-
PDF
- Split View
-
Views
-
Cite
Cite
Doreen Zhu, Parminder K Judge, Natalie Staplin, Richard Haynes, William G Herrington, Design considerations for future renoprotection trials in the era of multiple therapies for chronic kidney disease, Nephrology Dialysis Transplantation, Volume 40, Issue Supplement_1, January 2025, Pages i70–i79, https://doi.org/10.1093/ndt/gfae210
Close - Share Icon Share
PLAIN ENGLISH SUMMARY
In the last 5–10 years, several large high-quality research trials testing new treatments versus a dummy treatment in patients with kidney disease have provided new discoveries, particularly among people with diabetes. Some of these trials included patients with a wide variety of kidney diseases and therefore provided important information on how effective the treatment is, and whether it is safe to use for many people (and not just those with a specific type of kidney disease). The findings are particularly important as they suggest that, once established, kidney disease progresses in similar ways regardless of the initiating cause. These new treatments importantly slow kidney disease progression but, even when used together, do not arrest the loss of kidney function. New research is still needed to test new potential treatments.
Now that we have several drugs that can be used to treat kidney disease, there are new challenges when designing and conducting new trials. These include the reduced risk of kidney disease progression and heart disease (because of the new treatments available). Future research trials need to include a sufficiently large number of patients to be able to answer research questions reliably. In addition, different types of people and diseases should be included. In an age of increasing regulation and bureaucracy, conducting such trials is challenging. Simplifying the design and conduct of future trials by focusing only on the necessary components needed to answer the research key question(s) is important. Such trials reduce the burden of participation for patients and busy clinical staff, whilst still ensuring careful focus on patient safety and data quality. We hope more high-quality trials that are sufficiently large, inclusive and simple will be conducted in the future, so that kidney teams can offer better care to their patients.
Nephrology has benefited from conducting increasingly large high-quality trials in the last 5–10 years. In addition to the long-standing known benefits of renin–angiotensin system inhibitors, we now have multiple pharmacotherapies that provide kidney and/or cardiovascular protection for certain types of patient with chronic kidney disease (CKD). These include sodium-glucose co-transporter 2 inhibitors (SGLT2i), a non-steroidal mineralocorticoid receptor antagonist and a glucagon-like peptide-1 receptor agonist. Trials of SGLT2i have had particularly important impact, as wide eligibility criteria in pivotal trials have enabled safety and efficacy across a wide range of causes of CKD to be demonstrated. These findings support the concept of final common pathways of CKD progression and should encourage similar trial designs recruiting broad ranges of patients at risk of CKD progression. This is important as these new drugs do not completely arrest CKD progression nor do they mitigate the full excess of cardiovascular disease.
In the current era of multiple therapies to manage risk of CKD progression, trial design and conduct also need to consider new challenges. These include falling event rates, establishing standard of care for participants pre-randomization and improving the inclusion of trial participants understudied in previous trials. Streamlining trial design and conduct and reducing participation burden for patients and clinicians is increasingly important to facilitate larger sample sizes and to optimize adherence to study interventions and follow-up. Potential other solutions include maintaining a focus on wide generalizability (to include understudied patient groups) and empowering patients to volunteer for trials (through public and patient involvement and large-scale invitation methods), as well as innovations in trial design (including use of pre-randomization run-in periods to implement standard of care and factorial or platform trials to assess multiple treatments simultaneously).
NEPHROLOGY HAS BENEFITED FROM LARGE HIGH-QUALITY TRIALS IN THE LAST 5–10 YEARS
Chronic kidney disease (CKD) is a major public health problem that is associated with increased morbidity and mortality from kidney failure and cardiovascular disease [1]. 9 to 13% of the world's population has CKD and, as the population ages and the prevalence of risk factors rises, the burden of CKD is growing in both high-income countries (HICs) and low- and middle-income countries (LMICs) [2, 3]. Slowing CKD progression is highly desirable and until recently there has been an imbalance between the clinical need of patients with CKD and the amount of reliable evidence to inform practice. Historically, there may have been an underappreciation of the value of uncertainty, as many treatments have been adopted into nephrology practice before adequate evidence of efficacy of safety are available [4]. Arguably, trial designs may have also prioritized overdetailed phenotyping over adequate sample size [5]. As a result, the number, size and quality of clinical trials in nephrology is well-documented to have lagged behind those in other specialties, inciting concerted efforts from the community to close the gap [6, 7].
Within the last 5–10 years, nephrology has benefited from more large-scale, placebo-controlled randomized control trials (RCTs), leading to major pharmacological advancements that have been practice changing. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and a non-steroidal mineralocorticoid receptor antagonist (MRA) can be added to clinically appropriate renin–angiotensin system inhibition (RASi) to provide cardiorenal protection in those with type 2 diabetes [8–11]. Moreover, renoprotection RCTs with broad eligibility criteria have also improved treatment options for CKD patients without diabetes and guidelines now recommend SGLT2i to lower cardiorenal risk across a range of primary kidney diagnoses [12–14].
As an example of the scale of recent data, a collaborative meta-analysis of SGLT2i trials included approximately 90 000 participants from 13 large double-blind placebo-controlled RCTs, including 4 dedicated CKD trials of nearly 26 000 patients (20 931 with diabetes and 4967 without diabetes) [12]. Many patients from the non-CKD RCTs of SGLT2i included patients with decreased estimated glomerular filtration rate (eGFR). The totality of the evidence found a central role for SGLT2i in the treatment of CKD as well as reducing risk of serious acute kidney injury, irrespective of diabetes status or primary kidney diagnosis. Initial SGLT2i trials in CKD populations prioritized the study of patients with proteinuric diabetic kidney disease [8, 9]. However, the most recent trials (DAPA-CKD [Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease] and EMPA-KIDNEY [The Study of Heart and Kidney Protection With Empagliflozin]) included a broader range of patients with lower eGFR, with EMPA-KIDNEY providing new information in patients with low levels of albuminuria [9, 10].
Data on the non-steroidal MRA finerenone has also been generated at scale. Pooling results from two large placebo-control trials in patients with diabetic kidney disease resulted in findings from 13 026 patients followed for 3 years [11, 15]. This pooled analyses (FIDELITY [Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial Programme Analysis]) demonstrated that finerenone conferred lower cardiorenal risk when taken in addition to RASi in patients with CKD and type 2 diabetes with a serum potassium ≤4.8 mmol/L at screening [16]. Although only a small proportion of participants (<10%) took SGLT2i at baseline, the treatment effects were similar in the types of patients prescribed a SGLT2i and those who were not. Ongoing and future trials will help determine whether MRAs and other drugs that target the same pathway (such as aldosterone synthase inhibitors) can also offer cardiorenal benefits for patients with non-diabetic kidney disease [17–19].
