-
PDF
- Split View
-
Views
-
Cite
Cite
Timo Mayerhöfer, Andrew D Shaw, Christian J Wiedermann, Michael Joannidis, Fluids in the ICU: which is the right one?, Nephrology Dialysis Transplantation, Volume 38, Issue 7, July 2023, Pages 1603–1612, https://doi.org/10.1093/ndt/gfac279
Close - Share Icon Share
ABSTRACT
The administration of fluids is one of the most common interventions in the intensive care unit. The effects and side effects of intravenous fluids depend on the amount administered and their specific composition. Intravenous fluid solutions are either considered crystalloids (for example 0.9% saline, lactated Ringer's solution) or colloids (artificial colloids such as gelatins, and albumin). This narrative review summarizes the physiological principles of fluid therapy and reviews the most important studies on crystalloids, artificial colloids and albumin in the context of critically ill patients.
BACKGROUND
The history of intravenous (IV) fluid resuscitation dates back to 1832 and the cholera epidemic in London, where saline solutions were first administered [1, 2]. The development of fluid therapy continued in the year 1885, when Sidney Ringer developed a physiologic salt solution, which was later modified by Alexis Hartman. In contrast to 0.9% ‘normal’ saline, these preliminary solutions (now termed balanced or buffered solutions) contained chloride in reduced concentrations and various other electrolytes [3].
Subsequently, albumin and synthetic colloids were introduced as possible resuscitation fluids. Their theoretical advantage over crystalloids is based on the principle of oncotic pressure, whereby a greater volume expansion effect may be achieved. However, their clinical benefit over and above crystalloid solutions is a controversial topic [4].
The use of IV fluids in critically ill patients can generally be divided into resuscitation, replacement and maintenance. While resuscitation fluids are especially important in the initial phase of treatment, to regain hemodynamic stability [3], and are administered as boluses, maintenance fluids are mostly given as continuous infusions and aim to cover a patient's daily requirements [5, 6]. Replacement fluids have a special status and are designed to compensate for specific losses (e.g. electrolytes).
In the initial phase critically ill patients in the intensive care unit (ICU) often require large amounts of fluid for stabilization. However, not only too little, but also too much fluid may do harm [7, 8]. This realization led to fluids being considered as drugs, with effects and adverse effects depending on their composition and amount (dose) administered [9]. Therefore, many studies have attempted to answer the question of whether certain amounts and specific compositions of fluids provide benefits to patients in specific situations.
This review focuses primarily on resuscitation of critically ill patients, new studies in the field, ongoing topics of discussion and the state of the art in fluid administration in critical care.
Physiology of fluid administration
In general, the main goal of fluid administration is to ensure adequate tissue perfusion by increasing intravascular volume. According to the Frank Starling mechanism, in a normal heart the resulting increase in preload leads to an increased cardiac output (Fig. 1). The final goal is thus adequate oxygen delivery.
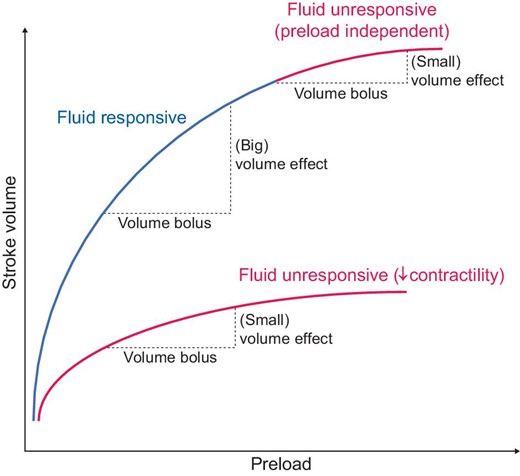
The Frank–Starling Mechanism. Reduced fluid responsiveness may occur either due to reduced contractility during heart failure or because the plateau of the Starling curve is reached.
The most common clinical scenarios that require immediate fluid resuscitation are hypovolemic shock due to hemorrhage by trauma or major surgery, or due to extravascular loss in systemic inflammatory reactions such as in sepsis or burn patients [10]. The response to fluid administration depends on cardiac function and on baseline preload. Therefore, even patients with normal cardiac function may become non-responsive to fluid, if the flat part of the Frank–Starling curve has already been reached after increasing pre-load (Fig. 1).
The second determinant of the efficacy of fluid administration is the duration of intravascular volume expansion following fluid administration. The physiological principle of early fluid therapy was also based on experiments by Starling. The vascular semipermeable membrane and the interplay between hydrostatic pressure (predominantly on the arterial side) and oncotic pressure (predominantly on the venous side) are responsible for fluid shifts between the various physiological compartments, according to this principle. These ideas have recently been revised with greater appreciation of the role of the endothelial glycocalyx [11]. This is a complex layer with multiple functions; it consists of glycoproteins and proteoglycans on the luminal side, which play a key role in regulating vascular permeability. In critically ill patients, especially in sepsis or severe trauma, this layer may be damaged, leading to alterations in vascular permeability resulting in increased trans-capillary escape of albumin, the major oncotic constituent in the plasma. Increased rates of fluid loss from the intravascular space into the extravascular space have a direct impact on the need for fluid administration, because the duration of its effect is diminished.
