-
PDF
- Split View
-
Views
-
Cite
Cite
Brendon L Neuen, Emily K Yeung, Janani Rangaswami, Muthiah Vaduganathan, Combination therapy as a new standard of care in diabetic and non-diabetic chronic kidney disease, Nephrology Dialysis Transplantation, Volume 40, Issue Supplement_1, January 2025, Pages i59–i69, https://doi.org/10.1093/ndt/gfae258
Close - Share Icon Share
PLAIN ENGLISH SUMMARY
Combination therapy, involving the use of multiple medications together, is becoming a new standard of care for chronic kidney disease (CKD). For people with CKD, combination therapy offers the promise of preventing kidney failure and reducing the risk of heart problems. This approach is appealing because different drugs target distinct mechanisms involved in CKD progression. For instance, some target immune responses, others reduce kidney inflammation and scarring, while others improve blood pressure within the kidneys. Data from large clinical trials suggest that each treatment works effectively on its own, regardless of other medications people are taking. Combining therapies can also reduce the risk of side effects of individual medications. This review highlights the evidence for combination therapy in CKD, explores how to improve its use, and discusses how future studies may answer remaining questions.
A range of therapies now exists to reduce the risk of kidney failure and cardiovascular events in people with type 2 diabetes, including renin–angiotensin system blockade, sodium-glucose cotransporter 2 (SGLT2) inhibitors, non-steroidal mineralocorticoid receptor antagonists, and glucagon-like peptide-1 receptor agonists. With multiple clinical trials underway, it is likely that at least some of these therapies—as well as additional agents such as endothelin receptor antagonists—will further demonstrate kidney-protective effects in people with CKD who do not have diabetes in the near future. For conditions such as IgA nephropathy, several therapies have recently been approved or are being evaluated in late phase trials. Thus combination therapy is emerging as a new standard for diabetic and non-diabetic chronic kidney disease (CKD). This approach is supported by randomized data suggesting that each therapeutic class offers independent and additive benefits in diabetic kidney disease, regardless of background therapy. Notably, the reduction in hyperkalaemia and fluid retention with SGLT2 inhibitors may enhance the tolerability and safety of other treatments. In this review, we present the rationale for combination therapy with evidence-based kidney therapies in diabetic and non-diabetic CKD. We also summarize randomized evidence supporting a multi-medicine approach, address safety considerations, review ongoing trials, and propose frameworks for implementing treatments aligned with patient risk to optimize person-centred care and reduce long-term risks of kidney failure and related complications.
INTRODUCTION
There has been remarkable progress in treatments to improve outcomes for people living with chronic kidney disease (CKD) in the last 5 years. Three large-scale kidney outcome trials have demonstrated the impact of sodium-glucose cotransporter 2 inhibitors (SGLT2i) on reducing the risk of kidney failure, cardiovascular events, and extending survival across a broad spectrum of CKD risk and phenotypes [1, 2, 3]. Two large-scale outcome trials have demonstrated that the non-steroidal mineralocorticoid receptor antagonist (ns-MRA) finerenone reduces the risk of CKD progression and cardiovascular events in patients with type 2 diabetes and CKD [4]. Therapeutic indications for glucagon-like peptide-1 receptor agonists (GLP-1RA) continue to expand, with semaglutide shown to reduce CKD progression and mortality in type 2 diabetes and CKD in the FLOW trial [5]. In non-diabetic CKD, treatment options are expanding rapidly, from tolvaptan for autosomal polycystic kidney disease to budesonide and sparsentan for IgA nephropathy. For glomerular diseases, especially IgA nephropathy, many more therapies are being evaluated in late-phase clinical trials [6].
The rapidity of these therapeutic advances is welcome news to patients, clinicians, and health systems. However, they also pose new challenges. How can we optimally implement multiple therapies to maximize benefits to individuals and population health? How can we address cost and other barriers to implementation that might contribute to health disparities? In whom, when, and how should we offer combination therapy to prevent kidney function decline, kidney failure, cardiovascular events, and extend survival?
Here, we review the rationale for combination therapy with evidence-based kidney therapies for diabetic and non-diabetic CKD. We also outline the randomized evidence supporting a combination therapy approach, safety considerations, and potential frameworks for implementation to reduce long-term risk of kidney failure and other associated complications.
EFFICACY RATIONALE FOR COMBINATION THERAPY IN CKD
Despite optimal use of a highly effective therapy on top of standard of care, rates of kidney function decline remain high for many patients with CKD. Across the three SGLT2i kidney outcome trials, ∼10% of participants who were randomized to SGLT2i experienced CKD progression or died due to cardiovascular or kidney disease over a median follow-up of 2.0–2.6 years [7]. In EMPA-KIDNEY, the annualized rate of decline in estimated glomerular filtration rate (eGFR) in patients with urine albumin: creatinine ratios (uACR) of >300 to <1000 mg/g, 1000 to <2000, and ≥2000 mg/g in the empagliflozin arm were −2.17, −3.31, and −5.60 ml/min/1.73 m2/year, respectively (Fig. 1) [8]. Indeed, despite an average 30% reduction in albuminuria with canagliflozin in the CREDENCE trial, residual albuminuria at 6 months post-randomization was still the most powerful predictor of long-term risk of kidney failure (Fig. 1) [9]. These observations underscore the need for multiple therapies for many individuals with CKD to reduce residual cardio–kidney risk and achieve the best possible outcomes.