To add to these promising developments, meta-analyses of cardiovascular outcome RCTs suggest clear cardiovascular benefits and possible renoprotective effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in people with type 2 diabetes and at risk of cardiovascular disease [20]. FLOW (Evaluate Renal Function with Semaglutide Once Weekly), a dedicated kidney outcomes RCT, has released top-line results in a press release which reported that allocation to semaglutide, compared with placebo, led to a 24% reduction in the primary composite outcome of kidney disease progression, major cardiovascular events or all-cause mortality in 3533 patients with CKD, albuminuria, and type 2 diabetes [21, 22].
Although recent randomized data have provided major advances in how we can manage CKD, these new treatments slow but do not arrest CKD progression, and substantial residual cardiorenal risk remains. New therapies are needed to further improve and refine management. The focus of future RCTs should be determining the efficacy of new interventions on top of standard of care for a broad range of people in CKD, including those who have previously been under-studied and thereby under-served (particularly those with non-diabetic causes of CKD).
CORE PRINCIPLES OF DESIGNING AND CONDUCTING TRIALS
The core principles of trial design are randomized allocation of intervention and complete follow-up to eliminate systematic bias, plus sufficiently large sample size to minimize random error. Recruitment of populations that will ensure a widely generalizable result is also encouraged [23, 24]. Lastly, it is important that trials include adequate blinding, participants remain adherent to their allocated intervention (wherever appropriate), and relevant clinically important outcomes are ascertained and analysed scientifically (Table 1).
Summary of core principles of randomized control trial design and conduct with examples.
| . | Key design objective . | Approaches to usually avoid . | Examples of problems and/or bias arising from not meeting a key design objective . |
|---|---|---|---|
| Sample size | Large | Small | Schiff et al. randomized 160 patients with acute kidney injury to daily or conventional haemodialysis and appeared to show that a more intensive regimen reduced mortality [57]. However, the VA/NIH Acute Renal Failure Trial Network Study and RENAL trials (1124 and 1508 critically unwell patients with acute kidney injury respectively) demonstrated that treatment with higher intensity continuous renal replacement therapy did not affect mortality [58, 59]. |
| Follow-up | Sufficiently long duration and complete | Short (or stopped too early) and incomplete | The ACCELERATE trial of evacetrapib versus placebo and the REVEAL trial of anacetrapib versus placebo in patients at high risk of vascular disease: ACCELERATE recruited 12 092 participants and was stopped early after 2.2 years for futility [60]. In contrast, REVEAL involved 30 449 participants and demonstrated a highly significant reduction in coronary events after a median follow-up of 4.1 years (the planned trial duration) [42]. The sufficiently long duration of REVEAL provided adequate statistical power to identify moderate benefits. |
| Blinding | Placebo-controlled Double-blind | Non-controlled open-label | SYMPLICITY-HTN trials of renal denervation versus standard care [61, 62]. The open-label trial design appeared to show that renal denervation results in large reductions in clinic systolic blood pressure measurements but a subsequent much larger double-blind design demonstrated no significant reductions in either clinic or ambulatory systolic blood pressure with renal denervation when implemented across many centres. |
| Eligibility criteria | Simple wide inclusion criteria with minimal exclusion criteria, and adopting the uncertainty principle | Over-precise inclusion and exclusion criteria, and including types of patient highly likely to start open label treatment | The 4S and HPS trials of simvastatin versus placebo: 4S demonstrated that simvastatin reduced cardiovascular events in patients with angina or previous myocardial infarction and a blood cholesterol 5.5–8.0 mmol/L [63]. However, patients with diabetes are at increased risk of cardiovascular events even though their blood cholesterol levels are similar to those in the general population. The broad eligibility criteria of HPS provided evidence that simvastatin is also reduces vascular risk in people with diabetes even if they do not already have manifest coronary disease or high cholesterol concentrations [64]. |
| Trial designs which include steps to enrich groups considered more likely to respond (perhaps by assessing ‘responses’ to biomarkers during run-in before randomization) make recruitment complicated and may inadvertently exclude patients who may still benefit from ever being studied properly. Studying a wide range of patients, carefully phenotyping patients at baseline (including during run-ins) and pre-specifying subgroup analyses may be a more optimum approach. HPS included a pre-randomization assessment of low-density lipoprotein response during run-in, but still included all participants in the trial (irrespective of response). This enabled a subgroup analyses at the end of the trial that clearly demonstrated that relative benefits on cardiovascular outcomes were unmodified in those with small versus large ‘responses’ to simvastatin [64]. | |||
| Adherence | Maintain adherence throughout | High rates of drop-out or drop-in | The EVOLVE trial of cinacalcet versus placebo in patients with secondary hyperparathyroidism [65]: during the course of this dialysis trial, nephrologists became less uncertain about the benefits of cinacalcet (which was already available for use) even though clinical evidence had not been reported. Commercially available cinacalcet was prescribed to 20% of participants who were allocated to the placebo group. This drop-in contributed to the erosion of study power for the primary assessment from 90% to 54% (meaning the chance of a false negative result rose from 1 in 10 to about 1 in 2) [66]. |
| Study Outcome | Pre-specified, clinically important and sensitive to study treatment | ‘Surrogate’ primary outcomes that lead to inadequate tests of efficacy | A meta-analysis of 10 trials of 315 patients examining dual RAS blockade for slowing progression of diabetic kidney disease demonstrated that dual therapy reduces albuminuria more than monotherapy [67]. However, much larger trials of dual therapy did not identify any significant improvements in clinical outcomes and larger trials revealed an increased risk of hyperkalaemia [68]. |
