-
PDF
- Split View
-
Views
-
Cite
Cite
Gregory L Damhorst, Kari J Broder, Elizabeth C Overton, Ronelio Rara, Lindsay M Busch, Eileen M Burd, Andrew S Webster, Colleen S Kraft, Ahmed Babiker, Clinical Utilization of DiaSorin Molecular Polymerase Chain Reaction in Pneumocystis Pneumonia, Open Forum Infectious Diseases, Volume 9, Issue 1, January 2022, ofab634, https://doi.org/10.1093/ofid/ofab634
Close - Share Icon Share
Abstract
Pneumocystis jirovecii polymerase chain reaction (PCR) testing is a sensitive diagnostic tool but does not distinguish infection from colonization. Cycle threshold (CT) may correlate with fungal burden and could be considered in clinical decision making. Clinical use of PCR and significance of CT values have not previously been examined with the DiaSorin Molecular platform.
Retrospective review of P jirovecii PCR, CT values and clinical data from 18 months in a multihospital academic health system. The diagnostic performance of PCR with respect to pathology and correlation of CT with severity were examined.
Ninety-nine of 1006 (9.8%) assays from 786 patients in 919 encounters were positive. Among 91 (9.9%) encounters in which P jirovecii pneumonia (PJP) was treated, 41 (45%) were influenced by positive PCR. Negative PCR influenced discontinuation of therapy in 35 cases. Sensitivity and specificity of PCR were 93% (95% CI, 68%–100%) and 94% (95% CI, 91%–96%) with respect to pathology. CT values from deep respiratory specimens were significantly different among treated patients (P = .04) and those with positive pathology results (P < .0001) compared to patients not treated and those with negative pathology, respectively, and was highly predictive of positive pathology results (area under the curve = 0.92). No significant difference was observed in comparisons based on indicators of disease severity.
Pneumocystis jirovecii PCR was a highly impactful tool in the diagnosis and management of PJP, and use of CT values may have value in the treatment decision process in select cases. Further investigation in a prospective manner is needed.
Pneumocystis jirovecii is a ubiquitous fungal organism that can cause pneumonia in immunocompromised hosts [1, 2]. Traditional diagnosis of P jirovecii pneumonia (PJP) is based on microscopic examination of sputum, bronchoalveolar lavage (BAL), or lung tissue biopsies. The sensitivity of microscopic examination ranges broadly from <50% to >90%, depending on staining procedure, specimen type, and quality [3, 4].
Polymerase chain reaction (PCR) allows for sensitive detection of P jirovecii, but at the expense of specificity for active disease. Pneumocystis jirovecii DNA can be detected in specimens derived from asymptomatic individuals where its presence is likely to represent colonization rather than active disease. A positive PCR assay in an individual without overt pneumonia is therefore a diagnostic dilemma that requires a broader assessment to determine if treatment is appropriate. This ambiguity underlies the difficulty in quantifying diagnostic performance of P jirovecii PCR as there is no perfect gold standard for comparison. Microscopic examination of specimens obtained by bronchoscopy, while still not perfectly sensitive, might be considered the closest approximation of “ground truth,” as deeper sampling (such as BAL specimens and transbronchial biopsy) may be more sensitive for achieving diagnosis compared to induced sputum [5]. But since not every patient suspected of PJP undergoes bronchoscopy, analysis of only those cases involving bronchoscopy is likely to introduce sampling bias and may inaccurately reflect the performance of PCR in a broader population. Some studies have alternatively employed the judgment of a clinician as a reference standard—either the treatment team managing the patient in real time or a retrospective adjudicator [6–9].
Several studies report the diagnostic performance of P jirovecii PCR with varying reference standards and have demonstrated a high sensitivity and specificity of PCR from BAL fluid when compared to microscopic evaluation or measurements of the commonly used serum biomarkers lactate dehydrogenase (LDH) and (1-3)-β-d-glucan (BDG) [10–13]. Furthermore, quantification of organism burden by examination of the PCR cycle threshold (CT) has been considered a tool to improve the specificity of PCR for discrimination between PJP and colonization [14]. This has exclusively been performed using in-house PCR assays not commercially available [7–9, 15–21] with rare exception [6]. The Emory University Hospital Microbiology Laboratory uses a thoroughly validated, laboratory-developed real-time PCR assay using primers that amplify the P jirovecii mtLSU gene with a CFR610- and BHQ-2–labeled detection probe (DiaSorin Molecular LLC, Cypress, California) on the commercially available LIAISON MDX thermocycler. Use of this platform for the detection of P jirovecii DNA has only been described in 1 prior report and showed a high sensitivity and negative predictive value when compared to a P jirovecii immunofluorescence assay on BAL samples from immunocompromised patients [22].