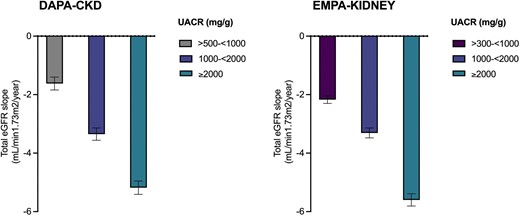
Annual rate of decline in eGFR as measured by total slope in dapagliflozin and empagliflozin treated participants by baseline albuminuria in the DAPA-CKD and EMPA-KIDNEY trials (error bars represent standard errors).
Pathophysiology underlying the development and progression of any given cause of CKD is complex. In general, most effective treatments that modulate one pathway of injury can be anticipated to have a moderate effect on disease progression. Thus, different therapies targeting distinct pathways of injury are required to stabilize kidney function. For example, while renin–angiotensin system (RAS) blockade and SGLT2i reduce glomerular hyperfiltration (among their other effects), ns-MRA may have more prominent effects on inflammation and fibrosis, while GLP-1RA address the underlying metabolic abnormalities that are pathognomonic of diabetic kidney disease [10]. In non-diabetic CKD there is a similar need to address different mechanisms of disease progression and injury, from reducing or preventing the production of aberrantly glycosylated IgA1 in IgA nephropathy, to reducing glomerular inflammation by targeting the alternative complement pathway, and abrogating pro-fibrotic signalling pathways within the kidney [11]. Each of the evidence-based kidney therapies in diabetic and non-diabetic CKD with their differing mechanisms of action suggests a ‘pillars of care’ framework may be the optimal way to address multiple mechanism of disease progression and injury and improve clinical outcomes (Fig. 2).
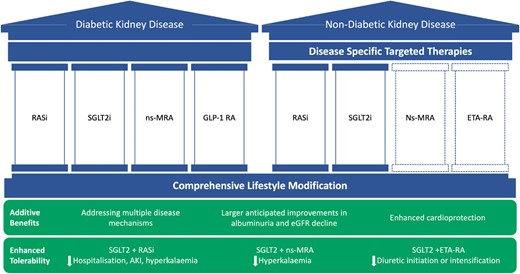
Combination therapy and the pillars of care for diabetic and non-diabetic kidney disease. RASi: renin–angiotensin system blockade; ETA-RA: endothelin type A receptor antagonist; AKI: acute kidney injury.
A third efficacy rationale for combination therapy in CKD is that cardiovascular mortality is a major competing risk for kidney failure, especially in patients with diabetes, and specific cardiovascular events are differentially modified by each evidence-based kidney therapy. For example, compared to people with normal or near normal kidney function, the risk of both atherosclerotic and non-atherosclerotic cardiovascular events is increased in patients with CKD [12]. While SGLT2i reduce major adverse cardiovascular events, this is driven primarily by heart failure and sudden cardiac death, with no clear effect on myocardial infarction or stroke [13]. Similarly, the cardiovascular benefits of ns-MRA appear most pronounced on heart failure outcomes [14]. In contrast to both these agents, GLP-1RA reduce myocardial infarction and stroke in patients with type 2 diabetes [15]. The magnitude of effects on intermediate markers or cardiometabolic risk also vary across key evidence-based kidney therapies—from neutral effects on blood glucose with ns-MRA [16], to moderate reductions in blood pressure with SGLT2i [17], to substantial reductions in glycated haemoglobin and body weight with GLP-1RA [18]. Thus, the varied intermediate effects of each evidence-based kidney therapies and the potential to prevent different complications provides another compelling rationale for combination therapy in individuals with CKD.
RANDOMIZED EVIDENCE SUPPORTING INDEPENDENT AND ADDITIVE BENEFITS OF COMBINATION THERAPY IN CKD
Preclinical and clinical randomized evidence indicate that the combined use of evidence-based kidney therapies may have additive effects compared to individual therapies alone.
The effects of combination kidney therapies have been assessed in several preclinical studies. In a hypertensive animal model, combined treatment with empagliflozin and finerenone resulted in more than additive benefits on albuminuria and improved cardiac and kidney histopathology compared to treatment with either therapy alone [19]. In a preclinical randomized trial using an animal model of Alport syndrome, treatment with ramipril, empagliflozin, and finerenone resulted in greater improvements in cardio–kidney histopathology and longer mean survival compared to treatment with ramipril and empagliflozin in combination, or with empagliflozin or ramipril monotherapy [20]. In particular, the addition of finerenone to ramipril and empagliflozin resulted in further suppression of residual interstitial inflammation and fibrosis. Similarly greater benefits on kidney histology have been observed in animal models of type 2 diabetes with ramipril, empagliflozin, and atrasentan compared to each agent individually [21]. These findings provide important mechanistic support for additive end-organ protection with combined use of evidence-based kidney therapies.