| Statistical analyses | Intention-to-treat | On-treatment | The Coronary Drug Project evaluated lipid-influencing drugs in the long-term treatment of coronary heart disease [69]. In an exploratory analysis, the difference in mortality between patients who did or did not take ≥80% of their allocated placebo was greater than the mortality difference in patients who did or did not take ≥80% of clofibrate after 5 years follow-up. Censoring participants when they stop taking the study intervention can significantly distort the balance of characteristics between intervention groups which was established by randomization (i.e. result in non-randomized comparisons). |
| . | Key design objective . | Approaches to usually avoid . | Examples of problems and/or bias arising from not meeting a key design objective . |
|---|---|---|---|
| Sample size | Large | Small | Schiff et al. randomized 160 patients with acute kidney injury to daily or conventional haemodialysis and appeared to show that a more intensive regimen reduced mortality [57]. However, the VA/NIH Acute Renal Failure Trial Network Study and RENAL trials (1124 and 1508 critically unwell patients with acute kidney injury respectively) demonstrated that treatment with higher intensity continuous renal replacement therapy did not affect mortality [58, 59]. |
| Follow-up | Sufficiently long duration and complete | Short (or stopped too early) and incomplete | The ACCELERATE trial of evacetrapib versus placebo and the REVEAL trial of anacetrapib versus placebo in patients at high risk of vascular disease: ACCELERATE recruited 12 092 participants and was stopped early after 2.2 years for futility [60]. In contrast, REVEAL involved 30 449 participants and demonstrated a highly significant reduction in coronary events after a median follow-up of 4.1 years (the planned trial duration) [42]. The sufficiently long duration of REVEAL provided adequate statistical power to identify moderate benefits. |
| Blinding | Placebo-controlled Double-blind | Non-controlled open-label | SYMPLICITY-HTN trials of renal denervation versus standard care [61, 62]. The open-label trial design appeared to show that renal denervation results in large reductions in clinic systolic blood pressure measurements but a subsequent much larger double-blind design demonstrated no significant reductions in either clinic or ambulatory systolic blood pressure with renal denervation when implemented across many centres. |
| Eligibility criteria | Simple wide inclusion criteria with minimal exclusion criteria, and adopting the uncertainty principle | Over-precise inclusion and exclusion criteria, and including types of patient highly likely to start open label treatment | The 4S and HPS trials of simvastatin versus placebo: 4S demonstrated that simvastatin reduced cardiovascular events in patients with angina or previous myocardial infarction and a blood cholesterol 5.5–8.0 mmol/L [63]. However, patients with diabetes are at increased risk of cardiovascular events even though their blood cholesterol levels are similar to those in the general population. The broad eligibility criteria of HPS provided evidence that simvastatin is also reduces vascular risk in people with diabetes even if they do not already have manifest coronary disease or high cholesterol concentrations [64]. |
| Trial designs which include steps to enrich groups considered more likely to respond (perhaps by assessing ‘responses’ to biomarkers during run-in before randomization) make recruitment complicated and may inadvertently exclude patients who may still benefit from ever being studied properly. Studying a wide range of patients, carefully phenotyping patients at baseline (including during run-ins) and pre-specifying subgroup analyses may be a more optimum approach. HPS included a pre-randomization assessment of low-density lipoprotein response during run-in, but still included all participants in the trial (irrespective of response). This enabled a subgroup analyses at the end of the trial that clearly demonstrated that relative benefits on cardiovascular outcomes were unmodified in those with small versus large ‘responses’ to simvastatin [64]. | |||
| Adherence | Maintain adherence throughout | High rates of drop-out or drop-in | The EVOLVE trial of cinacalcet versus placebo in patients with secondary hyperparathyroidism [65]: during the course of this dialysis trial, nephrologists became less uncertain about the benefits of cinacalcet (which was already available for use) even though clinical evidence had not been reported. Commercially available cinacalcet was prescribed to 20% of participants who were allocated to the placebo group. This drop-in contributed to the erosion of study power for the primary assessment from 90% to 54% (meaning the chance of a false negative result rose from 1 in 10 to about 1 in 2) [66]. |
| Study Outcome | Pre-specified, clinically important and sensitive to study treatment | ‘Surrogate’ primary outcomes that lead to inadequate tests of efficacy | A meta-analysis of 10 trials of 315 patients examining dual RAS blockade for slowing progression of diabetic kidney disease demonstrated that dual therapy reduces albuminuria more than monotherapy [67]. However, much larger trials of dual therapy did not identify any significant improvements in clinical outcomes and larger trials revealed an increased risk of hyperkalaemia [68]. |
| Statistical analyses | Intention-to-treat | On-treatment | The Coronary Drug Project evaluated lipid-influencing drugs in the long-term treatment of coronary heart disease [69]. In an exploratory analysis, the difference in mortality between patients who did or did not take ≥80% of their allocated placebo was greater than the mortality difference in patients who did or did not take ≥80% of clofibrate after 5 years follow-up. Censoring participants when they stop taking the study intervention can significantly distort the balance of characteristics between intervention groups which was established by randomization (i.e. result in non-randomized comparisons). |
VA/NIH: Veterans Affairs/National Institutes of Health; RENAL: Randomized Evaluation of Normal versus Augmented Level; ACCELERATE: Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes; REVEAL: Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification; SYMPLICITY-HTN: Renal Denervation in Patients With Uncontrolled Hypertension; 4S: Scandinavian Simvastatin Study; HPS: Heart Protection Study; EVOLVE: EValuation Of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events.
Summary of core principles of randomized control trial design and conduct with examples.