Here we describe diagnostic performance and clinical impact of the use of a P jirovecii real-time PCR assay on the DiaSorin Molecular LIAISON MDX thermocycler. We report diagnostic performance with respect to histopathology or cytopathology (hereafter referred to as pathology) and describe the relationship between CT values and indicators of disease severity.
METHODS
Patients and Medical Record Data
We included results from the Emory University Hospital Microbiology Laboratory, which services 6 hospitals and affiliated outpatient clinics in the Atlanta metropolitan area. Data from adult patients were considered from the implementation of the assay on 28 March 2019 through 18 September 2020. Query of the electronic medical record included specimen descriptors and dates, respiratory support (eg, supplemental oxygen and mechanical ventilation), serum biomarkers, pathology results, and antimicrobial agents. Manual review of the electronic medical record determined the patient’s underlying condition, dates of relevant therapy, use of adjunctive steroids, time of discharge or death, and the radiologist’s interpretation of computed tomography or chest radiography preceding the Pneumocystis PCR result (Supplementary Materials). The study was considered exempt from institutional review board review.
Sample Preparation and PCR Assay
Preparation of respiratory specimens and PCR reagents are described in the Supplementary Materials. Reaction master mix was pipetted into a reaction well in the DiaSorin universal disc followed by the addition of prepared specimen. CT values were obtained from LIAISON MDX Studio Software.
Definition of Treatment Decision Opportunity
All PCR results were assumed to correspond to an opportunity to initiate treatment for PJP except for any results collected during treatment for PJP that followed a prior positive PCR result. Electronic medical records were reviewed to determine PJP empiric and directed therapy duration and medical team treatment decisions.
Pathology Gold Standard
All available pathology reports from each encounter were filtered to include cytology of BAL fluid and tissue pathology from transbronchial biopsy.
Statistical Analysis
A single CT value for each unique treatment decision was employed in our analysis. Redundant CT values resulting from replicate assays of a single specimen or assays of multiple specimens collected on the same date for a single patient were excluded and only the lowest CT value from all available results was analyzed. PCR and pathology results were only paired if specimens were obtained within 2 days of each other (irrespective of which specimen was obtained first). When multiple PCR results and/or pathology results were available for a unique encounter, a pathology result was matched with the PCR result corresponding closest in time. No pathology result or PCR result was used twice, and unmatched results were excluded from the analysis.
Following chart review and tabulation of patient data in Microsoft Excel, statistical analysis was completed in MATLAB (The MathWorks, Inc, Natick, Massachusetts), R version 4.0.2 (Vienna, Austria) and the RStudio interface version 1.3.1073 (Boston, Massachusetts) software packages. Between-group comparisons were made with a 2-sided t test. Sensitivity, specificity, negative and positive predictive values, and their corresponding 95% confidence intervals (CIs) were calculated using the R package epiR. Receiver operating characteristic curves were constructed using the MATLAB function perfcurve.
RESULTS
PCR Assays
Among 786 unique patients and 919 encounters, there were 1080 unique results including 120 (11.1%) in which P jirovecii DNA was detected (positive PCR) and 960 (88.9%) in which DNA was not detected (negative PCR). Twenty-one of 120 (17.5%) positive results were excluded from analysis: 20 did not represent opportunities for a treatment decision, and in 1 case CT was not available. Fifty-three of 960 (5.5%) negative results were excluded due to redundancy (see Supplementary Materials for details of result exclusion). This resulted in a total of 1006 results included in the final analysis across 785 unique patients in 918 encounters: 99 positive results with corresponding CT values (Figure 1A) and 907 negative results considered to represent treatment decisions (“cases”). Characteristics of these patients and specimens are included in Table 1.