Multiple randomized trials have evaluated the effects of combination treatment with SGLT2i and GLP-1RA or agents that block the actions of aldosterone in people with CKD. Collectively, these trials showed that combination treatment reduced cardiometabolic risk factors such as blood pressure, body weight, and glycated haemoglobin to a greater extent than either therapy alone. In the DECREASE trial of 66 patients with obesity and type 2 diabetes, dapagliflozin and exanetide reduced albuminuria excretion by 40%, which was larger than dapagliflozin (18%) and exanetide (16%) alone [22]. In the ROTATE study, which studied 46 patients with CKD regardless of diabetes, the combination of dapagliflozin and eplerenone reduced albuminuria by 50%, which was larger than either therapy alone [23]. Finally, in a phase 2 trial of patients with CKD (irrespective of diabetes), combined treatment the aldosterone synthase inhibitor BI690517 reduced albuminuria by almost 40%, with or without empagliflozin [24]. Thus, multiple lines of evidence suggest additive kidney protection can be anticipated using these therapies in combination.
With respect to clinical outcomes, pooled subgroup data from completed large-scale randomized trials suggest that the effects of SGLT2i, ns-MRA, and GLP-1RA in people with type 2 diabetes are consistent irrespective of background therapy. A meta-analysis of four large-scale cardiovascular and kidney outcome trials reported that SGLT2i reduced CKD progression consistently in patients with type 2 diabetes receiving and not receiving RAS blockade at baseline [25]. Because key SGLT2i and GLP-1RA trials were conducted at approximately the same time before the benefits of each class were known, there were too few patients using GLP-1RA to evaluate treatment effects by baseline GLP-1RA use in any individual SGLT2i trial. However, across 12 trials within the SGLT2i Meta-Analysis Cardio-Renal Trialists Consortium (SMART-C), ∼3000 participants were receiving GLP-1RA [26]. In a SMART-C collaborative meta-analysis, SGLT2i reduced CKD progression (defined as 40% decline in eGFR, kidney failure or death due to kidney failure) and improved the annualized rate of eGFR decline as measured by chronic slope by ∼60%, regardless of GLP-1RA use. In the AMPLITUDE-O and FLOW trials, ∼15% of participants were receiving SGLT2 inhibitors at baseline, with randomization stratified by SGLT2i use. In these trials, the effects of efpeglenatide and semaglutide on cardiovascular and kidney outcomes were similarly consistent regardless of SGLT2i use at baseline [27, 28, 29]. Finally, the effects of the ns-MRA finerenone on cardiovascular and kidney outcomes in the pooled FIDELIO and FIGARO trials were also consistent regardless of SGLT2i and GLP-1RA use [30, 31].
Taken together, the preclinical data, additive effects on intermediate outcomes, and consistent benefits on clinical outcomes with each of individual evidence-based kidney therapy regardless of background treatment, suggests independent and additive effects with each drug class and supports the concept of their combined use to reduce residual cardio–kidney risk.
SAFETY RATIONALE FOR COMBINATION THERAPY IN CKD
An emerging concept within the framework of combination therapy is that individual evidence-based kidney therapies may enhance the tolerability or safety of other pillars of care.
Across both CKD and heart failure, there is emerging evidence that newer components of guideline-recommended care, such as angiotensin receptor neprilysin inhibitors and SGLT2i, reduce the incidence of hyperkalaemia. In patients with heart failure with reduced ejection fraction, sacubitril/valsartan reduced the risk of hyperkalaemia compared to enalapril in the PARADIGM trial [32]. Canagliflozin reduced the risk of serious hyperkalaemia (defined as a central laboratory serum potassium >6.0 mmol/l) by 23% compared to placebo in patients with type 2 diabetes and CKD in CREDENCE [33]. A subsequent collaborative meta-analysis of almost 50 000 patients confirmed a ∼15% reduction in serious hyperkalaemia with SGLT2i with consistent effects across type 2 diabetes, heart failure, and kidney outcome trials [34]. Consistent with these observations, use of an SGLT2i was associated with a lower risk of hyperkalaemia in the FIDELIO trial, including in those participants randomized to finerenone [35].
The reduction in hyperkalaemia with SGLT2i is likely to enable more persistent use of RAS blockade and ns-MRA to further improve cardio–kidney outcomes. In a pooled analysis of CREDENCE and DAPA-CKD, SGLT2i reduced the risk of discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in patients with CKD [36]. In EMPEROR-Reduced, empagliflozin reduced the risk of discontinuation of steroidal MRA in heart failure with reduced ejection fraction [37]. Finally, in the ROTATE-3 randomized trial of 48 patients with CKD, treatment with dapagliflozin attenuated the increases in serum potassium observed with eplerenone [23]. Thus, available randomized evidence indicates that combined use of SGLT2i with agents that block the renin–angiotensin–aldosterone system may have distinct safety advantages by mitigating the risk of hyperkalaemia.