| . | Key design objective . | Approaches to usually avoid . | Examples of problems and/or bias arising from not meeting a key design objective . |
|---|---|---|---|
| Sample size | Large | Small | Schiff et al. randomized 160 patients with acute kidney injury to daily or conventional haemodialysis and appeared to show that a more intensive regimen reduced mortality [57]. However, the VA/NIH Acute Renal Failure Trial Network Study and RENAL trials (1124 and 1508 critically unwell patients with acute kidney injury respectively) demonstrated that treatment with higher intensity continuous renal replacement therapy did not affect mortality [58, 59]. |
| Follow-up | Sufficiently long duration and complete | Short (or stopped too early) and incomplete | The ACCELERATE trial of evacetrapib versus placebo and the REVEAL trial of anacetrapib versus placebo in patients at high risk of vascular disease: ACCELERATE recruited 12 092 participants and was stopped early after 2.2 years for futility [60]. In contrast, REVEAL involved 30 449 participants and demonstrated a highly significant reduction in coronary events after a median follow-up of 4.1 years (the planned trial duration) [42]. The sufficiently long duration of REVEAL provided adequate statistical power to identify moderate benefits. |
| Blinding | Placebo-controlled Double-blind | Non-controlled open-label | SYMPLICITY-HTN trials of renal denervation versus standard care [61, 62]. The open-label trial design appeared to show that renal denervation results in large reductions in clinic systolic blood pressure measurements but a subsequent much larger double-blind design demonstrated no significant reductions in either clinic or ambulatory systolic blood pressure with renal denervation when implemented across many centres. |
| Eligibility criteria | Simple wide inclusion criteria with minimal exclusion criteria, and adopting the uncertainty principle | Over-precise inclusion and exclusion criteria, and including types of patient highly likely to start open label treatment | The 4S and HPS trials of simvastatin versus placebo: 4S demonstrated that simvastatin reduced cardiovascular events in patients with angina or previous myocardial infarction and a blood cholesterol 5.5–8.0 mmol/L [63]. However, patients with diabetes are at increased risk of cardiovascular events even though their blood cholesterol levels are similar to those in the general population. The broad eligibility criteria of HPS provided evidence that simvastatin is also reduces vascular risk in people with diabetes even if they do not already have manifest coronary disease or high cholesterol concentrations [64]. |
| Trial designs which include steps to enrich groups considered more likely to respond (perhaps by assessing ‘responses’ to biomarkers during run-in before randomization) make recruitment complicated and may inadvertently exclude patients who may still benefit from ever being studied properly. Studying a wide range of patients, carefully phenotyping patients at baseline (including during run-ins) and pre-specifying subgroup analyses may be a more optimum approach. HPS included a pre-randomization assessment of low-density lipoprotein response during run-in, but still included all participants in the trial (irrespective of response). This enabled a subgroup analyses at the end of the trial that clearly demonstrated that relative benefits on cardiovascular outcomes were unmodified in those with small versus large ‘responses’ to simvastatin [64]. | |||
| Adherence | Maintain adherence throughout | High rates of drop-out or drop-in | The EVOLVE trial of cinacalcet versus placebo in patients with secondary hyperparathyroidism [65]: during the course of this dialysis trial, nephrologists became less uncertain about the benefits of cinacalcet (which was already available for use) even though clinical evidence had not been reported. Commercially available cinacalcet was prescribed to 20% of participants who were allocated to the placebo group. This drop-in contributed to the erosion of study power for the primary assessment from 90% to 54% (meaning the chance of a false negative result rose from 1 in 10 to about 1 in 2) [66]. |
| Study Outcome | Pre-specified, clinically important and sensitive to study treatment | ‘Surrogate’ primary outcomes that lead to inadequate tests of efficacy | A meta-analysis of 10 trials of 315 patients examining dual RAS blockade for slowing progression of diabetic kidney disease demonstrated that dual therapy reduces albuminuria more than monotherapy [67]. However, much larger trials of dual therapy did not identify any significant improvements in clinical outcomes and larger trials revealed an increased risk of hyperkalaemia [68]. |
| Statistical analyses | Intention-to-treat | On-treatment | The Coronary Drug Project evaluated lipid-influencing drugs in the long-term treatment of coronary heart disease [69]. In an exploratory analysis, the difference in mortality between patients who did or did not take ≥80% of their allocated placebo was greater than the mortality difference in patients who did or did not take ≥80% of clofibrate after 5 years follow-up. Censoring participants when they stop taking the study intervention can significantly distort the balance of characteristics between intervention groups which was established by randomization (i.e. result in non-randomized comparisons). |
| . | Key design objective . | Approaches to usually avoid . | Examples of problems and/or bias arising from not meeting a key design objective . |
|---|---|---|---|
| Sample size | Large | Small | Schiff et al. randomized 160 patients with acute kidney injury to daily or conventional haemodialysis and appeared to show that a more intensive regimen reduced mortality [57]. However, the VA/NIH Acute Renal Failure Trial Network Study and RENAL trials (1124 and 1508 critically unwell patients with acute kidney injury respectively) demonstrated that treatment with higher intensity continuous renal replacement therapy did not affect mortality [58, 59]. |
| Follow-up | Sufficiently long duration and complete | Short (or stopped too early) and incomplete | The ACCELERATE trial of evacetrapib versus placebo and the REVEAL trial of anacetrapib versus placebo in patients at high risk of vascular disease: ACCELERATE recruited 12 092 participants and was stopped early after 2.2 years for futility [60]. In contrast, REVEAL involved 30 449 participants and demonstrated a highly significant reduction in coronary events after a median follow-up of 4.1 years (the planned trial duration) [42]. The sufficiently long duration of REVEAL provided adequate statistical power to identify moderate benefits. |
| Blinding | Placebo-controlled Double-blind | Non-controlled open-label | SYMPLICITY-HTN trials of renal denervation versus standard care [61, 62]. The open-label trial design appeared to show that renal denervation results in large reductions in clinic systolic blood pressure measurements but a subsequent much larger double-blind design demonstrated no significant reductions in either clinic or ambulatory systolic blood pressure with renal denervation when implemented across many centres. |
| Eligibility criteria | Simple wide inclusion criteria with minimal exclusion criteria, and adopting the uncertainty principle | Over-precise inclusion and exclusion criteria, and including types of patient highly likely to start open label treatment | The 4S and HPS trials of simvastatin versus placebo: 4S demonstrated that simvastatin reduced cardiovascular events in patients with angina or previous myocardial infarction and a blood cholesterol 5.5–8.0 mmol/L [63]. However, patients with diabetes are at increased risk of cardiovascular events even though their blood cholesterol levels are similar to those in the general population. The broad eligibility criteria of HPS provided evidence that simvastatin is also reduces vascular risk in people with diabetes even if they do not already have manifest coronary disease or high cholesterol concentrations [64]. |
| Trial designs which include steps to enrich groups considered more likely to respond (perhaps by assessing ‘responses’ to biomarkers during run-in before randomization) make recruitment complicated and may inadvertently exclude patients who may still benefit from ever being studied properly. Studying a wide range of patients, carefully phenotyping patients at baseline (including during run-ins) and pre-specifying subgroup analyses may be a more optimum approach. HPS included a pre-randomization assessment of low-density lipoprotein response during run-in, but still included all participants in the trial (irrespective of response). This enabled a subgroup analyses at the end of the trial that clearly demonstrated that relative benefits on cardiovascular outcomes were unmodified in those with small versus large ‘responses’ to simvastatin [64]. | |||
| Adherence | Maintain adherence throughout | High rates of drop-out or drop-in | The EVOLVE trial of cinacalcet versus placebo in patients with secondary hyperparathyroidism [65]: during the course of this dialysis trial, nephrologists became less uncertain about the benefits of cinacalcet (which was already available for use) even though clinical evidence had not been reported. Commercially available cinacalcet was prescribed to 20% of participants who were allocated to the placebo group. This drop-in contributed to the erosion of study power for the primary assessment from 90% to 54% (meaning the chance of a false negative result rose from 1 in 10 to about 1 in 2) [66]. |
| Study Outcome | Pre-specified, clinically important and sensitive to study treatment | ‘Surrogate’ primary outcomes that lead to inadequate tests of efficacy | A meta-analysis of 10 trials of 315 patients examining dual RAS blockade for slowing progression of diabetic kidney disease demonstrated that dual therapy reduces albuminuria more than monotherapy [67]. However, much larger trials of dual therapy did not identify any significant improvements in clinical outcomes and larger trials revealed an increased risk of hyperkalaemia [68]. |
| Statistical analyses | Intention-to-treat | On-treatment | The Coronary Drug Project evaluated lipid-influencing drugs in the long-term treatment of coronary heart disease [69]. In an exploratory analysis, the difference in mortality between patients who did or did not take ≥80% of their allocated placebo was greater than the mortality difference in patients who did or did not take ≥80% of clofibrate after 5 years follow-up. Censoring participants when they stop taking the study intervention can significantly distort the balance of characteristics between intervention groups which was established by randomization (i.e. result in non-randomized comparisons). |
VA/NIH: Veterans Affairs/National Institutes of Health; RENAL: Randomized Evaluation of Normal versus Augmented Level; ACCELERATE: Assessment of Clinical Effects of Cholesteryl Ester Transfer Protein Inhibition With Evacetrapib in Patients at a High Risk for Vascular Outcomes; REVEAL: Randomized Evaluation of the Effects of Anacetrapib through Lipid-modification; SYMPLICITY-HTN: Renal Denervation in Patients With Uncontrolled Hypertension; 4S: Scandinavian Simvastatin Study; HPS: Heart Protection Study; EVOLVE: EValuation Of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events.