This leads to a need for further fluid administration [12]. This situation may be associated with an increased risk of fluid overload (Fig. 2).
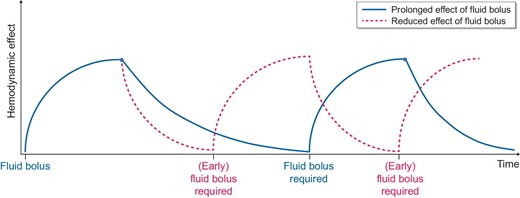
Schematic illustration of patterns of fluid response in different disease states; the red curve shows a faster decline of the effect of repeated fluid boluses (e.g. during sepsis due to increased vascular permeability). This leads to an earlier requirement of fluid boluses and consequently carries the risk of fluid overload.
Another important aspect among those mentioned above, especially when it comes to repeated fluid bolus administration, is the peripheral arterial resistance [13]. In a study by Monge García et al. in septic shock patients, fluid administration resulted in an improved cardiac output but reduced arterial load, and therefore did not improve blood pressure [14]. Consequently, studies indicate that further fluid boluses are not as effective as in the initial phase even in so-called responders [15].
Rationale of fluid administration
An international survey revealed that impaired tissue perfusion or low cardiac output were the most common reasons for fluid administration [16]. The Fluid challENges in Intensive CarE (FENICE) trial showed that both the amount and choice of fluid administration vary widely across countries [17].
This study also showed that hypotension and oliguria were the most frequent indications for fluid prescription, but many clinicians did not use any specific targets to regulate further fluid administration [17]. However, it is also true that there are no universally accepted targets for the administration of resuscitation fluids, which makes it challenging for clinicians to determine the right amount of fluid therapy for the individual patient [10]. This increases the potential for over-administration of IV fluid. Studies clearly indicate that not only too little but also too much fluid may lead to additional harm in the critically ill [8]. Administration of too much fluid may lead to the formation of edema, which can negatively affect the function of various organs such as the lungs or the kidneys, particularly in sepsis and acute respiratory distress syndrome (ARDS) [18, 19]. This is also the case in the perioperative setting [20], where hypervolemia has been associated with increased mortality rates.
In the early phase of treatment it is undoubtedly important to act quickly, but as the resuscitation phase moves into the maintenance phase, a more nuanced approach to fluid therapy is warranted, with specific therapeutic targets becoming more important [21].
The right amount of fluid is still a matter of an ongoing discussion. For initial resuscitation in septic shock the Surviving Sepsis Campaign recommends 30 mL/kg in the first 3 h [6]. However, after this initial fluid bolus, repeated lactate measurements (to determine its clearance) are recommended as parameters for adequate therapy. In addition, other clinical examinations such as central venous saturation, echocardiography, invasive hemodynamic monitoring or a simple assessment of capillary refill time are available as potential targets for the individualization of fluid strategies [6]. In ARDS patients a restrictive fluid management strategy (leading to an equal balance over 7 days) resulted in improved weaning from mechanical ventilation [22]. On the contrary, restrictive fluid management in the perioperative setting resulted in increased rates of acute kidney injury (AKI) and renal replacement therapy (RRT) [23].
Interestingly, the recently published Conservative vs. Liberal Approach to fluid therapy of Septic Shock in Intensive Care (CLASSIC) trial investigating the effect of restricted volume administration by accepting higher thresholds of lactate and mottling, as well as lower mean arterial pressures than usually recommended, did not show any difference in outcome compared with standard fluid therapy [24]. However, the median difference in the cumulative fluid balance between the two groups was only 744 mL (1676 mL versus 2420 mL) after 5 days and in a subanalysis of patients who had received <30 mL/kg fluids before randomization (n = 520) the 90-day mortality was 5.3% higher than in the standard-fluid group [25]. Although these results were not statistically significant a restrictive fluid strategy may be harmful for patients with reduced effective circulating volume.
CRYSTALLOIDS
The most commonly used resuscitation fluids are certainly crystalloids. There is evidence that supports the idea that the choice of crystalloid for IV fluid therapy does matter, and in fact that it makes a clinically important difference. Most data suggest that IV fluids containing a relatively physiological chloride concentration are probably the right initial fluid choice in adult ICU patients. A notable exception are patients with traumatic brain injury, patients with prolonged vomiting and excessive losses of gastric juice, and patients who are hyponatremic as well as hypochloremic. These patients should receive 0.9% saline [26].
Electrolyte content of available crystalloid preparations
In general, there are two broad categories of crystalloid preparations available for IV use in the ICU. These categories are physiologically buffered (balanced) preparations that contain electrolytes and buffers in such concentrations that they approximate those found in normal human plasma. The other category is neither buffered nor balanced, and it does not contain physiological concentrations of electrolytes. They are, however, mostly isotonic and, thus, will not lyse red blood cells when administered intravenously. Fluids such as 0.9% saline may lead to generation of a hyperchloremic metabolic acidosis which is dose dependent [27]. It is the chloride load that is thought to lead to the adverse effects observed in patients given moderate to large doses of high chloride–containing fluids such as 0.9% saline. The underlying pathophysiology is not entirely clear, and opinion differs on whether the precise mechanism is related to arteriolar vasoconstriction or an immune-mediated problem that makes patients more susceptible to damage-associated and pathogen-associated molecular pattern–induced insults.