The kidney-protective effects of endothelin type A receptor antagonists have been most clearly demonstrated in patients with diabetic kidney disease in the SONAR trial [38], however, the use of this class of agent has been limited its tendency to cause fluid retention. This has led to concerns about a potential increased risk of heart failure [39]. However, data from SGLT2i trials suggest that SGLT2i reduce diuretic initiation and intensification [40]. Further in post hoc analyses from SONAR, treatment with an SGLT2i appeared to offset fluid retention associated with atrasentan [41]. In a phase 2 trial, there was no increased risk of fluid retention with combination dapagliflozin and low dose zibotentan in patients with CKD, and additive reductions in albuminuria with both agents compared to either alone [42]. Therefore, emerging data indicate that SGLT2i may enable safer use of endothelin type A receptor antagonists, a concept that is being further evaluated in the ZENITH-HP trial (Table 1).
Ongoing and planned trials of combination therapy and recent trials with varying use of background evidence-based kidney therapies.
| Trial . | Number of participants . | Population . | Intervention and control . | Primary outcome . | Participants receiving RAS blockade (%) . | Participants receiving SGLT2i (%) . | Status . |
|---|---|---|---|---|---|---|---|
| CKD progression trials | |||||||
| FLOW | 3534 | T2D and CKD | Semaglutide vs. placebo | ≥50% decline in eGFR, kidney failure or death due to kidney failure or cardiovascular disease | 95.3 | 15.5 | Completed and reported in May 2024 |
| FIND-CKD | 1598 | Non-diabetic CKD | Finerenone vs. placebo | Total eGFR slope from baseline to 32 months | 99.8 | 16.9 | Estimated completion in 2026 |
| ARTIC | ∼2500 | CKD | Dapagliflozin/baxdrostat vs. dapagliflozin | Change in eGFR from baseline to 24 months + 6 weeks off-treatment | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| EASi-KIDNEY | ∼11 000 | CKD | BI 690517 (aldosterone synthase inhibitor)/empagliflozin vs. empagliflozin | CKD progression, heart failure hospitalization or cardiovascular death | ∼100 | 100 | Recruitment to commence in 2024 |
| ZENITH-HP | ∼1500 | CKD | Zibotentan/dapagliflozin vs. dapagliflozin | Change in eGFR from baseline to 24 months | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| Heart failure and CKD | |||||||
| BALANCED-HF | ∼4800 | Heart failure and CKD | Balcinrenone (MR modulator)/dapagliflozin vs. dapagliflozin | Cardiovascular death or worsening heart failure event | TBD | 100 | Currently recruiting |
| Glomerular diseases | |||||||
| ALIGN | 320 + 64 in dedicated SGLT2i stratum | IgA nephropathy | Atrasentan vs. placebo | Change in proteinuria (UPCR) at 36 weeks Confirmatory endpoint: change in eGFR up to week 136 (4 weeks off-treatment) | ∼100 | 16.6 (dedicated SGLT2i stratum) | Estimated completion 2026 Interim analysis complete and reported |
| ASSIST | 52 | IgA nephropathy | Atrasentan (cross over trial) | Change in proteinuria (UPCR) at 12 weeks | ∼100 | 100 | Estimated completion October 2025 |
| Type 1 diabetes | |||||||
| ASPIRE | 36 | T1D | Ambrisentan and sotagliflozin alone then in combination | Change in albuminuria (UACR) | ∼100 | 100 | Not yet recruiting |
| Trial . | Number of participants . | Population . | Intervention and control . | Primary outcome . | Participants receiving RAS blockade (%) . | Participants receiving SGLT2i (%) . | Status . |
|---|---|---|---|---|---|---|---|
| CKD progression trials | |||||||
| FLOW | 3534 | T2D and CKD | Semaglutide vs. placebo | ≥50% decline in eGFR, kidney failure or death due to kidney failure or cardiovascular disease | 95.3 | 15.5 | Completed and reported in May 2024 |
| FIND-CKD | 1598 | Non-diabetic CKD | Finerenone vs. placebo | Total eGFR slope from baseline to 32 months | 99.8 | 16.9 | Estimated completion in 2026 |
| ARTIC | ∼2500 | CKD | Dapagliflozin/baxdrostat vs. dapagliflozin | Change in eGFR from baseline to 24 months + 6 weeks off-treatment | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| EASi-KIDNEY | ∼11 000 | CKD | BI 690517 (aldosterone synthase inhibitor)/empagliflozin vs. empagliflozin | CKD progression, heart failure hospitalization or cardiovascular death | ∼100 | 100 | Recruitment to commence in 2024 |
| ZENITH-HP | ∼1500 | CKD | Zibotentan/dapagliflozin vs. dapagliflozin | Change in eGFR from baseline to 24 months | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| Heart failure and CKD | |||||||
| BALANCED-HF | ∼4800 | Heart failure and CKD | Balcinrenone (MR modulator)/dapagliflozin vs. dapagliflozin | Cardiovascular death or worsening heart failure event | TBD | 100 | Currently recruiting |
| Glomerular diseases | |||||||
| ALIGN | 320 + 64 in dedicated SGLT2i stratum | IgA nephropathy | Atrasentan vs. placebo | Change in proteinuria (UPCR) at 36 weeks Confirmatory endpoint: change in eGFR up to week 136 (4 weeks off-treatment) | ∼100 | 16.6 (dedicated SGLT2i stratum) | Estimated completion 2026 Interim analysis complete and reported |
| ASSIST | 52 | IgA nephropathy | Atrasentan (cross over trial) | Change in proteinuria (UPCR) at 12 weeks | ∼100 | 100 | Estimated completion October 2025 |
| Type 1 diabetes | |||||||
| ASPIRE | 36 | T1D | Ambrisentan and sotagliflozin alone then in combination | Change in albuminuria (UACR) | ∼100 | 100 | Not yet recruiting |
T2D: type 2 diabetes; MR; mineralocorticoid receptor; UPCR: urinary protein: creatinine ratio; UACR: urinary albumin: creatinine ratio; T1D: type 1 diabetes.