| Characteristic . | PCR-Positive, No. (%) . | PCR-Negative, No. (%) . |
|---|---|---|
| No. of patients | 92 | 705 |
| No. of encounters | 97 | 827 |
| Inpatient | 93 (96) | 594 (72) |
| Outpatient | 4 (4) | 233 (28) |
| Specimen type, No. | 99 | 907 |
| Bronchoalveolar lavage | 51 (52) | 661 (73) |
| Induced sputum | 21 (21) | 55 (6) |
| Sputum | 20 (20) | 107 (12) |
| Mini-bronchoalveolar lavage | 4 (4) | 58 (6) |
| Bronchial washing | 2 (2) | 9 (1) |
| Endotracheal aspirate | 1 (1) | 12 (1) |
| Bronchial brush | 0 (0) | 2 (0) |
| Tracheal aspirate | 0 (0) | 3 (0) |
| Underlying condition | ||
| HIV/AIDS | 40 (43) | … |
| Solid organ transplant | 20 (22) | … |
| Autoimmune/inflammatory disease | 10 (11) | … |
| Solid organ malignancy | 7 (8) | … |
| Hematologic malignancy | 14 (15) | … |
| Stem cell transplant | 5 (5) | … |
| No apparent immunocompromise | 4 (4) | … |
| Cirrhosis/ESLD | 3 (3) | … |
| Congenital immunodeficiency | 1 (1) | … |
| Characteristic . | PCR-Positive, No. (%) . | PCR-Negative, No. (%) . |
|---|---|---|
| No. of patients | 92 | 705 |
| No. of encounters | 97 | 827 |
| Inpatient | 93 (96) | 594 (72) |
| Outpatient | 4 (4) | 233 (28) |
| Specimen type, No. | 99 | 907 |
| Bronchoalveolar lavage | 51 (52) | 661 (73) |
| Induced sputum | 21 (21) | 55 (6) |
| Sputum | 20 (20) | 107 (12) |
| Mini-bronchoalveolar lavage | 4 (4) | 58 (6) |
| Bronchial washing | 2 (2) | 9 (1) |
| Endotracheal aspirate | 1 (1) | 12 (1) |
| Bronchial brush | 0 (0) | 2 (0) |
| Tracheal aspirate | 0 (0) | 3 (0) |
| Underlying condition | ||
| HIV/AIDS | 40 (43) | … |
| Solid organ transplant | 20 (22) | … |
| Autoimmune/inflammatory disease | 10 (11) | … |
| Solid organ malignancy | 7 (8) | … |
| Hematologic malignancy | 14 (15) | … |
| Stem cell transplant | 5 (5) | … |
| No apparent immunocompromise | 4 (4) | … |
| Cirrhosis/ESLD | 3 (3) | … |
| Congenital immunodeficiency | 1 (1) | … |
Abbreviations: ESLD, end-stage liver disease; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
| Characteristic . | PCR-Positive, No. (%) . | PCR-Negative, No. (%) . |
|---|---|---|
| No. of patients | 92 | 705 |
| No. of encounters | 97 | 827 |
| Inpatient | 93 (96) | 594 (72) |
| Outpatient | 4 (4) | 233 (28) |
| Specimen type, No. | 99 | 907 |
| Bronchoalveolar lavage | 51 (52) | 661 (73) |
| Induced sputum | 21 (21) | 55 (6) |
| Sputum | 20 (20) | 107 (12) |
| Mini-bronchoalveolar lavage | 4 (4) | 58 (6) |
| Bronchial washing | 2 (2) | 9 (1) |
| Endotracheal aspirate | 1 (1) | 12 (1) |
| Bronchial brush | 0 (0) | 2 (0) |
| Tracheal aspirate | 0 (0) | 3 (0) |
| Underlying condition | ||
| HIV/AIDS | 40 (43) | … |
| Solid organ transplant | 20 (22) | … |
| Autoimmune/inflammatory disease | 10 (11) | … |
| Solid organ malignancy | 7 (8) | … |
| Hematologic malignancy | 14 (15) | … |
| Stem cell transplant | 5 (5) | … |
| No apparent immunocompromise | 4 (4) | … |
| Cirrhosis/ESLD | 3 (3) | … |
| Congenital immunodeficiency | 1 (1) | … |
| Characteristic . | PCR-Positive, No. (%) . | PCR-Negative, No. (%) . |
|---|---|---|
| No. of patients | 92 | 705 |
| No. of encounters | 97 | 827 |
| Inpatient | 93 (96) | 594 (72) |
| Outpatient | 4 (4) | 233 (28) |
| Specimen type, No. | 99 | 907 |
| Bronchoalveolar lavage | 51 (52) | 661 (73) |
| Induced sputum | 21 (21) | 55 (6) |