The fundamental reason for random allocation of treatment in RCTs is to ensure that each type of patient is allocated in similar proportions to the different treatment strategies. If randomization is performed correctly, known and unknown prognostic factors that could influence the development of the outcomes being investigated are balanced between groups, ensuring that any differences observed can be attributed to the allocation of interventions. Non-randomized studies can explore the associations between exposures (e.g. prescription of a drug) and clinical disease outcomes, but are unable to guarantee elimination of systematic biases, and are not appropriate for studying new unlicensed drugs [25]. Genotypes that mimic a drug's mode of action can help raise hypotheses, but are also not a definitive test that an intervention is safe and effective. Well-designed RCTs will remain the gold standard approach to identify and quantify the beneficial and adverse effects of interventions with moderate effects and influence clinical practice guidelines.
In CKD, multiple pathophysiological mechanisms are responsible for disease progression and the associated complications. This means a single treatment is likely to only modify one mechanism leading to only small or moderate effects. Clinically important outcomes can also take a long time to accrue (this is true of both kidney failure and cardiovascular events). As sample size calculations need to assume only moderate effects, and trials need to be sufficiently long to accrue meaningful clinical outcomes, CKD trials need to be large and long enough to ensure an adequate test of the primary question [5].
Broad inclusion, minimal exclusion criteria and upholding the uncertainty principle (both the patient and their doctor must be substantially uncertain as to which treatment option is optimum) are important to facilitate widely generalizable trial results [26, 27]. Over-precise eligibility criteria may exclude patients with CKD who might benefit from the test interventions, resulting in understudied groups of patients (as well as overcomplicated trial procedures). Instead, eligibility criteria should aim to restrict entry only to those with definite indications or definite contraindications for the test intervention and identify a population at risk of the outcomes of interest. Pre-specified subgroup analyses can assess whether treatment effects differ between different types of patient, particularly if there is a biological plausible rationale to consider that to be the case.
It is important to remember that randomization alone does not wholly guard against bias. Flaws in the design, conduct, analysis or reporting of RCTs in the CKD population can produce biased estimates of treatment effect jeopardizing the validity of their findings [26, 27]. If participants and/or clinicians are aware of treatment assignments (i.e. an open label RCT) then the treatment effect estimates could be biased, particularly for subjectively assessed outcomes. Therefore, all RCTs should be adequately blinded, unless the outcome ascertainment is unlikely to be biased (e.g. the 28-day mortality outcome in COVID-19 trials) [28].
Low adherence to a study intervention in an RCT will quickly erode a trial's ability to test the study hypothesis and distort estimates of the effect of the intervention. As a rule of thumb, every one participant randomly allocated to active treatment who stops study intervention (drop-out) or every one participant randomly allocated to placebo who starts open-label active treatment (drop-in) has the equivalent effect to recruiting two fewer patients in the first place [24]. Use of a pre-randomization run-in phase allows participants and clinicians time to reconsider a particular patient's participation and is a good test of compliance [29]. Those participants who are unlikely, or unable, to take study treatment long-term can be excluded prior to randomization. This is effectively enrichment for participants most likely to adhere to trial treatments and follow-up (thereby guarding against loss of power).
Lastly, bias can be introduced by inappropriate choice of trial outcomes or of statistical analyses. All RCTs should have pre-specified outcomes that are both clinically important and sensitive to the study intervention. Results from intention-to-treat analyses where all participants who were enrolled and randomly allocated to treatment groups are included regardless of whether they take any or none of their allocated treatment should be emphasized.
NEW CHALLENGES FACING FUTURE RENOPROTECTION RCTs IN THE ERA OF MULTIPLE THERAPIES FOR CKD
Falling event rates
The problem
As effective interventions are developed and population-level risks fall, it becomes increasingly challenging to conduct reliable assessments of new or existing interventions. Lower absolute risks of kidney failure in the CKD population mean that larger trial sample sizes are required to generate the necessary numbers of outcomes of interest, and follow-up may also need to be longer. As the size and duration of trials increases, the cost and complexity of trial conduct rises. A temptation develops to study only those at highest risk, or to limit the duration of follow-up by use of surrogate outcomes. However, once a new treatment obtains a marketing approval in a restricted subtype of patients, it becomes more challenging to conduct definitive trials, to the detriment of the wider range of patients that may benefit [30, 31]. Smaller and shorter trials also inadequately assess safety, because these drugs will typically be taken by an individual for many years [32, 33]. Therefore, small short trials in specific rarer subpopulations may be a false solution to the problem of falling event rates for kidney failure [34].