Ongoing and planned trials of combination therapy and recent trials with varying use of background evidence-based kidney therapies.
| Trial . | Number of participants . | Population . | Intervention and control . | Primary outcome . | Participants receiving RAS blockade (%) . | Participants receiving SGLT2i (%) . | Status . |
|---|---|---|---|---|---|---|---|
| CKD progression trials | |||||||
| FLOW | 3534 | T2D and CKD | Semaglutide vs. placebo | ≥50% decline in eGFR, kidney failure or death due to kidney failure or cardiovascular disease | 95.3 | 15.5 | Completed and reported in May 2024 |
| FIND-CKD | 1598 | Non-diabetic CKD | Finerenone vs. placebo | Total eGFR slope from baseline to 32 months | 99.8 | 16.9 | Estimated completion in 2026 |
| ARTIC | ∼2500 | CKD | Dapagliflozin/baxdrostat vs. dapagliflozin | Change in eGFR from baseline to 24 months + 6 weeks off-treatment | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| EASi-KIDNEY | ∼11 000 | CKD | BI 690517 (aldosterone synthase inhibitor)/empagliflozin vs. empagliflozin | CKD progression, heart failure hospitalization or cardiovascular death | ∼100 | 100 | Recruitment to commence in 2024 |
| ZENITH-HP | ∼1500 | CKD | Zibotentan/dapagliflozin vs. dapagliflozin | Change in eGFR from baseline to 24 months | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| Heart failure and CKD | |||||||
| BALANCED-HF | ∼4800 | Heart failure and CKD | Balcinrenone (MR modulator)/dapagliflozin vs. dapagliflozin | Cardiovascular death or worsening heart failure event | TBD | 100 | Currently recruiting |
| Glomerular diseases | |||||||
| ALIGN | 320 + 64 in dedicated SGLT2i stratum | IgA nephropathy | Atrasentan vs. placebo | Change in proteinuria (UPCR) at 36 weeks Confirmatory endpoint: change in eGFR up to week 136 (4 weeks off-treatment) | ∼100 | 16.6 (dedicated SGLT2i stratum) | Estimated completion 2026 Interim analysis complete and reported |
| ASSIST | 52 | IgA nephropathy | Atrasentan (cross over trial) | Change in proteinuria (UPCR) at 12 weeks | ∼100 | 100 | Estimated completion October 2025 |
| Type 1 diabetes | |||||||
| ASPIRE | 36 | T1D | Ambrisentan and sotagliflozin alone then in combination | Change in albuminuria (UACR) | ∼100 | 100 | Not yet recruiting |
| Trial . | Number of participants . | Population . | Intervention and control . | Primary outcome . | Participants receiving RAS blockade (%) . | Participants receiving SGLT2i (%) . | Status . |
|---|---|---|---|---|---|---|---|
| CKD progression trials | |||||||
| FLOW | 3534 | T2D and CKD | Semaglutide vs. placebo | ≥50% decline in eGFR, kidney failure or death due to kidney failure or cardiovascular disease | 95.3 | 15.5 | Completed and reported in May 2024 |
| FIND-CKD | 1598 | Non-diabetic CKD | Finerenone vs. placebo | Total eGFR slope from baseline to 32 months | 99.8 | 16.9 | Estimated completion in 2026 |
| ARTIC | ∼2500 | CKD | Dapagliflozin/baxdrostat vs. dapagliflozin | Change in eGFR from baseline to 24 months + 6 weeks off-treatment | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| EASi-KIDNEY | ∼11 000 | CKD | BI 690517 (aldosterone synthase inhibitor)/empagliflozin vs. empagliflozin | CKD progression, heart failure hospitalization or cardiovascular death | ∼100 | 100 | Recruitment to commence in 2024 |
| ZENITH-HP | ∼1500 | CKD | Zibotentan/dapagliflozin vs. dapagliflozin | Change in eGFR from baseline to 24 months | ∼100 | 100 | Currently recruiting; estimated completion in 2027 |
| Heart failure and CKD | |||||||
| BALANCED-HF | ∼4800 | Heart failure and CKD | Balcinrenone (MR modulator)/dapagliflozin vs. dapagliflozin | Cardiovascular death or worsening heart failure event | TBD | 100 | Currently recruiting |
| Glomerular diseases | |||||||
| ALIGN | 320 + 64 in dedicated SGLT2i stratum | IgA nephropathy | Atrasentan vs. placebo | Change in proteinuria (UPCR) at 36 weeks Confirmatory endpoint: change in eGFR up to week 136 (4 weeks off-treatment) | ∼100 | 16.6 (dedicated SGLT2i stratum) | Estimated completion 2026 Interim analysis complete and reported |
| ASSIST | 52 | IgA nephropathy | Atrasentan (cross over trial) | Change in proteinuria (UPCR) at 12 weeks | ∼100 | 100 | Estimated completion October 2025 |
| Type 1 diabetes | |||||||
| ASPIRE | 36 | T1D | Ambrisentan and sotagliflozin alone then in combination | Change in albuminuria (UACR) | ∼100 | 100 | Not yet recruiting |
T2D: type 2 diabetes; MR; mineralocorticoid receptor; UPCR: urinary protein: creatinine ratio; UACR: urinary albumin: creatinine ratio; T1D: type 1 diabetes.