| Sputum | 20 (20) | 107 (12) |
| Mini-bronchoalveolar lavage | 4 (4) | 58 (6) |
| Bronchial washing | 2 (2) | 9 (1) |
| Endotracheal aspirate | 1 (1) | 12 (1) |
| Bronchial brush | 0 (0) | 2 (0) |
| Tracheal aspirate | 0 (0) | 3 (0) |
| Underlying condition | ||
| HIV/AIDS | 40 (43) | … |
| Solid organ transplant | 20 (22) | … |
| Autoimmune/inflammatory disease | 10 (11) | … |
| Solid organ malignancy | 7 (8) | … |
| Hematologic malignancy | 14 (15) | … |
| Stem cell transplant | 5 (5) | … |
| No apparent immunocompromise | 4 (4) | … |
| Cirrhosis/ESLD | 3 (3) | … |
| Congenital immunodeficiency | 1 (1) | … |
Abbreviations: ESLD, end-stage liver disease; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
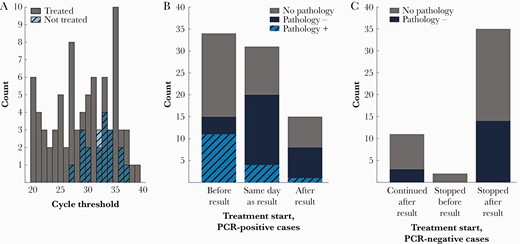
A, Histograms of cycle threshold values grouped by treatment decision. B, Treatment initiation relative to positive polymerase chain reaction (PCR) results. C, Treatment status following negative PCR results.
Clinical Impact
Among 80 PCR-positive cases treated for PJP, 34 (42.5%) had already initiated treatment prior to the result, 31 (38.8%) were started on treatment the same day as the result, and 15 (18.8%) were started following the result (11 the day following; 1 each on day 2, 3, and 4, respectively, after result; and 1 treated during outpatient follow-up in an unspecified time frame) (Figure 1B). One case where treatment was initiated following the PCR result had positive pathology and 4 cases where treatment was initiated the same day as the PCR result had positive pathology (in all 5 cases, pathology specimens were derived from the same bronchoscopy as the PCR specimen).
Among 907 PCR-negative cases, 48 (5.4%) received any duration of Pneumocystis-directed therapy. Empiric treatment was stopped following the negative PCR result in 35 cases (72.9%; Figure 1C). Only 5 (14.3%) of these continued to receive treatment beyond the day following PCR result (2 were stopped on the second day, 2 on the third day, and 1 on the sixth day following PCR result). Fourteen of 35 (40.0%) PCR-negative cases followed by cessation of treatment had corresponding pathology within 2 days; all 14 were negative for microscopic evidence of P jirovecii.
Clinical Treatment Decision
A total of 1006 P jirovecii PCR results (99 detected, 907 not detected) and corresponding medical records were reviewed to determine if PJP treatment had been administered and/or intended. The intention to administer a full course of treatment was evident for 80 (80.8%) PCR-positive and 11 (1.2%) PCR-negative cases. Treatment was never administered in 19 (19.2%) PCR-positive cases and either never administered or discontinued in 896 (98.8%) PCR-negative cases. Among PCR-positive patients who were not treated, 2 died within 2 weeks but with syndromes not likely explained by PJP and 3 others were discharged to hospice within 60 days. Nine patients had absent or stable respiratory symptoms at follow-up (range of follow-up, 21–147 days after PCR result), of which 1 was treated for PJP 97 days later and another was treated for PJP 179 days after the PCR result. Three specimens (2 from the same patient 12 days apart) were thought to be from residual DNA after prior PJP treatment. One patient was lost to follow-up (see Supplementary Table 1 for more details).