Evidence that electrolyte concentration is important
Recently, data have been published that suggest the concentration of electrolytes in the plasma is highly predictive of risk of moderate to severe AKI after heart surgery. Demirjian et al. recently reported a model (capable of almost 90% accuracy) that is derived entirely from the first basic metabolic panel drawn after admission to the ICU [28]. Since the principal determinant of electrolyte concentration in the plasma of patients during and immediately after heart surgery is the electrolyte delivered to them in the form of IV fluid, it is reasonable to conclude that these two phenomena are at least associated.
Chowdhury et al. reported a double-blind crossover comparison of Plasmalyte© versus 0.9% saline in which 2 L of crystalloid therapy were administered to human volunteers and then after a 2-week washout period the other fluid was administered [29]. These investigators measured renal blood flow both at the overall organ level and at the cortico-glomerular interface. They demonstrated an almost immediate and highly statistically significant reduction in blood flow in subjects given 0.9% saline, but not with the buffered preparation, which induced a mild increase.
Observational literature
In 2012 Shaw et al. published an observational study in surgical patients that reported an association between resuscitation with 0.9% saline and adverse clinical outcomes [30]. This paper also suggested that patients receiving saline received more interventions for metabolic acidosis, including newly initiated RRT. Subsequently, others reported further associations between the use of high chloride fluid preparations and adverse outcomes in different patient populations, including evidence of a dose response [31].
Interventional literature
In 2012 Yunos et al. reported a before-and-after study in which the incidence of moderate to severe AKI was measured after access to high chloride–containing fluids was restricted [32]. The incidence of this endpoint was significantly lower in the period after which these fluids were effectively removed from the ICUs contributing data to the study. At this point in time substantial circumstantial evidence had accumulated that the need for high quality interventional clinical trials was clear. The effect size of the hazard (high chloride fluids) is likely small, and thus large trials would be needed. Also, the ability of the host to tolerate (i.e. resist) the hazard is likely dependent on how sick the recipient is.
Over the next couple of years, pilot trials (SPLIT, SALT) were published examining the feasibility of conducting large scale trials that might address the problem. These initial trials hinted that there may be a mortality signal that would confirm the observational data, but by themselves were not conclusive [26, 33].
In 2018 Semler et al. published the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) in the New England Journal of Medicine (NEJM) [34]. This study was a cluster crossover trial conducted in the adult ICUs of their institution in which each ICU was randomized to start with either 0.9% saline, or a buffered crystalloid solution for all fluid therapy in that ICU for the whole month (Table 1). The next month each ICU crossed over to the other fluid and this pattern then continued until the end of the trial. The endpoint in the trial was major adverse kidney events at Day 30 (MAKE30) and included death, new dialysis and sustained worsened renal function (2× baseline creatinine) at Day 30. There was a statistically significant reduction in the composite endpoint in the buffered crystalloid group, with the death component driving the difference.
Summary of key multicenter randomized controlled studies that compare normal saline with balanced crystalloids.
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of study fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Crystalloids | |||||||
| SMART trial (2018) | 15 802 ICU patients | Balanced crystalloids vs 0.9% saline | 0 (median, 24 h prior to ICU admission) | 1 L (median, until discharge) | Multicenter, cluster randomized, multiple crossover | 30d MAKE | Lower MAKE rate in crystalloid group |
| SALT-ED (2018) | 13 347 non-critically ill | Balanced crystalloids vs 0.9% saline | NA | 1 L (median, study period) | Single center, multi-crossover | Hospital-free days | No difference, less MAKE in crystalloid group |
| BaSICS trial (2021) | 11 052 critically ill | Balanced crystalloids vs 0.9% saline | 1 L (median, 24 h before enrollment) | 2.9 L (median, first 3 days) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| PLUS trial (2022) | 5037 critically ill | Balanced crystalloids vs 0.9% saline | 571 mL (median, 0.9% NaCl), 0 (median, balanced solutions) | 3.7–3.9 L (median, study period) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of study fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Crystalloids | |||||||
| SMART trial (2018) | 15 802 ICU patients | Balanced crystalloids vs 0.9% saline | 0 (median, 24 h prior to ICU admission) | 1 L (median, until discharge) | Multicenter, cluster randomized, multiple crossover | 30d MAKE | Lower MAKE rate in crystalloid group |
| SALT-ED (2018) | 13 347 non-critically ill | Balanced crystalloids vs 0.9% saline | NA | 1 L (median, study period) | Single center, multi-crossover | Hospital-free days | No difference, less MAKE in crystalloid group |
| BaSICS trial (2021) | 11 052 critically ill | Balanced crystalloids vs 0.9% saline | 1 L (median, 24 h before enrollment) | 2.9 L (median, first 3 days) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| PLUS trial (2022) | 5037 critically ill | Balanced crystalloids vs 0.9% saline | 571 mL (median, 0.9% NaCl), 0 (median, balanced solutions) | 3.7–3.9 L (median, study period) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
NA, not applicable.