CLINICAL PRACTICE GUIDELINES AND CONSENSUS RECOMMENDATIONS
The American Heart Association Cardio-Kidney-Metabolic syndrome construct highlights the key role of excess/dysfunctional adiposity upstream to the development of a large proportion of metabolic syndrome, hypertension, diabetes, and dyslipidaemia, and eventual cardiovascular or kidney disease [43]. In this context, combination therapies offer a pathway to address the totality of cardio–kidney-metabolic risk across multiple enhancers in a holistic fashion.
Clinical practice guidelines and consensus recommendations acknowledge the potential to address residual cardio–kidney risk with the use of multiple evidence-based therapies. The American Diabetes Standards of Care explicitly addresses combination therapy with SGLT2i and GLP-1RA, suggesting this may be considered for additive risk reduction to improve cardiovascular and kidney outcomes in patients with type 2 diabetes with or at high risk of atherosclerotic cardiovascular disease [44]. The Kidney Disease: Improving Global Outcomes (KDIGO) diabetes and CKD clinical practice guidelines advocate for the addition of ns-MRA to reduce cardiorenal risk in patients with residual albuminuria despite RAS blockade and an SGLT2i [45]. KDIGO guidelines also recommend the use of GLP-1RA as the preferred glucose-lowering agent on top of RAS blockade and SGLT2i to reduce the risk of cardiovascular events [45]. Thus, major guidelines that provide strong recommendations for the use of SGLT2i, GLP-1RA, and ns-MRA individually also recognize the potential role for combination therapy.
THERAPEUTIC IMPLEMENTATION
Long-term projections based on large-scale randomized data indicates the potential of combination therapy to substantially improve long-term health outcomes in patients with type 2 diabetes and CKD. In an analysis of the major SGLT2i, ns-MRA, and GLP-1RA trials, combined treatment with RAS blockade, SGLT2i, ns-MRA and GLP-1RA was associated with a 35% relative risk reduction in major cardiovascular events, 55% reduction in hospitalization for heart failure, and 58% reduction in CKD progression compared to RAS blockade alone in patients with type 2 diabetes and CKD (Fig. 3) [46].
![Estimated treatment effects of SGLT2i, GLP-1RA, and ns-MRA alone, and in combination, when added to RAS blockade and risk factor control (conventional care) in people with type 2 diabetes and CKD. Adapted from Neuen et al. Circulation 2024 [46]. HR: hazard ratio; CI: confidence interval. MACE: major adverse cardiovascular events.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/40/Supplement_1/10.1093_ndt_gfae258/1/m_gfae258fig3.jpeg?Expires=1749745226&Signature=P6f5fU3il-moVy3Y3~lHWZL4XYQoSo1YNNE0Dg6DtyVPAiC4kjpqkymFN1CK~u4-74cvYJ1fRMjqmFBJYIEkQJgb8fRemrnjgseVlQtsVNYsKys3TM6v3h3YKkqwZ55bJVhld8r4TPsPWxYAGIj-GDMSIno53JjhJgEUGdXoXJGn3TX23FHUYRQ8nuLevd0tovOXKZOYLvEFhpppDji3pu1QL9rTDoCtoQxKJUPC5KeA4L8c1IMBqsPVMaoANDivyJ7LGSkIv2MkYqV3ZRpvlHOM5CFy4ay5JrCoiE6FKkQ-YtGSentFm~ZeLb8qlLfPHruLOFJ2JuXJy0U0vXbTpw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Estimated treatment effects of SGLT2i, GLP-1RA, and ns-MRA alone, and in combination, when added to RAS blockade and risk factor control (conventional care) in people with type 2 diabetes and CKD. Adapted from Neuen et al. Circulation 2024 [46]. HR: hazard ratio; CI: confidence interval. MACE: major adverse cardiovascular events.