Diagnostic Performance
There were 534 BAL specimens, 62 transbronchial biopsies, and 3 endobronchial biopsies with a pathology report available and PCR results from any specimen within 2 days (Table 2). Pneumocystis jirovecii PCR from BAL specimens exhibited 93.3% (95% CI, 68.1%–99.8%) sensitivity and 93.6% (95% CI, 91.2%–95.6%) specificity. Only 3 of 62 transbronchial biopsies identified P jirovecii by microscopic examination and the corresponding PCR was positive in all 3 of these cases.
Performance of Pneumocystis jirovecii Polymerase Chain Reaction With Reference to Pathology
| Pathology . | Positive, No. (%) . | Negative, No. (%) . | Pneumocystis jirovecii PCR . | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) . | Specificity (95% CI) . | PPV (95% CI) . | NPV (95% CI) . | |||
| BAL | 15 | 519 | 0.93 (.68–1.00) | 0.94 (.91–.96) | 0.30 (.17–.45) | 1.00 (.99–1.00) |
| P jirovecii PCR+ | 14 (93.3) | 33 (6.4) | … | … | … | … |
| P jirovecii PCR– | 1 (6.7) | 486 (93.6) | … | … | … | … |
| Transbronchial biopsy | 3 | 59 | 1.00 (.29–1.00) | 0.97 (.88–1.00) | 0.60 (.28–.85) | 1.00 |
| P jirovecii PCR+ | 3 (100) | 2 (3.4) | … | … | … | … |
| P jirovecii PCR– | 0 (0) | 57 (97) | … | … | … | … |
| Pathology . | Positive, No. (%) . | Negative, No. (%) . | Pneumocystis jirovecii PCR . | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) . | Specificity (95% CI) . | PPV (95% CI) . | NPV (95% CI) . | |||
| BAL | 15 | 519 | 0.93 (.68–1.00) | 0.94 (.91–.96) | 0.30 (.17–.45) | 1.00 (.99–1.00) |
| P jirovecii PCR+ | 14 (93.3) | 33 (6.4) | … | … | … | … |
| P jirovecii PCR– | 1 (6.7) | 486 (93.6) | … | … | … | … |
| Transbronchial biopsy | 3 | 59 | 1.00 (.29–1.00) | 0.97 (.88–1.00) | 0.60 (.28–.85) | 1.00 |
| P jirovecii PCR+ | 3 (100) | 2 (3.4) | … | … | … | … |
| P jirovecii PCR– | 0 (0) | 57 (97) | … | … | … | … |
Abbreviations: BAL, bronchoalveolar lavage; CI, confidence interval; NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value.
Performance of Pneumocystis jirovecii Polymerase Chain Reaction With Reference to Pathology
| Pathology . | Positive, No. (%) . | Negative, No. (%) . | Pneumocystis jirovecii PCR . | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) . | Specificity (95% CI) . | PPV (95% CI) . | NPV (95% CI) . | |||
| BAL | 15 | 519 | 0.93 (.68–1.00) | 0.94 (.91–.96) | 0.30 (.17–.45) | 1.00 (.99–1.00) |
| P jirovecii PCR+ | 14 (93.3) | 33 (6.4) | … | … | … | … |
| P jirovecii PCR– | 1 (6.7) | 486 (93.6) | … | … | … | … |
| Transbronchial biopsy | 3 | 59 | 1.00 (.29–1.00) | 0.97 (.88–1.00) | 0.60 (.28–.85) | 1.00 |
| P jirovecii PCR+ | 3 (100) | 2 (3.4) | … | … | … | … |
| P jirovecii PCR– | 0 (0) | 57 (97) | … | … | … | … |
| Pathology . | Positive, No. (%) . | Negative, No. (%) . | Pneumocystis jirovecii PCR . | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) . | Specificity (95% CI) . | PPV (95% CI) . | NPV (95% CI) . | |||
| BAL | 15 | 519 | 0.93 (.68–1.00) | 0.94 (.91–.96) | 0.30 (.17–.45) | 1.00 (.99–1.00) |
| P jirovecii PCR+ | 14 (93.3) | 33 (6.4) | … | … | … | … |
| P jirovecii PCR– | 1 (6.7) | 486 (93.6) | … | … | … | … |
| Transbronchial biopsy | 3 | 59 | 1.00 (.29–1.00) | 0.97 (.88–1.00) | 0.60 (.28–.85) | 1.00 |
| P jirovecii PCR+ | 3 (100) | 2 (3.4) | … | … | … | … |
| P jirovecii PCR– | 0 (0) | 57 (97) | … | … | … | … |
Abbreviations: BAL, bronchoalveolar lavage; CI, confidence interval; NPV, negative predictive value; PCR, polymerase chain reaction; PPV, positive predictive value.