Solutions
Potential solutions that would facilitate larger longer renoprotection RCTs are focusing on wide generalizability, innovations in streamlining trial design and conduct, and adopting methods for widescale patient invitation. A population-based approach with simple wide eligibility criteria that includes those intolerant or without indications for standard of care therapies ensures the trial results are generally applicable to the majority of the CKD population. For example, rather than excluding all CKD patients who were not taking a RASi for good clinical reasons, participants in EMPA-KIDNEY had to be on investigator-judged ‘clinically appropriate’ dose of RASi by randomization [10].
Future renoprotection trials should ‘streamline’ data collection by assessing a limited number of critical data elements—only those needed to answer the central research question and keep participants safe (Fig. 1) [26, 35]. A limited number of dedicated large sites enrolling a substantial number of participants at each site reduces the monitoring burden and ensures high-quality data as the study coordinators repeatedly perform the same trial procedures. Technology can be harnessed to further streamline trial conduct through the use of web-based or app-based data collection, remote monitoring (including central statistical monitoring to identify atypical data patterns) and ‘virtual’ trial visits. Wherever possible, the trial procedures should be integrated into routine nephrology care. Short focused study visits help ensure optimal adherence and follow-up (through minimized participation burden). The consequence of streamlining is that large high quality trials can be conducted at a feasible cost and recruitment in diverse populations is more attainable.
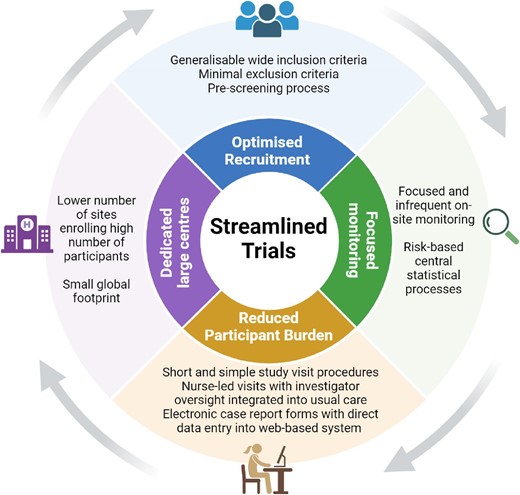
Widescale invitation is a time and cost-effective method to ensure an adequate sample size. In the field of diabetes, an academic (non-commercial) aspirin/omega fatty acid placebo-controlled trial based in the UK randomized and followed 15 000 adults for 7 years. This was made possible by innovative and extremely cost-effective direct to participant methods. Such mail-based methodology can be adopted for assessment of older interventions with known safety profiles that do not require intensive clinical monitoring of participants [36]. ASCEND (A Study of Cardiovascular Events iN Diabetes) used central and local diabetes registers to identify potentially eligible patients and automated methods to send invitation packs by mail directly to participants’ home addresses. Direct-to-participant drug supply and follow-up removed the need for local research sites. Similar mail-based follow-up (supplemented by flags in routine healthcare data) has been used in a large kidney transplant trial [37, 38]. Implementing more of such designs in nephrology could provide reliable answers to key questions which do not attract industry levels of funding. The approach also minimizes the burden of participation for both participants and the study team. This in turn optimizes completeness of follow-up.
If large-scale central identification of potential participants from regional registries is not possible (as is the case in many countries), a prescreening model at local research sites can be used to accelerate recruitment [26]. Nephrology lends itself to identifying potential participants due to the existence of often long-established locally held databases and/or electronic health records (e.g. using clinic blood/urine test results). From these types of data, patients with CKD who fit the eligibility criteria for a trial can be identified and pre-invited by local research centres at scale [39]. Those potentially eligible are then provided with information about the trial and asked if they would agree to attend a future study visit. In this way, CKD patients are empowered with the opportunity to volunteer and local study staff form a pool of interested patients to demonstrate feasibility of recruitment while other time-consuming aspects of the study (e.g. acquiring relevant approvals) are being completed.
Establishing the standard of care
The problem
Having more pharmacotherapy options for the CKD population, whilst welcome, could lead to considerable variation in the standard of care in future renoprotection trials. The optimum standard of care for an individual with CKD of a specific aetiology (particularly diabetes), level of kidney function and albuminuria will be some combination of RASi, SGLT2i, MRA and GLP-1RA depending on local approvals for use and access to treatment, as well as individual patient tolerances and preferences. New treatments may not translate into clinical care promptly (particularly in LMICs) [40]. If future trials mandated the same standard of care for all participants (e.g. RASi and SGLT2i in all before randomization) then this could lead to the continued exclusion of understudied CKD patients to date where uncertainty about efficacy and safety of these interventions remains (e.g. SGLT2i in patients with polycystic kidney disease) [41].
Solutions
Potential solutions to this challenge include focusing on establishing investigator-judged ‘clinically appropriate’ standards of care during pre-screening, screening and run-in processes. An individualized ‘clinically appropriate’ standard of care is the optimum treatment regimen based on the treatment indications and tolerances of an individual in their usual clinical setting. Focusing on establishing ‘clinically appropriate’ rather than perfectly consistent standards of care is pragmatic and allows future trials to recruit a broad range of CKD patients globally. Alternatively, if it is scientifically important that all participants should be on background treatment with a particular treatment, this could be supplied specifically by the trial.
A pre-randomization run-in period can facilitate such an approach (Fig. 2) [29]. In the HPS3-TIMI55-REVEAL (Randomised Evaluation of the Effects of Anacetrapib through Lipid-modification) lipid trial, participants were issued with a 12-week supply of atorvastatin (the low-density lipoprotein cholesterol–lowering standard of care) during such a run-in phase [42]. This also provided time for local clinicians to be provided with relevant clinical details collected at the screening visits and indicate whether their patients were suitable for randomization [43, 44].
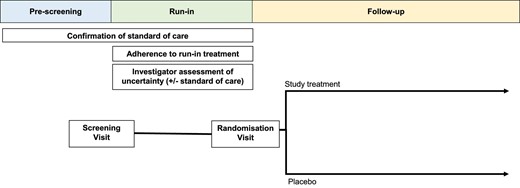
Outline of a parallel two-arm randomized control trial design including pre-screening and run-in processes.
Improving generation of information for understudied patient groups
The problem (i)
Patients who find it difficult to volunteer because of logistic reasons (e.g. those in full-time employment or with disabilities) or who are generally less willing to participate (e.g. ethnic minorities or women) continue to be under-represented in RCTs across many specialties including nephrology [45, 46]. Improving the diversity of trial participants may lead to more complete data that broadens the understanding of differences in treatment responses and reduces disparities in outcomes.