Summary of key multicenter randomized controlled studies that compare normal saline with balanced crystalloids.
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of study fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Crystalloids | |||||||
| SMART trial (2018) | 15 802 ICU patients | Balanced crystalloids vs 0.9% saline | 0 (median, 24 h prior to ICU admission) | 1 L (median, until discharge) | Multicenter, cluster randomized, multiple crossover | 30d MAKE | Lower MAKE rate in crystalloid group |
| SALT-ED (2018) | 13 347 non-critically ill | Balanced crystalloids vs 0.9% saline | NA | 1 L (median, study period) | Single center, multi-crossover | Hospital-free days | No difference, less MAKE in crystalloid group |
| BaSICS trial (2021) | 11 052 critically ill | Balanced crystalloids vs 0.9% saline | 1 L (median, 24 h before enrollment) | 2.9 L (median, first 3 days) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| PLUS trial (2022) | 5037 critically ill | Balanced crystalloids vs 0.9% saline | 571 mL (median, 0.9% NaCl), 0 (median, balanced solutions) | 3.7–3.9 L (median, study period) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of study fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Crystalloids | |||||||
| SMART trial (2018) | 15 802 ICU patients | Balanced crystalloids vs 0.9% saline | 0 (median, 24 h prior to ICU admission) | 1 L (median, until discharge) | Multicenter, cluster randomized, multiple crossover | 30d MAKE | Lower MAKE rate in crystalloid group |
| SALT-ED (2018) | 13 347 non-critically ill | Balanced crystalloids vs 0.9% saline | NA | 1 L (median, study period) | Single center, multi-crossover | Hospital-free days | No difference, less MAKE in crystalloid group |
| BaSICS trial (2021) | 11 052 critically ill | Balanced crystalloids vs 0.9% saline | 1 L (median, 24 h before enrollment) | 2.9 L (median, first 3 days) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
| PLUS trial (2022) | 5037 critically ill | Balanced crystalloids vs 0.9% saline | 571 mL (median, 0.9% NaCl), 0 (median, balanced solutions) | 3.7–3.9 L (median, study period) | Multicenter, double-blind randomized controlled | 90-day mortality | No difference in mortality or AKI |
NA, not applicable.
In the same issue of the NEJM, Self et al. published the the Saline Against Lactated ringer's or plasma-lyTe in the Emergency Department (SALT-ED) trial in which the same design and same endpoint was used to investigate the effect of fluid choice in Emergency Department patients admitted to the hospital but not the ICU [35]. In this group of (less sick) patients, the endpoint was again reduced in patients given buffered fluid, this time driven by sustained reductions in renal function rather than death.
In 2021 Zampieri et al. reported the results of a trial in over 10 000 ICU patients in which the comparison was saline or buffered fluid [36]. This trial also assessed rate of administration of fluid. Nether intervention was associated with an overall reduction in mortality. However, in patients being treated for traumatic brain injury there was a reduction in mortality in the saline group. Regardless of the mechanism there is now good rationale for selecting 0.9% saline as first choice of IV fluid in TBI patients.
In 2022 Finfer et al. reported the results of the Plasma-Lyte versUs Saline (PLUS) study trial, which compared Plasmalyte© versus saline in ICU patients [37]. This also failed to detect a difference in mortality. Superficially then it may appear there is no difference in outcomes if these trials are accurate. However, closer examination suggests that there was likely insufficient separation of the interventions (due to the nature of postoperative ICU populations who have already received large quantities of what is largely buffered fluid) and a total dose of fluid that is unlikely to have been able to lead to a detectable difference. Perhaps a more accurate conclusion therefore is that both these trials were unable to find a difference because there was an insufficient dose of the putative hazard delivered, and too much contamination in the intervention groups [38]. A post hoc analysis of the Balanced Solution versus Saline in Intensive Care (BaSICS) study trial found a high probability that the use of balanced solution improves 90-day mortality in patients without ‘contamination’ and who received only balanced fluids [39]. Similar results were obtained by a secondary analysis of the SMART trial [40]. Both of the above-mentioned problems are avoided by using a cluster crossover design and allowing naturally occurring randomization (i.e. date of ICU admission) to minimize bias in the trial. On the same day that PLUS was published, the BaSICs and PLUS investigators together reported a meta-analysis of the high quality trial data in this clinical space that concluded there is a 90% chance that 0.9% saline is associated with an increase in mortality in adult ICU patients (risk ratio 0.96, 95% confidence interval 0.91–1.01; I2 = 12.1%) [41]. In addition, it must be mentioned that these trials used the corresponding fluid not only for resuscitation, but also for maintenance and replacement. This might lead to an overestimation of the effect on fluid boluses during fluid resuscitation alone.