Significance of CT Values
Forty-seven bronchoscopy-derived specimens also had corresponding pathology examinations, and comparison based on whether P jirovecii was detected microscopically demonstrated a significant difference in CT values (median [range], 21.8 [19.7–35.0] vs 33.3 [20.2–37.2]; P < .0001; Figure 2A). CT value was also highly predictive of a positive histopathology result (AUC = 0.92; Figure 2B). Additional comparisons of PCR CT values were stratified by sample type: deep respiratory sampling derived from bronchoscopy (n = 53) or mini-BAL (n = 4), and all other specimens (sputum, n = 41; endotracheal [ET] aspirate, n = 1). Among samples derived through deep sampling, CT values were significantly different in the group that was treated compared to the group that was not treated (median [range], 30.5 [19.7–38.9] vs 33.3 [28.6–37.0]; P = .04; Figure 2C). The same comparison was not significantly different for sputum/ET aspirate specimens (median [range], 28.6 [19.7–38.0] vs 31.5 [27.3–33.7]; P = .22; Figure 2D).
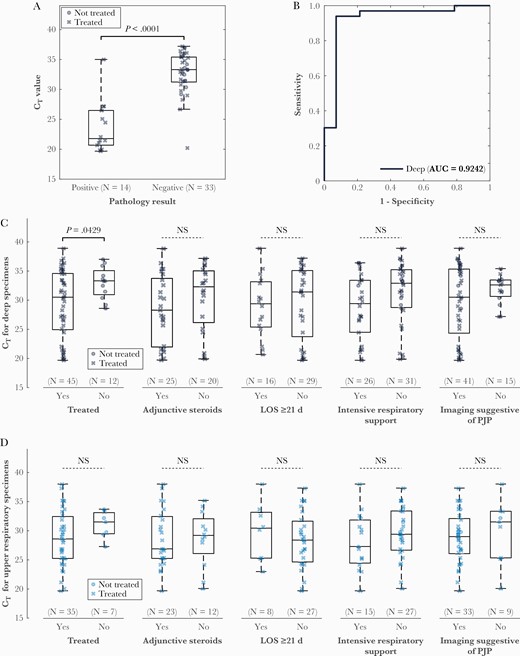
Boxplot (A) and receiver operating characteristic (ROC) curves (B) for cycle threshold (CT) value from deep specimens with reference to pathology. P value determined by t test was <.0001 and area under the ROC curve was 0.92. C, There were significant differences in CT values from deep respiratory specimens when polymerase chain reaction–positive cases that were treated were compared to cases that were not treated, but no significant differences were observed in comparisons based on use of adjunctive steroids, length of stay, need for intensive respiratory support, or imaging suggestive of Pneumocystis jirovecii pneumonia. D, No differences were observed in any comparison from upper respiratory specimens. Abbreviations: AUC area under the curve; CT, cycle threshold; LOS, length of stay; NS, not significant; PJP, Pneumocystis jirovecii pneumonia.
There were no statistically significant differences between CT values in groups stratified by steroid administration, length of stay less than and greater than 21 days, need for advanced respiratory support (mechanical ventilation, high-flow nasal cannula, venturi mask, or non-rebreather), or imaging features suggestive of PJP (Figure 2C and 2D). Finally, subgroup analyses in persons living with human immunodeficiency virus (HIV) (Supplementary Figure 2), transplant recipients (Supplementary Figure 3), and persons without HIV (Supplementary Figure 4) were limited by small group sizes.