Solutions (i)
Barriers to more inclusive diverse participation in clinical trials can best be identified and addressed by drawing on the expertise of patients and the public from different backgrounds. Patient and public involvement in the design and conduct of future renoprotection trials promotes a partnership between patients and ensures trials address questions that are important to patients [47]. It is also likely to have a positive impact on the engagement, recruitment and retention of participants. Establishing public advisory groups with representatives from a diverse group of patients are particularly helpful and can provide invaluable feedback (including addressing logistical barriers to participation) over the lifecycle of the trial.
Importantly, streamlining design and conduct ensures future renal trials can be delivered to diverse patient populations by avoiding overcomplicated procedures and ensuring feasible costs. Integrating trial procedures into routine care could also remove barriers caused by inconvenience that limit the participation of previously under-served CKD patients. Other innovations in trial conduct that help eliminate logistical barriers include providing mail-based (e.g. questionnaires by post) and hybrid (e.g. virtual trial visits with couriered study treatment) participation options (introduced above). Furthermore, collaborations with primary care networks could provide study sites and investigators in areas centrally located to diverse communities to support recruitment of minority groups.
The problem (ii)
Although recent high-quality randomized data have led to considerable improvements in CKD treatment options, there are still substantial gaps in knowledge resulting from trials focusing on specific diseases or patient characteristics. There is a great need to study patients under-represented in previous renoprotection trials including subtypes of CKD patients (e.g. polycystic kidney disease who have been excluded from the large SGLT2i trials) or patients who may progress slowly (e.g. those with low levels of albuminuria).
Solution (ii)
A potential solution is to include rarer subtypes of patients or those progressing slowly in the large definitive renoprotective trials and perform pre-specified subgroup analyses to provide some information about whether the effects of treatment are likely to different importantly by key subgroups of interest. eGFR slope is known to correlate well with kidney failure or established eGFR-based dichotomous surrogate outcomes (e.g. doubling of creatinine levels) when analysed appropriately (e.g. using shared parameter models) [48]. By transforming results onto a relative scale, such eGFR slope analyses have recently been used in EMPA-KIDNEY to compare effects of SGLT2i use across the range of albuminuria studied and provided evidence of renal efficacy even at low levels of albuminuria (thereby addressing the limitation of the trial's relatively short follow-up and number of kidney failure outcomes in this common and important group of patients with CKD) [49]. Crucially, although surrogates for efficacy like eGFR slope-based analyses may be employed as a method to perform trials in rare disease settings to address unmet need, there are no such substitutes for assessing safety. Therefore, trial designs employing eGFR slope based primary outcomes should be discouraged when testing new interventions that are likely to modify final common pathways of CKD progression. Sufficiently large streamlined RCTs with wide eligibility criteria powered to assess clinical cardiorenal outcomes should be prioritized for new interventions. Such an approach will build a more robust evidence-base on which to base our clinical care for the full range of patients with CKD.
Assessing the efficacy and safety of multiple therapies
The problem
A potential challenge for future renoprotection trials is the assessment of efficacy and safety of multiple therapies administered in combination. Although RASi, SGLT2i, MRA and GLP-1RA are proposed to have different but potentially complementary mechanisms of action, there are still limited data for combinations of three or more drug classes in the CKD population. It is important to note that subgroup analyses comparing patients on different baseline therapies cannot reliably assess interactions between the different therapies because these subgroups are not defined by the therapies but by the types of patients who take the therapies (i.e. there are differences between the patients in these subgroups other than their different baseline therapies). Traditional parallel two-arm RCTs cannot definitively investigate the efficacy and safety of more than one treatment approach. This may be important if there is a biologically plausible reason why combining two interventions may be synergistic or conversely may not be safe, or if there is a need to demonstrate the separate efficacy of each intervention.
The haemodynamic effects of multiple therapies also requires careful design considerations as the initial eGFR decline (acute dip) of RASi, SGLT2i and aldosterone pathway inhibition may be additive [50]. Acute dips reduce a trial's sensitivity to detect long terms benefits on CKD progression.
Solutions
Factorial RCTs add multiple simultaneous randomizations to a traditional parallel-group design and could be a useful way to examine combinations of treatments that target different pathways (i.e. independent mechanisms of action). For example, in a 2 × 2 factorial RCT participants are randomized to one of two levels of factor 1 (e.g. placebo A vs treatment A) and to one of two levels of factor 2 (e.g. placebo B vs treatment B). Provided the effects of each treatment are moderate, there is the potential to test more than one primary question within a single protocol with little impact on the statistical power of each analysis. These approaches have been common in large clinical outcome cardiology and diabetes trials [51–54]. More recently, it has been adopted in a phase 2 renal trial to demonstrate that an aldosterone synthase inhibitor has added efficacy on albuminuria when combined with a SGLT2i [50].
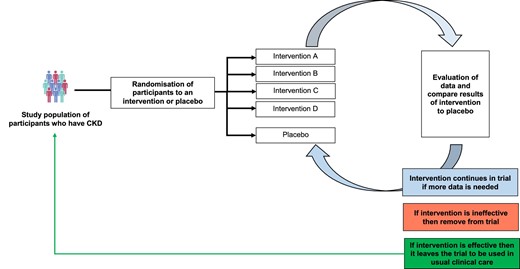
Platform RCTs also could be used to examine the effects of multiple therapies in comparison with a common control group (Fig. 3) [55]. RECOVERY (Randomised Evaluation of COVID-19 Therapy) highlighted the benefits of this design and rapidly identified 4 effective treatments for patients hospitalized for COVID-19, and demonstrated that several other commonly used treatments were not effective [28]. The disease-focused open-ended design with the flexibility to add new interventions are all key drivers of this trial's success. A common feature of platform RCTs is a master protocol that defines key clinical trial elements, to which new arms are added or removed as the trial progresses. If an intervention is demonstrated to be more effective than placebo then it can be rolled out to benefit patients, creating a potential cycle of improvement. Future renoprotection trials that adopt this design could develop new therapies in a timely and affordable fashion. However, key challenges include the higher statistical burden compared with traditional trials [56].
To address the challenge of acute dips, trials could employ a pre-randomization run-in which establishes background renoprotective standard of care; such run-ins need to be sufficiently long to ensure the acute dip with RASi and SGLT2i has occurred before randomization [50]. This helps ensure any acute dip after randomization is attributable to the new test intervention. An alternative or complimentary design is to use post-treatment measurement of eGFR at the end of the trial after stopping study intervention for a few days/weeks as the final eGFR measurement (i.e. measure final eGFR off the intervention when the acute dip is likely to have reversed) [18].
SUMMARY
Nephrology has benefited from increasing numbers of large high-quality trials in the last 5–10 years but there is significant residual cardiorenal risk and not all patients with CKD can benefit. New renoprotection trials are needed that are randomized, sufficiently large and long, with broad eligibility criteria to generate definitive evidence on the efficacy and safety of potential treatments. Once trials have studied a wide range of patients, focused subgroup analyses can compare effects in different groups of participants to assess precision medicine questions. In the new era of multiple therapies for CKD, trial design and conduct also need to consider new challenges (Table 2). Streamlining trial design is a key potential solution to enable even larger trials and reduce participation burden for optimal adherence and follow-up. Other considerations for future trials include maintaining a focus on generalizability (to include understudied patient groups), empowering patients to volunteer for trials (through public and patient involvement and large-scale invitation methods), as well as innovations in trial design (including use of pre-randomization run-in periods to implement standard of care, and factorial or platform trials to assess multiple treatments simultaneously).
Summary of the extra challenges in the design and conduct of randomized control trials in the era of multiple therapies.
| Challenges . | Potentially false solutions (to be avoided where possible) . | Potential solutions . |
|---|---|---|
| Falling event rates | Study only those at highest risk; limit the duration of follow-up; use surrogate outcomes | Broad eligibility criteria; streamlining of trial design and conduct; widescale invitation |
| Establishing the standard of care | Mandating a consistent standard of care for all participants | Focus on ‘clinically appropriate’ standards of care for trial participants (which may vary) or provide all participants with the internationally recommended standard of care; pre-screening; pre-randomization run-in period |
| Improving generation of information for understudied patient groups | ||
| (i) Patients who find it difficult to volunteer or who are generally less willing to participate (ii) Rare subtypes of CKD patients or who progress slowly | (i) Complex trial procedures (ii) Use of unreliable surrogate markers to explore treatment effects in rare subgroups | (i) Patient and public involvement; integrating trial procedures with usual clinical care; mail-based or hybrid participation options to empower patients to be able to volunteer; collaborations with primary care networks (ii) Use of robust surrogate markers to explore treatment effects in rare subgroups |
| Assessing the efficacy and safety of multiple therapies | Subgroup analyses comparing types of patients on different baseline therapies | Embrace different trial designs such as factorial trials and platform trials |
| Challenges . | Potentially false solutions (to be avoided where possible) . | Potential solutions . |
|---|---|---|
| Falling event rates | Study only those at highest risk; limit the duration of follow-up; use surrogate outcomes | Broad eligibility criteria; streamlining of trial design and conduct; widescale invitation |
| Establishing the standard of care | Mandating a consistent standard of care for all participants | Focus on ‘clinically appropriate’ standards of care for trial participants (which may vary) or provide all participants with the internationally recommended standard of care; pre-screening; pre-randomization run-in period |
| Improving generation of information for understudied patient groups | ||
| (i) Patients who find it difficult to volunteer or who are generally less willing to participate (ii) Rare subtypes of CKD patients or who progress slowly | (i) Complex trial procedures (ii) Use of unreliable surrogate markers to explore treatment effects in rare subgroups | (i) Patient and public involvement; integrating trial procedures with usual clinical care; mail-based or hybrid participation options to empower patients to be able to volunteer; collaborations with primary care networks (ii) Use of robust surrogate markers to explore treatment effects in rare subgroups |
| Assessing the efficacy and safety of multiple therapies | Subgroup analyses comparing types of patients on different baseline therapies | Embrace different trial designs such as factorial trials and platform trials |
Summary of the extra challenges in the design and conduct of randomized control trials in the era of multiple therapies.
| Challenges . | Potentially false solutions (to be avoided where possible) . | Potential solutions . |
|---|---|---|
| Falling event rates | Study only those at highest risk; limit the duration of follow-up; use surrogate outcomes | Broad eligibility criteria; streamlining of trial design and conduct; widescale invitation |
| Establishing the standard of care | Mandating a consistent standard of care for all participants | Focus on ‘clinically appropriate’ standards of care for trial participants (which may vary) or provide all participants with the internationally recommended standard of care; pre-screening; pre-randomization run-in period |
| Improving generation of information for understudied patient groups | ||
| (i) Patients who find it difficult to volunteer or who are generally less willing to participate (ii) Rare subtypes of CKD patients or who progress slowly | (i) Complex trial procedures (ii) Use of unreliable surrogate markers to explore treatment effects in rare subgroups | (i) Patient and public involvement; integrating trial procedures with usual clinical care; mail-based or hybrid participation options to empower patients to be able to volunteer; collaborations with primary care networks (ii) Use of robust surrogate markers to explore treatment effects in rare subgroups |
| Assessing the efficacy and safety of multiple therapies | Subgroup analyses comparing types of patients on different baseline therapies | Embrace different trial designs such as factorial trials and platform trials |
| Challenges . | Potentially false solutions (to be avoided where possible) . | Potential solutions . |
|---|---|---|
| Falling event rates | Study only those at highest risk; limit the duration of follow-up; use surrogate outcomes | Broad eligibility criteria; streamlining of trial design and conduct; widescale invitation |
| Establishing the standard of care | Mandating a consistent standard of care for all participants | Focus on ‘clinically appropriate’ standards of care for trial participants (which may vary) or provide all participants with the internationally recommended standard of care; pre-screening; pre-randomization run-in period |
| Improving generation of information for understudied patient groups | ||
| (i) Patients who find it difficult to volunteer or who are generally less willing to participate (ii) Rare subtypes of CKD patients or who progress slowly | (i) Complex trial procedures (ii) Use of unreliable surrogate markers to explore treatment effects in rare subgroups | (i) Patient and public involvement; integrating trial procedures with usual clinical care; mail-based or hybrid participation options to empower patients to be able to volunteer; collaborations with primary care networks (ii) Use of robust surrogate markers to explore treatment effects in rare subgroups |
| Assessing the efficacy and safety of multiple therapies | Subgroup analyses comparing types of patients on different baseline therapies | Embrace different trial designs such as factorial trials and platform trials |
FUNDING
This paper was published as part of a supplement financially supported by Bayer AG and the scientific content has not been influenced in any way by the sponsor.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
There is departmental funding from Boehringer Ingelheim to run the EMPA-KIDNEY and EASi-KIDNEY trials but a long-standing departmental policy to decline honoraria.






Comments