However, the risk of kidney failure and other complications is highly heterogenous, depending on age, diabetes, and the aetiology of CKD itself [47]. Most patients with type 2 diabetes and CKD will die, primarily due to cardiovascular disease, before reaching kidney failure, and thus prevention of cardiovascular disease is a major therapeutic priority. But for many glomerular and cystic kidney diseases, lifetime risk of kidney failure is extremely high [48]. For very elderly patients addressing frailty and quality of life may be the dominant clinical priorities. Consequently, it is unlikely that all patients with CKD will require treatment with the same number and combination of therapies.
Strategies to match the intensity of treatment to absolute risk, individualizing the sequence of therapies based on the prevailing comorbidities and concerns of the individual, and tailoring the combination of therapies to the dominant event(s) of interest are needed. A strategy of using the KDIGO heat map, or another validated risk score, has been proposed to identify those who might benefit most from a more intensive up-front treatment with combination evidence-based kidney therapies (Fig. 4) [49]. Such an approach may be appealing as it prioritizes those who are likely to obtain the greatest absolute risk reductions, matches the intensity of treatment to risk, and may be a more cost-effective approach. Because the long-term benefits in preventing kidney failure and cardiovascular events are likely to be substantial for individuals with early CKD (i.e. preserved kidney function and severely increased albuminuria), these people should be prioritized [49]. As most evidence-based kidney therapies cause acute reductions in GFR that differ from their long-term effects [50], closer monitoring is also likely to be appropriate for those individuals with lower GFR who are initiating multiple therapies in a shorter timeframe [51]. Prioritizing specific agents early based on a compelling need to address specific comorbidities (e.g. GLP-1RA if weight and/or glycaemia is a clinical priority) may also be appropriate.
![Framework for implementation of combination guideline-directed medical therapies in type 2 diabetes and CKD. Adapted from Neuen et al. Circulation 2024 [49]. GDMT: guideline-directed medical therapy; KFRE: kidney failure risk equation.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/40/Supplement_1/10.1093_ndt_gfae258/1/m_gfae258fig4.jpeg?Expires=1749745226&Signature=I2x4HbztOFv-Iil6esORMzSMaWEGWjKFtaVFQM8k6lI76yJ2StS4U0mW5zUzz5lO7RF9RkQwU2qonC-ZBvF0-9PLDpfZbRv1kgEKNHONenPVloyKUSOd8ObR5alRAZ~lg5hXqlgFcwRsPlwnR9GFahgAX3L2KM~3ZRSE3PKRFwPd05e8yzhX8IumBdd0Y7Omm4abwkXQRPHFTxQE-QhxWYRWWrWoXr0O3lsKMFyf8k~YgM5HRugwCZTaRKl6nU7mIEaOOTKTrTsIleBmUNY3vm8tcZLxefsiXnxat3KVMgIJlU8JIk8CZwQorTHlbWm8Ke4PW08wR5E3olIDhzk1Gw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Framework for implementation of combination guideline-directed medical therapies in type 2 diabetes and CKD. Adapted from Neuen et al. Circulation 2024 [49]. GDMT: guideline-directed medical therapy; KFRE: kidney failure risk equation.
However, polypharmacy is common among people living with CKD, increasing with kidney disease stage, and is associated with adverse effects on quality of life, medication non-adherence, and adverse drug reactions [52, 53]. Studies suggest the top five classes of medications used by CKD patients are lipid lowering agents, beta blockers, glucose-lowering agents, analgesics, and diuretic agents, none of which have kidney-protective properties, representing a significant opportunity for optimization of therapy [54]. It is therefore important that clinicians focus on high-value prescribing (including potentially deprescribing medications of limited benefit on clinical outcomes) and prioritize proven therapies. Ongoing work to develop and refine practical strategies for therapeutic implementation should be a major clinical research priority.
COMBINATION THERAPY IN NON-DIABETIC CKD
Despite major therapeutic advances, many important questions remain unanswered. Evidence for a pillared approach is strongest in type 2 diabetes and CKD, where multiple therapies have been proven to improve clinical outcomes, with fewer data for non-diabetic CKD.
Additional evidence for a pillared approach to non-diabetic CKD is evolving. The FIND-CKD trial is evaluating the effect of finerenone on a primary outcome of total eGFR slope over 32 months in ∼1600 patients with non-diabetic CKD, almost 60% of whom have an underlying glomerular disease (Table 1) [55]. Optimal use and dosing of RAS blockade was required for entry into the trial, and ∼17% were receiving SGLT2i at baseline. Thus FIND-CKD has the potential to expand the role of finerenone, such that three of the four ‘pillars’ of therapy for CKD may be shared across diabetic and non-diabetic CKD: RAS blockade, SGLT2i, and ns-MRA. The role of GLP-1RA in people with non-diabetic CKD may also expand. Data from the SELECT trial of >17 000 people with obesity and cardiovascular disease without diabetes suggests that semaglutide reduces albuminuria and kidney function decline, although this was a low kidney-risk population [56]. Most recently, semaglutide reduced albuminuria by approximately 50% in a trial of approximately 100 people with overweight or obesity and albuminuric CKD [57].