COLLOIDS
Colloids are IV fluids that contain high molecular weight microscopic substances suspended in crystalloid solutions. Colloids were introduced into clinical practice because of their theoretical ability to remain in the intravascular space for longer periods of time than crystalloids, due to the presence of macromolecules in solution, which create greater osmotic pressure in the circulation. Because of their high molecular weight, colloidal substances penetrate the healthy semipermeable endothelial barrier only slowly. While crystalloid electrolyte solutions distribute evenly to intravascular and extravascular spaces, colloids are intended to achieve a selective expansion of the intravascular space due to oncotic gradients. This effect is partially lost with inflammation-altered vascular permeability.
An advantage of colloidal solutions in fluid therapy is their volume-saving effect. Theoretically, the volume of the colloid required to maintain the same intravascular filling is up to three times smaller than that of crystalloids. However, this advantage is lost when the endothelial glycocalyx is damaged by inflammatory conditions, although to a different extent. Indeed, despite their theoretical advantages, colloids have not generally proved effective in critically ill patients. At present, there are no data to support the routine use of colloid for volume resuscitation [42].
Colloids can be divided into two groups: ‘semi-synthetic’ [hydroxyethyl starch (HES), gelatin and dextran solutions] and ‘natural’ (human albumin solution). Both types of colloids have found widespread clinical use because of stronger volume expanding effects than could be achieved with crystalloids. Semi-synthetic colloids were preferred because they were comparable in efficacy but cheaper and more readily available than their natural counterparts. Of the semi-synthetic colloids, it was HES that was preferentially used in many countries for decades [43].
Semi-synthetic colloids
Hydroxyethyl starch
HES solutions obtained by hydroxyethyl substitution of amylopectin molecules in potato or maize starch are available with different molecular weights, molar degrees of substitution and tonicities. A higher concentration and molecular weight of HES are associated with a stronger osmotic effect. A higher degree of substitution and a higher C2/C6 ratio provide better protection against blood amylases at the expense of increased accumulation in reticuloendothelial tissues such as kidney, liver and skin [44]. Adverse effects associated with the use of HES include deterioration of kidney function, coagulopathy, persistent itching of the skin and allergic reactions [45].
Numerous studies have demonstrated nephrotoxic effects of hyperoncotic HES solutions. In three large randomized controlled trials comparing HES and crystalloids, the volume of HES administered was only to 20% smaller than in the crystalloid group, much less than expected, and the small reduction in the volume of fluid administered does not appear to be clinically relevant (Table 2). In fact, this putative benefit is offset by the known side effects of HES such as renal failure. Because of possible adverse effects, the use of HES should also be generally discouraged, including in the critical care and perioperative settings. Neither form of HES solution should probably be used in patients with trauma or any form of brain injury as well [45].
Summary of key studies comparing artificial colloids or albumin with other fluids.
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Artificial colloids | |||||||
| CRISTAL Randomized Trial (2013) | 2857 critically ill | Colloids (gelatins, dextrans, HES, or 4% or 20% of albumin) vs crystalloids | Excluded | 2000 vs 3000 mL (during first 7 days, 1:1.5) | Multicenter, parallel group, randomized controlled | 28-day mortality | No difference, 90-day mortality was lower in colloid group |
| CHEST (2012) | 7000 critically ill | 6% HES vs 0.9% saline | NA, >1000 mL HES excluded | 1000 vs 1200 mL (1: 1.2) | Multicenter, double-blind randomized controlled | 90d mortality | No difference, HES was associated with more adverse events |
| 6S trial (2012) | 804 critically ill | HES 130/0.42 vs Ringer acetate | 2500 vs 2400 mL crystalloids (median), 500 vs 275 mL albumin (median) | 1500 vs 1500 mL (1:1.1) | Multicenter, parallel group, blinded, randomized controlled | 90-day mortality or end-stage kidney failure | Increased risk of death and more RRT in HES group |
| VISEP (2008) | 537 severe sepsis patients | 10% HES 200/0.5 vs Ringer lactate | 2000 mL crystalloids, 315 mL collids (median, 12 h prior), >1000 mL HES excluded | 70 mL/kg of body weight HES (1:1.3) | Multicenter, two-by-two, randomized controlled trial | 90-day mortality or mean score for organ failure | Increased rate of AKI and RRT in HES group |
| Albumin | |||||||
| SAFE | 6997 critically ill | Albumin 4% vs 0.9% saline | NA | 1200 vs 1500 mL (1:1.4) | Multicenter, double-blind randomized controlled | 28-day mortality | No difference, higher mortality in subgroup of patients with traumatic brain injury |
| ALBIOS | 1818 severe sepsis patients | Albumin 20% and crystalloids vs crystalloids | NA, 500 mL HES (median) | 4300 vs 4250 mL crystalloids | Multicenter, open-label, randomized controlled, Albumin target | 28-day mortality | No difference, benefit for subgroup of septic shock? |
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Artificial colloids | |||||||
| CRISTAL Randomized Trial (2013) | 2857 critically ill | Colloids (gelatins, dextrans, HES, or 4% or 20% of albumin) vs crystalloids | Excluded | 2000 vs 3000 mL (during first 7 days, 1:1.5) | Multicenter, parallel group, randomized controlled | 28-day mortality | No difference, 90-day mortality was lower in colloid group |