Serum Biomarkers
Serum LDH was measured 2022 times among 431 unique encounters. Two hundred fifty-two PCR results representing a unique treatment decision had an LDH measurement available within 2 days. Eighty-nine pathology results were accompanied by an LDH measurement within 2 days. These LDH measurements were 77.8% (95% CI, 40.0%–97.2%) sensitive and 63.8% (95% CI, 52.2%–74.2%) specific (cutoff value 350 U/L) with AUC 0.69 with respect to pathology result (Supplementary Table 2, Supplementary Figure 5). There was no correlation between CT values and LDH measurements obtained within 2 days of PCR and comparison of CT between groups above and below the LDH cutoff of 350 units/L (Supplementary Figure 6).
Serum BDG was measured 127 times among 105 unique encounters. Fourteen pathology results were accompanied by a serum BDG measurement within 2 days. Only 1 of these pathology results was positive for P jirovecii and the corresponding serum BDG was above the cutoff of 100 pg/mL. Two of 13 negative pathology results had BDG levels above the cutoff.
DISCUSSION
Due to its ubiquitous nature, PJP is a prevalent and significant opportunistic infection among immunocompromised hosts [1, 2]. Early and accurate diagnosis of PJP is crucial for favorable outcomes and prevention of inappropriate therapy and off-target antibiotic adverse events among vulnerable patients [23]. In the present study, we evaluated the diagnostic performance and clinical use of the DiaSorin Molecular PCR platform in patients suspected to have PJP. We analyzed a total of 1006 results representing unique treatment decision opportunities among 785 patients during an 18-month period. Clinical use of the DiaSorin Molecular P jirovecii PCR assay played a major role in decisions to treat or discontinue treatment for PJP at our intuitions. Comparisons to pathology results found PCR testing to have a moderate positive predictive value and high negative predictive value, and lower CT values were highly predictive of positive pathology results.
Several studies report the diagnostic performance of P jirovecii PCR with varying reference standards. A study of 448 HIV-negative immunocompromised patients found PCR to be 100% sensitive and 90.0% specific in induced sputum and 84.0% sensitive and 92.2% specific in BAL fluid with respect to indirect fluorescent antibody staining [10]. A study of 110 specimens (primarily BAL fluid) with PCR found 92.8% sensitivity and 90.6% specificity with respect to a clinical diagnosis of PJP [11]. A meta-analysis of 16 studies that employed both clinical factors and microscopic detection as the gold standard for diagnosis determined summary sensitivity of PCR from BAL fluid to be 98.3% and specificity 91.0% [12]. Our findings are consistent with the abovementioned prior literature.
Pneumocystis jirovecii pneumonia PCR testing and subsequent results played a major role in influencing clinical decisions to initiate or discontinue PJP treatment at our intuitions. In our cohort, 14 patients were started on treatment after the PCR result was reported and either had negative pathology or did not have a pathology result. Without PCR, these represent likely missed cases (the clinical team would likely not have treated the patient without the positive PCR result). There were also 27 cases without pathology or with negative pathology where the PCR result and treatment initiation occurred on the same day, and therefore it was more difficult to speculate whether treatment would have been initiated if PCR was not available as a diagnostic tool. We interpreted these as possible missed cases. A negative PCR result in 35 of 126 (27.8%) patients who were initiated on treatment suggests that PCR facilitated discontinuation of empiric antimicrobials. The primary therapeutic agent of PJP is trimethoprim-sulfamethoxazole. Its use is associated with high rates of gastrointestinal intolerance, hyperkalemia, acute kidney injury, leukopenia, and allergic reactions [24]. Hence, limiting inappropriate therapy, especially among immunocompromised patients on polypharmacy, is of great value.
It has been proposed that quantitative PCR, by characterizing fungal burden in a respiratory specimen, may be a discriminator of active disease from colonization. A small number of studies have described the direct use of CT values or quantitative estimation of organism number derived through reference standards [6–9, 15–21]. An overview of these studies and the assays utilized [25–28] is shown in Supplementary Table 3. Collectively, these analyses are problematic: Cutoff values vary by several orders of magnitude between studies, and even those studies with good internal consistency often require identification of 2 separate cutoff values (1 with high sensitivity and 1 with high specificity). Only 1 of the studies we identified had analyzed CT values from a commercially available assay [6]. Furthermore, recent experience with SARS-CoV-2 shows that CT values can vary significantly between and within methods, as demonstrated by a survey of 700 laboratories using SARS-CoV-2 proficiency testing material produced from the same batch, where within a single gene target for a single method, up to 12.0 cycle differences were seen across all laboratories [29]. The totality of this literature highlights the barriers to standardization across platforms and institutions.
We characterized the utility of P jirovecii PCR CT value from the DiaSorin Molecular assay in 2 ways. First, test performance of CT values relative to pathology demonstrated that low CT values are highly predictive of a positive pathology result (Figure 2A and 2B). Second, comparisons by t test revealed significant difference in distribution of CT values from deep respiratory specimens with respect to treatment decision, although there was significant overlap. There was not significant difference in this comparison among sputum and ET aspirate specimens. Further comparisons with indicators of disease severity did not exhibit statistically significant differences. For example, while deep respiratory specimens with CT values <25 all had imaging suggestive of PJP, many high CT values were observed in patients with suggestive imaging while sputum/ET aspirate specimens included a few specimens near or below 25 without suggestive imaging. This illustrates that CT values must be interpreted with caution.
Reasons that significant differences in CT values were not observed in comparisons based on severity indicators may be the presence of alternative or concurrent diagnoses or factors related to specimen collection. While lower CT values might be expected in individuals with more severe PJP, other processes causing respiratory failure, imaging features compatible with PJP, or prompting the use of steroids can confound these results. Meanwhile, specimen collection has inherent variability and can impact CT value results. For example, fungal concentration in a liquid specimen, which would influence CT values, is influenced by sampling technique and sample preparation. Lavage or bronchial brushing may exert different mechanical forces on the lower airways, resulting in differing yield. Fluid volume likely also influences fungal recovery as BAL technique can range from tens to hundreds of milliliters and inadequate volume may compromise recovery, whereas excess volume may dilute fungal organism and therefore influence CT value. Sputum is produced by an inherently different process (which is why it is not compared to bronchoscopy-derived samples in this study) and may differ based on patient effort, age, and underlying lung disease and can be contaminated with saliva. Other preanalytical factors—time from collection to analysis during which DNA could degrade and sample storage conditions—as well as when in the clinical course a specimen is obtained (especially if empiric treatment has been administered) and degree of host immune suppression also likely influence organism burden and impact the CT value.
Ultimately, histopathological examination does not perfectly represent ground truth for PJP diagnosis. It appears true from our experience that histopathological examination is not perfectly sensitive. Compared to the serum biomarker LDH, however, PCR appears to be more sensitive and specific. There were limited data for drawing conclusions about the performance of serum BDG. We do not present diagnostic performance with respect to clinical treatment decision because this is inherently flawed and may be influenced by clinician knowledge of the CT value (which is occasionally requested by the infectious diseases consultant) and the experience or expertise of the clinical teams making a treatment decision. Prospective adjudication in real time by clinicians experienced with the diagnosis of PJP and blinded to the PCR result may be a better approximation of ground truth.
These results demonstrate that data from additional studies are necessary to guide the clinician in proper use of CT value from Pneumocystis PCR. Platform and assay variability in PCR results across laboratories necessitates familiarity with data from within one’s own institution if CT values are to be used at all. Our analysis suggests that CT values may be most useful in a clinical scenario where pathology examination is limited (eg, poor sample quality) and a low CT value may prompt commitment to treatment. Our experience presented here suggests that negative PCR, but not high CT values in a PCR-positive individual, might be used to “rule out” PJP.
In summary, we found P jirovecii PCR to be a highly impactful tool in the diagnosis and management of PJP, and use of CT values may have value in the treatment decision process under select circumstances.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Patient consent. This study was designated as a quality improvement project by the Institutional Review Board at Emory University and therefore did not require patient written consent.
Potential conflicts of interest. C. S. K. has served on the advisory board for Rebiotix/Ferring. All other authors report no potential conflicts of interest.
The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments