PJP, Pneumocystis carinii pneumonia; RCT, randomized controlled trial; TMP-SMX, trimethoprim-sulfamethoxazole.
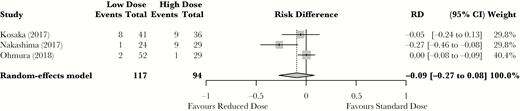
Standard-dose vs reduced-dose trimethoprim-sulfamethoxazole mortality meta-analysis. Results are reported as relative risk difference. Abbreviations: CI, confidence interval; RD, risk difference.
Adverse Events
Four studies reported on adverse events based on dose of TMP-SMX [11–13, 15] and were assessed for inclusion in our analysis, including the 3 previous Japanese studies [11–13]. Two studies used the Common Terminology Criteria for Adverse Events [16] (CTCAE) scale to report only grade 3 adverse events, whereas Nakashima et al. [12] defined their own adverse events. These 3 studies all considered that adverse events prompting termination of treatment were significant. The fourth study by Lee et al. [15] performed a retrospective study on the risk of TMP-SMX-induced psychosis. This study was not retained for analysis as it had a critical risk of bias using the Robins-1 tool. Although it did show an increased incidence of acute psychosis with standard doses, it did not report on any other adverse events and more importantly did not specify how patients were allocated to standard or reduced TMP-SMX doses. The final adverse event meta-analysis yielded a statistically significant absolute risk reduction of 18% (95% CI, –31% to –5%) in favor of the reduced-dose TMP-SMX arm (Figure 3).
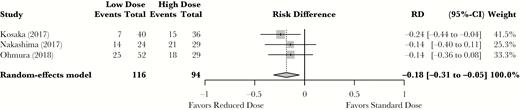
Standard-dose vs reduced-dose trimethoprim-sulfamethoxazole adverse events meta-analysis. Results are reported as relative risk difference. Abbreviations: CI, confidence interval; RD, risk difference.
Mortality in Studies Only Reporting on Reduced-Dose TMP-SMX Use
We found an additional 3 studies [17–19] that reported on reduced-dose TMP-SMX. These studies were limited to patients with HIV. Two [18, 19] were retrospective, whereas the third [17] was a randomized controlled trial comparing the use of TMP-SMX (mean dose of 12 mg/kg/d) and pentamidine. Of the 2 retrospective studies, 1 reported on the result of the implementation of a policy in a New Zealand hospital mandating that any further PJP therapy with TMP-SMX would aim for TMP doses of 10 mg/kg/d [18]. The second retrospective study was performed in Taiwan to assess hepatic adverse events from the use of TMP-SMX, and the median dose was 14.4 mg/kg/d [19]. None of these studies explicitly adjusted for renal function; however, they were retained for analysis as they were already reduced-dose treatment regimens, and in a population with low rates of chronic kidney disease. We pooled these studies with the reduced-dose TMP-SMX arms of the previously included studies to estimate the pooled mortality in patients who received lose-dose TMP-SMX (Figure 4). This pooled mortality rate was 9% (95% CI, 6% to 14%).
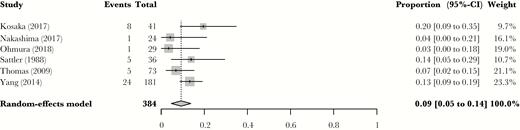
Reduced-dose mortality Pneumocystis carinii pneumonia mortality rates with reduced doses of trimethoprim-sulfamethoxazole, including single-arm cohorts with HIV patients. Results are reported in relative risk. Abbreviation: CI, confidence interval.
To compare the result of the reduced-dose TMP-SMX mortality estimate with a standard-dose estimate, we analyzed data extracted from 8 randomized controlled trials in which at least 1 treatment arm included TMP-SMX at a dose exceeding 15 mg/kg/d [5, 20–26]. These studies were all performed in patients with HIV before the widespread availability of antiretroviral therapy and had restrictive exclusion criteria. Exclusion criteria included neutropenia or concomitant therapy with myelosuppressive agents for all studies, and renal failure was an exclusion criterion in all but 1 [23]. A pooled mortality rate of 11% (95% CI, 4% to 18%) at 1–3 months (depending on the study) was obtained for the standard-dose arm, which is comparable to the pooled retrospective mortality in the single-arm reduced-dose studies (Figure 5).
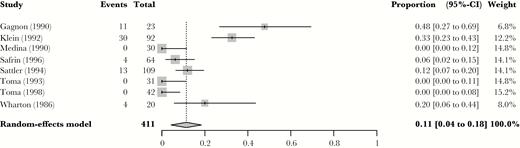
Mortality rate from historical control cohort of standard dose trimethoprim-sulfamethoxazole, as extracted from eligible randomized controlled trials on Pneumocystis carinii pneumonia therapy. Abbreviation: CI, confidence interval.
Step-Down Therapy
Two studies [27, 28] addressed outcomes related to “step-down therapy,” the practice of providing standard-dose therapy at initial presentation and later reducing the dose of TMP-SMX before the completion of therapy in an effort to minimize adverse events. Both studies were retrospective, and participants had a similar clinical status and degrees of immune suppression on admission. In Eeftinck Schattenkerk et al. [28], participants could be switched to a reduced dose of TMP-SMX if they developed an adverse event within 14 days of initiating treatment. Details were not provided on the severity of disease or type of adverse events that factored into the decision to step down to a reduced dose. In Creemers-Schild et al. [27], the decision to step down was based on clinical stability and was at the discretion of the treating physician. Even if they both reported on the safety of step-down strategies, using Robins-1 the 2 studies were deemed to be at a severe or critical risk of bias given that the treatments that were compared were conditioned on the outcome. Therefore, they were not meta-analyzed.