| CHEST (2012) | 7000 critically ill | 6% HES vs 0.9% saline | NA, >1000 mL HES excluded | 1000 vs 1200 mL (1: 1.2) | Multicenter, double-blind randomized controlled | 90d mortality | No difference, HES was associated with more adverse events |
| 6S trial (2012) | 804 critically ill | HES 130/0.42 vs Ringer acetate | 2500 vs 2400 mL crystalloids (median), 500 vs 275 mL albumin (median) | 1500 vs 1500 mL (1:1.1) | Multicenter, parallel group, blinded, randomized controlled | 90-day mortality or end-stage kidney failure | Increased risk of death and more RRT in HES group |
| VISEP (2008) | 537 severe sepsis patients | 10% HES 200/0.5 vs Ringer lactate | 2000 mL crystalloids, 315 mL collids (median, 12 h prior), >1000 mL HES excluded | 70 mL/kg of body weight HES (1:1.3) | Multicenter, two-by-two, randomized controlled trial | 90-day mortality or mean score for organ failure | Increased rate of AKI and RRT in HES group |
| Albumin | |||||||
| SAFE | 6997 critically ill | Albumin 4% vs 0.9% saline | NA | 1200 vs 1500 mL (1:1.4) | Multicenter, double-blind randomized controlled | 28-day mortality | No difference, higher mortality in subgroup of patients with traumatic brain injury |
| ALBIOS | 1818 severe sepsis patients | Albumin 20% and crystalloids vs crystalloids | NA, 500 mL HES (median) | 4300 vs 4250 mL crystalloids | Multicenter, open-label, randomized controlled, Albumin target | 28-day mortality | No difference, benefit for subgroup of septic shock? |
NA, not applicable.
Summary of key studies comparing artificial colloids or albumin with other fluids.
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Artificial colloids | |||||||
| CRISTAL Randomized Trial (2013) | 2857 critically ill | Colloids (gelatins, dextrans, HES, or 4% or 20% of albumin) vs crystalloids | Excluded | 2000 vs 3000 mL (during first 7 days, 1:1.5) | Multicenter, parallel group, randomized controlled | 28-day mortality | No difference, 90-day mortality was lower in colloid group |
| CHEST (2012) | 7000 critically ill | 6% HES vs 0.9% saline | NA, >1000 mL HES excluded | 1000 vs 1200 mL (1: 1.2) | Multicenter, double-blind randomized controlled | 90d mortality | No difference, HES was associated with more adverse events |
| 6S trial (2012) | 804 critically ill | HES 130/0.42 vs Ringer acetate | 2500 vs 2400 mL crystalloids (median), 500 vs 275 mL albumin (median) | 1500 vs 1500 mL (1:1.1) | Multicenter, parallel group, blinded, randomized controlled | 90-day mortality or end-stage kidney failure | Increased risk of death and more RRT in HES group |
| VISEP (2008) | 537 severe sepsis patients | 10% HES 200/0.5 vs Ringer lactate | 2000 mL crystalloids, 315 mL collids (median, 12 h prior), >1000 mL HES excluded | 70 mL/kg of body weight HES (1:1.3) | Multicenter, two-by-two, randomized controlled trial | 90-day mortality or mean score for organ failure | Increased rate of AKI and RRT in HES group |
| Albumin | |||||||
| SAFE | 6997 critically ill | Albumin 4% vs 0.9% saline | NA | 1200 vs 1500 mL (1:1.4) | Multicenter, double-blind randomized controlled | 28-day mortality | No difference, higher mortality in subgroup of patients with traumatic brain injury |
| ALBIOS | 1818 severe sepsis patients | Albumin 20% and crystalloids vs crystalloids | NA, 500 mL HES (median) | 4300 vs 4250 mL crystalloids | Multicenter, open-label, randomized controlled, Albumin target | 28-day mortality | No difference, benefit for subgroup of septic shock? |
| Study (year) . | Population . | Fluids . | Amount of fluid prior to randomization . | Amount of fluid . | Design . | Endpoint . | Outcome . |
|---|---|---|---|---|---|---|---|
| Artificial colloids | |||||||
| CRISTAL Randomized Trial (2013) | 2857 critically ill | Colloids (gelatins, dextrans, HES, or 4% or 20% of albumin) vs crystalloids | Excluded | 2000 vs 3000 mL (during first 7 days, 1:1.5) | Multicenter, parallel group, randomized controlled | 28-day mortality | No difference, 90-day mortality was lower in colloid group |
| CHEST (2012) | 7000 critically ill | 6% HES vs 0.9% saline | NA, >1000 mL HES excluded | 1000 vs 1200 mL (1: 1.2) | Multicenter, double-blind randomized controlled | 90d mortality | No difference, HES was associated with more adverse events |
| 6S trial (2012) | 804 critically ill | HES 130/0.42 vs Ringer acetate | 2500 vs 2400 mL crystalloids (median), 500 vs 275 mL albumin (median) | 1500 vs 1500 mL (1:1.1) | Multicenter, parallel group, blinded, randomized controlled | 90-day mortality or end-stage kidney failure | Increased risk of death and more RRT in HES group |
| VISEP (2008) | 537 severe sepsis patients | 10% HES 200/0.5 vs Ringer lactate | 2000 mL crystalloids, 315 mL collids (median, 12 h prior), >1000 mL HES excluded | 70 mL/kg of body weight HES (1:1.3) | Multicenter, two-by-two, randomized controlled trial | 90-day mortality or mean score for organ failure | Increased rate of AKI and RRT in HES group |
| Albumin | |||||||
| SAFE | 6997 critically ill | Albumin 4% vs 0.9% saline | NA | 1200 vs 1500 mL (1:1.4) | Multicenter, double-blind randomized controlled | 28-day mortality | No difference, higher mortality in subgroup of patients with traumatic brain injury |
| ALBIOS | 1818 severe sepsis patients | Albumin 20% and crystalloids vs crystalloids | NA, 500 mL HES (median) | 4300 vs 4250 mL crystalloids | Multicenter, open-label, randomized controlled, Albumin target | 28-day mortality | No difference, benefit for subgroup of septic shock? |
NA, not applicable.
In view of these serious risks, the European Medicines Agency [46] as well as the Food and Drug Administration [47] has recommended the suspension of the marketing authorization or required safety labeling and withdrew approval of certain fluids containing HES.
Gelatins
Compared with other synthetic colloids, gelatins are semi-synthetic polypeptide solutions derived from bovine collagen molecules with relatively small particle sizes (average molecular weight ∼35 000 Daltons) whose osmotic effect lasts for a shorter time than that of other colloids. There are no well-conducted randomized studies available comparing resuscitation with gelatin solutions [urea cross-linked polygeline (Haemacel) or succinylated gelatins (Gelofusine)] versus crystalloids. A systematic review of the limited studies found no advantage in administering gelatin compared with crystalloid solution [48]. The use of gelatin has been associated with severe anaphylaxis, potentially attributed to hypersensitivity against galactose-α-1,3 galactose [49]. Furthermore, it increases the risks of bleeding, AKI and mortality. Thus, gelatin is not recommended as resuscitation fluid in the acute care environment, especially in sepsis [6].
Dextrans
Dextrans are a mixture of glucose polymers of variable sizes, derived from bacteria Leuconostoc mesenteroides and commercially available as 10% Dextran-40 or 6% Dextran-70. They are approved for use in vascular surgery because of their rheologic properties, decreasing blood viscosity and potentially improving microvascular flow, especially after grafting. In critically ill patients no difference could be found in relevant clinical outcome parameters between dextrans and crystalloids in a systematic review of limited evidence [50]. As dextrans are associated with renal failure, allergic reactions and coagulopathy, these solutions should not be used for volume resuscitation.
Natural colloid—albumin
Albumin is a plasma protein that is produced in the liver and which in the physiological state is involved in a variety of homeostatic functions. These include maintainence of colloid osmotic pressure of the plasma for up to 75%–80%, acid-base homeostasis (as the most important component of the anion gap in the traditional Stewart's concept), and as the main transport protein for both endogenous substances and drugs. Albumin also plays a role as an antioxidant, anti-inflammatory and anti-apoptotic protein (Fig. 3).
![Albumin metabolism from Joannidis et al. Ten myths about albumin. Intensive Care Med 2022 [51].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/38/7/10.1093_ndt_gfac279/1/m_gfac279fig3.jpeg?Expires=1750189720&Signature=rWI7orUOze3LxVzxA5TEU4u5Ep8FrIUZhS7DG117N5bfH17CcWyHQ00kyRyLOnRD393H5Kxmt-IZ0YNwrp0CUlhy1kRAwPuyjnPRtqO8Ai1Hh4oUwFA-Vx3ae4GLPvtf3g9U32IV7a4fFNNPjmCPlHHteDp46XIuGulZTWhIFwCi-4B9dCPeDz6hMiVO0yNWKtmsq7J5OBTdr9R79jz5qarCV5YHczwb4kQTdqa~wLUqU7jphlkKPf8HmGIT22gbcZYurYGyfuskaaUWqHvW7dw74TIKuJ7pW3AmpmD4sXs0ZUWaGNpbZXjMjceAP0P7oA4Bv7zg0vUhYVucTgyUZg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Albumin metabolism from Joannidis et al. Ten myths about albumin. Intensive Care Med 2022 [51].
Albumin is considered a relatively safe, although more expensive, alternative for volume resuscitation of ICU patients with semi-synthetic colloids. Because of its superior volume effect compared with crystalloids, it is recommended for those clinical situations where increased intravenous fluid administration is required, such as in patients with liver cirrhosis or septic shock.
The effect of albumin on mortality in liver cirrhosis depends on the indication [51]. In contrast to beneficial effects in patients with spontaneous bacterial peritonitis, no such benefit has been seen in other infections [52]. In hospitalized patients with decompensated cirrhosis, recurrent albumin infusions to raise albumin levels to a target of 35 g/L did not improve outcome [53]. However, in patients with cirrhosis and septic shock, 5% albumin for volume resuscitation had a beneficial effect on hemodynamics and short-term mortality compared with 0.9% saline [54]. Clear recommendations for albumin are established for large volume ascites paracentesis, in hepato-renal syndrome and spontaneous bacterial peritonitis of patients with advanced liver disease, because albumin infusion can reduce the risk of AKI [51].
Conversely, hypo-oncotic 4% albumin solution can worsen the outcome in patients with traumatic brain injury [51]. A landmark study in 2004 showed that 4% albumin for fluid resuscitation in critically ill patients produced similar results to 0.9% saline at 28 days [55], although albumin significantly reduced the total amount of fluid administered (the ratio of saline to albumin was 1.4 for the first four days). Secondary analyses showed an increased risk of mortality in patients with traumatic brain injury [56], but in contrast there was a trend towards lower mortality in septic patients receiving albumin (P = .09). This led to the design of a randomized controlled trial in patients with severe sepsis, comparing the replacement of 20% albumin (to maintain plasma levels ≥3 g/dL) with crystalloids alone [57].
The Albumin Italian Outcome Sepsis (ALBIOS) study (published 10 years later) showed that albumin did not improve 28- and 90-day survival, but post hoc analysis found a reduction in 90-day mortality for the albumin group in patients with septic shock [58]. There is thus conflicting evidence on the role of albumin in critically ill patients with sepsis [51]. In addition, it should be noted that the ALBIOS trial did not investigate the effectiveness of albumin during fluid resuscitation. In ALBIOS, during the early phase of volume resuscitation, fluids were administered in both groups according to early goal-directed therapy. After randomization, patients in the albumin group received 300 mL of 20% albumin solution from Day 1 until Day 28 or ICU discharge (whichever came first); 20% albumin was administered on a daily basis, to maintain a serum albumin level of 30 g/L or more. In both groups, crystalloids were administered whenever it was clinically indicated by the attending physician.
Use of human albumin solutions has been investigated in different phases of restrictive fluid resuscitation. Albumin administration improves fluid removal during kidney replacement therapy [51]. Hyperoncotic human albumin solution facilitates restrictive fluid therapy and the effectiveness of de-resuscitative measures [59].
CONCLUSION AND CLINICAL IMPLICATIONS
Fluid administration has to be considered a pharmacologic intervention. The effects and adverse effects depend on the amount as well as the type of fluid administered. Though fluid overload has consistently been associated with increased mortality, the effects of fluid restriction vary depending on the clinical setting.
There is a sizeable body of observational, interventional and prospective clinical trial data that suggests that there is a clinically important effect of choice of crystalloid fluid on outcomes in adult ICU patients. With the exception of those with a traumatic brain injury or pronounced hypochloremia (who should receive 0.9% saline), ICU patients should receive a buffered crystalloid fluid for initial and subsequent IV fluid therapy (Fig. 4). Whether there are differences between the buffered fluid types themselves remains unknown, and the differences are likely to be so small that they will be almost impossible to detect with any degree of certainty.
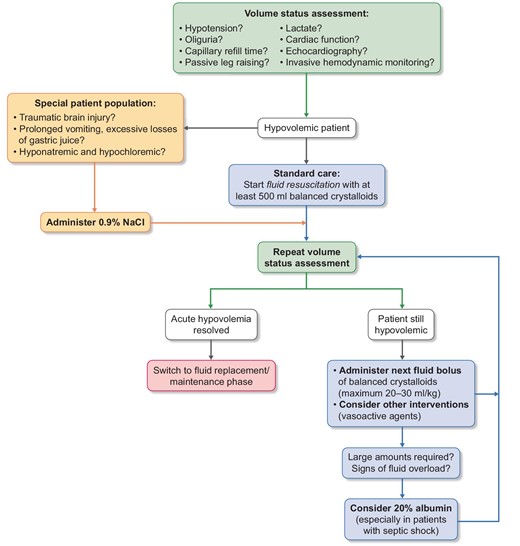
Main considerations for fluid resuscitation in hypovolemic critically ill patients.
If exceptional amounts of fluid are required, additional administration of 20% albumin may be considered and should be preferred to semi-synthetic colloids, especially in sepsis and septic shock patients.
DATA AVAILABILITY STATEMENT
No new data were generated or analysed in support of this research.
CONFLICT OF INTEREST STATEMENT
T.M. declares no competing interests. A.D.S. is a consultant for Edwards Lifesciences, FAST BioMedical, Fresenius, Astellas and AM Pharma. C.J.W. is a consultant for CSL Behring and has received fees for speaking from CSL Behring and Biotest. M.J. has received honoraria and/or research support from Baxter Healthcare Corp., AM-Pharma, CLS Behring, Fresenius, Gilead and Novartis.





Comments