The advent of disease-specific targeted therapies in specific glomerular diseases, for example APRIL and BAFF inhibitors in IgA nephropathy, provides an additional opportunity for combination therapy. With multiple therapies on the horizon in IgA nephropathy, how B-cell targeted therapies may be used alongside agents that target the complement pathway is uncertain, as is the optimal way to determine treatment response (e.g. markers of complement activity) and treatment duration. Another key challenge with disease-specific therapies in glomerular diseases is to identify those who might benefit most—and when—from targeting the underlying immunological basis of disease, compared to those who should be primarily treated with therapies that address maladaptive responses to injury (e.g. SGLT2i and ns-MRA). These questions are important areas for further study, and better strategies to guide selection and sequencing of therapies will be needed. Nevertheless, for most patients, disease-specific and disease-agnostic therapies are not mutually exclusive and should be viewed as complimentary (Fig. 2).
FUTURE DIRECTIONS IN COMBINATION THERAPY
An increasing number of trials are evaluating combination therapy with active-controlled designs (Table 1). Two phase 3 clinical trial programmes are evaluating the effects of aldosterone synthase inhibition with SGLT2i versus SGLT2i alone in patients with CKD. The ARTIC trial is recruiting ∼2500 patients with albuminuric CKD, evaluating the effect of baxdrostat/dapagliflozin versus dapagliflozin alone on a primary outcome of change in eGFR from baseline to 2 years with a final eGFR measurement off-treatment to account for the acute negative effect on GFR of baxdrostat [58]. The EASi-KIDNEY trial is testing the effect of vicadrostat/empagliflozin versus empagliflozin alone in ∼11 000 patients with CKD [59]. In the ZENITH-HP trial, the effect of zibotentan/dapagliflozin versus dapagliflozin alone is being studied in ∼1500 patients with albuminuric CKD, with a primary outcome of change in eGFR over 24 months [60]. Thus, ongoing trials will evaluate new therapies on top of almost universal use of RAS blockade and SGLT2i. Similar designs are being used across the spectrum of cardio–kidney-metabolic syndrome, including in glomerular diseases.
With multiple highly effective and safe therapies available to improve CKD outcomes, developing and validating new tools to assess treatment response that do not rely solely on albuminuria lowering are needed. To date, few biomarkers have translated into useful tools to assess treatment response [61], with anti-phospholipase A2 receptor antibodies being a notable exception. Identifying biomarkers that can evaluate treatment response and therefore guide therapeutic decision making could allow clinicians to personalize combination therapy in a way that is not currently possible.
Finally, there is a need for clinical trials evaluating different implementation strategies for combination therapies in CKD. Could a risk-based accelerated approach to therapeutic implementation reduce therapeutic inertia and translate into an improvement in clinical outcomes compared to usual care? Could lowering albuminuria as much and as quickly as possible reduce cardiovascular and kidney outcomes compared to usual care? In heart failure, the STRONG-HF trial demonstrated that in patients recently hospitalized for heart failure, high-intensity care, and rapid titration of guideline-directed medical therapy to recommended doses improved quality of life and reduced heart failure readmission or all-cause mortality [62]. Similar trials in dedicated CKD populations have the potential to substantially advance care by providing a practical approach to implementation of evidence-based kidney therapies.
CONCLUSION
The complex pathophysiology that underlies the development and progression of diabetic and non-diabetic CKD argues the need for multiple evidence-based therapies used in combination to reduce the lifetime risks of kidney failure, cardiovascular events, and mortality. Evidence for combination therapy is strongest in diabetic CKD, with four agents proven to improve kidney, cardiovascular, and mortality outcomes. A similar pillared approach is likely to emerge in non-diabetic CKD, with the additional dimension of disease-specific immunological therapies. Current evidence suggests additive effects and potential safety advantages with the combined use of available therapies, however, additional work to determine optimal timing, sequence, and intensity of evidence-based kidney therapies will help transition CKD management towards a multi-medicine strategy to reduce residual kidney and cardiovascular risk.
FUNDING
This paper was published as part of a supplement financially supported by Bayer AG and the scientific content has not been influenced in any way by the sponsor.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
BLN reports fees for travel support, advisory boards, scientific presentations, publication support and steering committee roles from AstraZeneca, Alexion, Bayer, Boehringer and Ingelheim, Janssen, Menarini, Novo Nordisk, and Travere Therapeutics. EKY has no relevant disclosures. JR reports consultant work for Boehringer Ingelheim/Lily, Edward Lifesciences, and Astra Zeneca and advisory board participation for Procyrion Inc (Aortix). MV has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, BMS, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.





Comments