DISCUSSION
Extrapolated from early and limited pediatric data, a standard dose of 15–20 mg/kg/d of TMP-SMX for the treatment of PJP has been routinely used in clinical practice and is recommended in major guidelines. This practice may not represent the best means of achieving clinical cure with minimal risk of adverse events. Standard-dose TMP-SMX became commonplace in an era before widespread solid organ transplantation and modern immunosuppressive treatments, which are now available for an ever-enlarging array of medical conditions. Present-day patients with PJP differ from the patient population that was treated 30–40 years ago and may do as well, if not better, with reduced doses of TMP-SMX. Most notably, in the modern era, we are treating older patients who have more preexisting renal insufficiency and greater medical complexity, concomitant polypharmacy, and a higher risk of drug–drug interactions and drug-induced harm, such as arrythmia or death from hyperkalemia. In this review, we have shown that there are no modern data to suggest that standard-dose TMP-SMX improves clinical outcomes in the treatment of PJP. On the other hand, standard doses lead to an absolute increase of 18% (95% CI, 5% to 31%) in the risk of grade ≥3 adverse events (a number needed to harm [NNH] of 6). Although step-down therapy may be an approach worth exploring, the current evidence for this practice is weak, and recommendations cannot be made based on this study. We believe that current conventional standard doses of TMP-SMX are not supported by evidence and that there could be a paradigm shift in the treatment of PJP toward reduced doses, with less toxicity. In fact, some centers have already switched to reduced-dose regimens, presumably based on these concerns [18].
Our study’s main strength was an exhaustive search of the literature, as even with a manual reference search, no further studies were identified. Furthermore, even though there was a paucity of studies looking at TMP-SMX dosing strategies for PJP, the studies included in our analysis came to a similar conclusion: Reduced-dose TMP-SMX appears safer than, and potentially as effective as, standard dose.
Although ours is the only meta-analysis on this topic, this study does have some significant limitations. Primarily, we were limited by the scarcity of high-quality cohorts and the complete lack of randomized controlled trials comparing different dosing strategies. This was most striking for non-HIV immunosuppressed patients, for whom the current TMP-SMX treatment recommendation was inferred from HIV cohorts without replication. A 2017 meta-analysis [29] on PJP outcomes in non-HIV patients previously reported a mortality rate of 30.6% (much higher than ours). However, that review did not compare antibiotic dosing regimens and included a larger percentage than ours of standard-risk patients who were specifically excluded from the HIV-era randomized controlled trials (RCTs; eg, hematology patients), making further comparisons difficult. These differences underline the heterogeneity in the non-HIV immunosuppressed population suffering from PJP, and that more research is required for this condition. Given that the evidence for the safety and efficacy of standard-dose TMP-SMX is weak, reduced-dose TMP-SMX represents a biologically plausible and less harmful alternative, even in non-HIV patients.
Another limitation is that our meta-analysis required that we pool studies with different dosing regimens and duration of follow-up for mortality outcomes. First, while 2 studies examined mortality at 30 days, Kosaka et al. [11] followed mortality up to 90 days. Even with a 90-day follow-up, Kosaka obtained similar overall mortality rates to the other studies, as it seems that most PJP-related deaths occurred within the first 30 days. This makes sense for a condition that is, in general, treated for 14–21 days. Second, Nakashima et al. [12] had a 10–20-mg/kg/d treatment arm, which overlapped with how we classified other studies’ reduced-dose arms. However, of the 3 studies, the reduced-dose arm in this study had the best outcomes. If the 10–20-mg/kg/d outcome were biased in any direction, it would have made the standard-dose group appear more favorable and would not negate our conclusion that reduced-dose TMP-SMX provides similar outcomes with lower adverse events.
Further, the lack of comparative cohorts in HIV patients could limit our ability to infer safety in this group, which is known for a higher Pneumocystis burden when they are infected [30]. However, the reduced-dose cohorts that our search strategy yielded showed no mortality difference compared with modern patients, and the pooled overall PJP mortality rate was similar to both the historically reported rates of 6%–11% [2] and the estimates we derived from conventional standard doses in RCTs. Nonetheless, specifically in HIV patients with CD4 <200 and a breakthrough PJP infection while on TMP-SMX prophylaxis, our results may not apply and should be interpreted with caution.
Finally, owing to the lack of randomized trials comparing standard and reduced TMP-SMX doses, inferences made from our work must be tempered. Nonetheless, medical professionals have used the current standard TMP-SMX dose suggested for PJP therapy based on similarly limited bodies of evidence. Our study suggests clinical equipoise about TMP-SMX dosing for this indication. Given the plausible increased risk of adverse events associated with standard dosing, our results are likely sufficient to challenge the current recommendations and support a call for randomized studies.
In conclusion, this review provides the current best evidence for dosing strategies of TMP-SMX in PJP. Although the evidence on the optimal dose of TMP-SMX in PJP is limited, the only evident trend in the literature is that doses closer to 10 mg/kg/d may provide satisfactory outcomes while significantly decreasing the number of treatment-limiting or serious treatment-emergent adverse events. For some patient populations, such as critical illness, or PJP emerging on TMP-SMX prophylaxis in patients with HIV, it may still be warranted to initiate higher doses early on in the treatment. However, this may come with the risk of requiring a change to an antibiotic with lower efficacy than TMP-SMX if and when adverse events emerge. Importantly, our analysis would support the conduct of a randomized controlled trial to definitively answer the question of optimal TMP-SMX dosing for PJP, particularly in non-HIV patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Selma, Darius, and Artémis for their unwavering support through Dr. Butler-Laporte’s residency.
Financial support. Drs. Lee and McDonald receive financial support by the Fonds de Recherche du Québec – Santé.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments