-
PDF
- Split View
-
Views
-
Cite
Cite
Luis M Ruilope, Bertram Pitt, Stefan D Anker, Peter Rossing, Csaba P Kovesdy, Roberto Pecoits-Filho, Pablo Pergola, Amer Joseph, Andrea Lage, Nicole Mentenich, Markus F Scheerer, George L Bakris, on behalf of the FIGARO-DKD Investigators, Kidney outcomes with finerenone: an analysis from the FIGARO-DKD study, Nephrology Dialysis Transplantation, Volume 38, Issue 2, February 2023, Pages 372–383, https://doi.org/10.1093/ndt/gfac157
Close - Share Icon Share
ABSTRACT
In FIGARO-DKD, finerenone reduced the risk of cardiovascular events in patients with type 2 diabetes (T2D) and stage 1–4 chronic kidney disease (CKD). In FIDELIO-DKD, finerenone improved kidney and cardiovascular outcomes in patients with advanced CKD. This analysis further explores kidney outcomes in FIGARO-DKD.
FIGARO-DKD (NCT02545049) included patients with urine albumin-to-creatinine ratio (UACR) 30–<300 mg/g and estimated glomerular filtration rate (eGFR) 25–90 mL/min/1.73 m2 or UACR 300–5000 mg/g and eGFR ≥60 mL/min/1.73 m2. Outcomes included two composite kidney endpoints, a composite of ≥40% decrease in eGFR from baseline sustained over ≥4 weeks, kidney failure or renal death, and a composite of ≥57% decrease in eGFR from baseline sustained over ≥4 weeks, kidney failure or renal death. Changes in albuminuria and eGFR slope were also analyzed. Kidney and CV outcomes were evaluated by baseline UACR.
A lower incidence rate for the eGFR ≥40% kidney composite endpoint was observed with finerenone compared with placebo, but the between-group difference was not significant [hazard ratio (HR) = 0.87; 95% confidence interval (CI): 0.76–1.01; P = .069]. A greater treatment effect was observed on the eGFR ≥57% kidney composite endpoint (HR = 0.77; 95% CI: 0.60–0.99; P = 0.041) with a 36% relative risk reduction for end-stage kidney disease. A larger magnitude of effect on kidney outcomes was observed with finerenone versus placebo for patients with severely increased albuminuria than with moderately increased albuminuria. Improvements in UACR, eGFR slope and cardiovascular risk were evident in both subgroups with finerenone.
The present analyses suggest that finerenone protects against kidney disease progression and cardiovascular events in patients with T2D and early- or late-stage CKD.
What is already known about this subject?
Increased albuminuria is an independent marker of progression to kidney failure and adverse cardiovascular (CV) outcomes, and patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) remain at high risk despite best-recommended therapies.
Finerenone is a novel, selective, nonsteroidal mineralocorticoid receptor antagonist that slowed CKD progression and improved CV outcomes versus placebo in patients with predominantly stage 3–4 CKD with severely increased albuminuria in the FIDELIO-DKD trial.
In the FIGARO-DKD trial, finerenone reduced the risk of CV events in patients with stage 2–4 CKD and moderately increased albuminuria or stage 1–2 CKD with severely increased albuminuria. Although there was a trend favoring finerenone for the first kidney composite endpoint, this difference was not statistically significant.
What this study adds?
The purpose of this analysis was to further evaluate the effects of finerenone on kidney outcomes in patients with CKD and T2D in FIGARO-DKD.
This exploratory analysis demonstrated that there are beneficial effects of finerenone on clinical kidney outcomes, including a 36% relative risk reduction of end-stage kidney disease compared with placebo.
In FIGARO-DKD, despite showing greater treatment effects on kidney composite outcomes in patients with severely rather than moderately increased albuminuria, finerenone reduced the risk of CV events and markers of CKD progression in patients with both severely increased albuminuria and moderately increased albuminuria.
What impact this may have on practice or policy?
Finerenone offers an important treatment advance for patients with CKD and T2D with both moderately or severely increased albuminuria to protect against CV events and kidney disease progression.
These findings emphasize the need for albuminuria screening in clinical practice for patients with T2D to enable earlier identification of CKD and initiation of appropriate treatment.
INTRODUCTION
Diabetes is one of the main causes of kidney failure [1], and approximately 40% of patients with type 2 diabetes (T2D) globally are estimated to be affected by chronic kidney disease (CKD) [2]. Patients with CKD and T2D are also at increased risk of cardiovascular (CV) morbidity and mortality; compared with T2D alone, and comorbid CKD increases the risk of all-cause and CV mortality approximately threefold [3]. Albuminuria is an independent and robust prognostic marker of progression to kidney failure as well as CV disease. The risk of CV mortality and heart failure (HF) in patients with diabetes increases as albuminuria progresses beyond a urine albumin-to-creatinine ratio (UACR) of 10 mg/g and as estimated glomerular filtration rate (eGFR) falls below 75 mL/min/1.73 m2 [4]. Standard-of-care in CKD and T2D includes control of blood glucose and blood pressure, and guidelines recommend treatment with a renin–angiotensin system (RAS) inhibitor and a sodium-glucose co-transporter-2 inhibitor (SGLT-2i) in most patients [5, 6]. However, patients with CKD and T2D remain at high risk of CV events and kidney disease progression despite best-recommended therapy, particularly for patients in whom albuminuria persists [7, 8]. Therefore, there remains a high therapeutic unmet need in these patients and an opportunity for drugs with mechanisms of action that extend beyond glomerular hemodynamics [7].
Finerenone is a novel, selective, nonsteroidal mineralocorticoid receptor antagonist (MRA) that slowed CKD progression and improved CV outcomes versus placebo in patients with predominantly stage 3–4 CKD with severely increased albuminuria in the Phase 3 FIDELIO-DKD trial [9]. FIGARO-DKD was a parallel study investigating the effect of finerenone in patients with earlier stages of CKD and included more patients with moderately increased albuminuria (UACR 30–<300 mg/g), a population under-represented in other studies in CKD. FIGARO-DKD showed a statistically significant benefit favoring finerenone versus placebo for the primary CV composite endpoint (time to CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalization for HF) [10]. However, although there was a trend favoring finerenone for the first secondary kidney composite endpoint in the testing hierarchy (time to kidney failure, sustained ≥40% decrease in eGFR or renal death), this difference was not statistically significant [10].
The purpose of this exploratory analysis was to further evaluate the effects of finerenone on cardiorenal outcomes in patients with CKD and T2D beyond the results of the primary analysis. This investigation included prespecified subgroup analyses by baseline albuminuria to evaluate for treatment-effect heterogeneity.
MATERIALS AND METHODS
Study design and participants
The study design and eligibility criteria of FIGARO-DKD (NCT02545049), an international, multicenter, Phase 3, randomized, double-blind, placebo-controlled, parallel group, event-driven trial, have been described in detail previously [11].
FIGARO-DKD enrolled patients aged ≥18 years with T2D and UACR 30 to <300 mg/g and eGFR 25–90 mL/min/1.73 m2 (stage 2–4 CKD) or UACR 300–5000 mg/g and an eGFR ≥60 mL/min/1.73 m2 (stage 1–2 CKD; Supplementary data, Fig. S1). In patients with UACR 30 to <300 mg/g, recruitment caps limited the proportion of patients with eGFR ≥60 mL/min/1.73 m2 to approximately 10% of the total population, and those with no history of CV disease to approximately 40% of the total patient number. Eligible patients received a maximum tolerated labeled dose of a RAS inhibitor therapy and had serum potassium ≤4.8 mmol/L at screening. Key exclusion criteria included known significant nondiabetic causes of kidney disease, symptomatic HF with reduced ejection fraction and receipt of dialysis or a kidney transplant.
Procedures and outcomes
The trial procedures and outcomes have been described in detail previously [10, 11]. Following run-in and adjustment of background medical therapies, eligible patients were randomly assigned (1:1) to receive finerenone or placebo (10 or 20 mg once daily). Following randomization, trial visits were conducted at months 1 and 4, then every 4 months until study completion. Treatment was withheld if serum potassium concentrations exceeded 5.5 mmol/L and restarted when serum potassium levels decreased to ≤5.0 mmol/L.
Kidney outcomes of interest for this analysis included an eGFR ≥40% kidney composite endpoint of time to kidney failure [defined as end-stage kidney disease (ESKD; the initiation of long-term dialysis for ≥90 days, or kidney transplantation) or an eGFR <15 mL/min/1.73 m2 sustained for ≥4 weeks], a sustained ≥40% decrease in eGFR from baseline for ≥4 weeks, or renal death; a similar eGFR ≥57% kidney composite considered a sustained ≥57% decrease in eGFR from baseline for ≥4 weeks. A CV composite endpoint of time to CV death, nonfatal myocardial infarction, nonfatal stroke or hospitalization for HF was also analyzed. All potential endpoints were prospectively adjudicated by an independent clinical event committee blinded to treatment assignment.
Efficacy and safety outcomes were also analyzed by UACR subgroups at baseline. For the UACR subgroups, patients were defined as having either moderately increased albuminuria (UACR: 30 to <300 mg/g) or severely increased albuminuria (UACR: 300 to 5000 mg/g). A small subgroup of patients had UACR <30 mg/g at baseline and were excluded from subgroup analyses because of the limited sample size.
Albuminuria regression was defined as a change from severely increased to moderately increased albuminuria, or moderately increased albuminuria to normal albuminuria, accompanied by a UACR decrease from baseline of ≥30%. Albuminuria progression for patients with moderately increased albuminuria at baseline was defined as a change to severely increased albuminuria, accompanied by a UACR increase from baseline of ≥30%.
Statistical analysis
Efficacy analyses were performed in the full analysis set (i.e. all randomized patients without critical Good Clinical Practice violations). Safety analyses were performed in the safety analysis set, consisting of all full analysis set patients who took ≥1 dose of study drug. A stratified log-rank test was used to analyze the superiority of finerenone versus placebo in time-to-event analyses. Treatment effects of time-to-event outcomes were expressed as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) from a stratified Cox proportional hazards model. Events were reported from randomization up to the end-of-study visit. Patients without an event were censored at the date of their last contact, with complete information on all components of their respective outcomes.
All eGFR and UACR measurements from a central laboratory were considered for efficacy analyses, irrespective of discontinuation of study treatment but excluding values after the onset of ESKD. The ratio of UACR from baseline to month 4 and chronic eGFR slope (annualized change from month 4 to premature discontinuation or end-of-study visit) were analyzed with an analysis of covariance (ANCOVA) model with covariates of treatment group, stratification factors and baseline value. Treatment differences in UACR and eGFR over the course of the trial were analyzed with a mixed model using separate unstructured covariance patterns for each treatment group and adjusted for stratification factors, baseline value, time and interaction of time with treatment and baseline value, respectively. Additional details on the efficacy and safety analyses have been published previously [10].
RESULTS
Patients
Overall, 7437 patients were randomized in FIGARO-DKD [10]. Following the prospective exclusion of 85 patients from all analyses due to critical violations of Good Clinical Practice, 7352 were included in this analysis. The trial concluded after a median follow-up of 3.4 years, with 7334 (99.8%) patients completing the study [10]. At baseline, 3414 (46.4%) had moderately increased albuminuria (UACR: 30 to <300 mg/g), and 3729 (50.7%) had severely increased albuminuria (UACR ≥300 mg/g); 207 (2.8%) patients had normal albuminuria (UACR <30 mg/g) and for two (<0.1%) patients no UACR value was available.
Baseline characteristics in patients according to urine albumin-to-creatinine ratio status at baseline
At baseline, mean eGFR levels reflected the trial inclusion criteria. Patients with moderately increased albuminuria had a lower mean eGFR compared with patients with severely increased albuminuria (55.7 versus 79.6 mL/min/1.73 m2), and the proportions of patients with baseline eGFR <60 mL/min/1.73 m2 were 65.3% and 11.7%, respectively (Supplementary data, Fig. S1 and Table 1).
| . | UACR at baselinea . | |
|---|---|---|
| . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) . |
| Characteristic . | (n = 3414) . | (n = 3729) . |
| Age, years, mean ± SD | 67.7 ± 8.6 | 60.6 ± 9.7 |
| Sex, n (%) Male Female | 2430 (71.2) 984 (28.8) | 2548 (68.3) 1181 (31.7) |
| Race, n (%) | ||
| White | 2532 (74.2) | 2591 (69.5) |
| Black/African | 128 (3.7) | 125 (3.4) |
| Asian | 633 (18.5) | 783 (21.0) |
| Otherb | 121 (3.5) | 230 (6.2) |
| SBP, mmHg, mean ± SD | 134.6 ± 14.4 | 137.1 ± 13.5 |
| DBP, mmHg, mean ± SD | 74.8 ± 9.7 | 78.7 ± 9.0 |
| BMI, kg/m2, mean ± SD | 31.2 ± 5.9 | 31.6 ± 6.1 |
| Duration of diabetes, years, mean ± SD | 15.5 ± 9.0 | 13.5 ± 7.9 |
| HbA1c, %, mean ± SD | 7.6 ± 1.3 | 7.9 ± 1.4 |
| Serum potassium, mEq/L, mean ± SD | 4.35 ± 0.42 | 4.31 ± 0.43 |
| eGFR, mL/min/1.73 m2, mean ± SD | 55.7 ± 18.8 | 79.6 ± 17.3 |
| eGFR, mL/min/1.73 m2, n (%) | ||
| ≥60 | 1183 (34.7) | 3292 (88.3) |
| 45 to <60 | 1126 (33.0) | 336 (9.0) |
| 25 to <45 | 1081 (31.7) | 98 (2.6) |
| <25 | 24 (0.7) | 2 (<0.1) |
| UACR, mg/g, median (IQR) | 113 (65–187) | 730 (459–1304) |
| History of CV disease, n (%) | 1837 (53.8) | 1367 (36.7) |
| Current smoker, n (%) | 431 (12.6) | 837 (22.4) |
| Medication use at baseline, n (%) | ||
| RAS inhibitors | 3409 (99.9) | 3725 (99.9) |
| Beta blockers | 1802 (52.8) | 1618 (43.4) |
| Diuretics | 1825 (53.5) | 1562 (41.9) |
| Statins | 2561 (75.0) | 2465 (66.1) |
| Potassium supplements | 120 (3.5) | 90 (2.4) |
| Potassium-lowering agents | 28 (0.8) | 18 (0.5) |
| Glucose-lowering therapies | 3318 (97.2) | 3676 (98.6) |
| Insulin | 1791 (52.2) | 2102 (56.4) |
| Metformin | 2104 (61.6) | 2835 (76.0) |
| SGLT-2i | 244 (7.1) | 358 (9.6) |
| GLP-1RA | 263 (7.7) | 272 (7.3) |
| . | UACR at baselinea . | |
|---|---|---|
| . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) . |
| Characteristic . | (n = 3414) . | (n = 3729) . |
| Age, years, mean ± SD | 67.7 ± 8.6 | 60.6 ± 9.7 |
| Sex, n (%) Male Female | 2430 (71.2) 984 (28.8) | 2548 (68.3) 1181 (31.7) |
| Race, n (%) | ||
| White | 2532 (74.2) | 2591 (69.5) |
| Black/African | 128 (3.7) | 125 (3.4) |
| Asian | 633 (18.5) | 783 (21.0) |
| Otherb | 121 (3.5) | 230 (6.2) |
| SBP, mmHg, mean ± SD | 134.6 ± 14.4 | 137.1 ± 13.5 |
| DBP, mmHg, mean ± SD | 74.8 ± 9.7 | 78.7 ± 9.0 |
| BMI, kg/m2, mean ± SD | 31.2 ± 5.9 | 31.6 ± 6.1 |
| Duration of diabetes, years, mean ± SD | 15.5 ± 9.0 | 13.5 ± 7.9 |
| HbA1c, %, mean ± SD | 7.6 ± 1.3 | 7.9 ± 1.4 |
| Serum potassium, mEq/L, mean ± SD | 4.35 ± 0.42 | 4.31 ± 0.43 |
| eGFR, mL/min/1.73 m2, mean ± SD | 55.7 ± 18.8 | 79.6 ± 17.3 |
| eGFR, mL/min/1.73 m2, n (%) | ||
| ≥60 | 1183 (34.7) | 3292 (88.3) |
| 45 to <60 | 1126 (33.0) | 336 (9.0) |
| 25 to <45 | 1081 (31.7) | 98 (2.6) |
| <25 | 24 (0.7) | 2 (<0.1) |
| UACR, mg/g, median (IQR) | 113 (65–187) | 730 (459–1304) |
| History of CV disease, n (%) | 1837 (53.8) | 1367 (36.7) |
| Current smoker, n (%) | 431 (12.6) | 837 (22.4) |
| Medication use at baseline, n (%) | ||
| RAS inhibitors | 3409 (99.9) | 3725 (99.9) |
| Beta blockers | 1802 (52.8) | 1618 (43.4) |
| Diuretics | 1825 (53.5) | 1562 (41.9) |
| Statins | 2561 (75.0) | 2465 (66.1) |
| Potassium supplements | 120 (3.5) | 90 (2.4) |
| Potassium-lowering agents | 28 (0.8) | 18 (0.5) |
| Glucose-lowering therapies | 3318 (97.2) | 3676 (98.6) |
| Insulin | 1791 (52.2) | 2102 (56.4) |
| Metformin | 2104 (61.6) | 2835 (76.0) |
| SGLT-2i | 244 (7.1) | 358 (9.6) |
| GLP-1RA | 263 (7.7) | 272 (7.3) |
207 patients with UACR <30 mg/g and 2 patients with missing UACR were excluded from this analysis.
Other = Native American, Native Hawaiian Islander, not reported and multiple.
BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; IQR, interquartile range; RAS, renin–angiotensin system; SBP, systolic blood pressure; SD, standard deviation; SGLT-2i, sodium-glucose co-transporter-2 inhibitor; UACR, urine albumin-to-creatinine ratio.
| . | UACR at baselinea . | |
|---|---|---|
| . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) . |
| Characteristic . | (n = 3414) . | (n = 3729) . |
| Age, years, mean ± SD | 67.7 ± 8.6 | 60.6 ± 9.7 |
| Sex, n (%) Male Female | 2430 (71.2) 984 (28.8) | 2548 (68.3) 1181 (31.7) |
| Race, n (%) | ||
| White | 2532 (74.2) | 2591 (69.5) |
| Black/African | 128 (3.7) | 125 (3.4) |
| Asian | 633 (18.5) | 783 (21.0) |
| Otherb | 121 (3.5) | 230 (6.2) |
| SBP, mmHg, mean ± SD | 134.6 ± 14.4 | 137.1 ± 13.5 |
| DBP, mmHg, mean ± SD | 74.8 ± 9.7 | 78.7 ± 9.0 |
| BMI, kg/m2, mean ± SD | 31.2 ± 5.9 | 31.6 ± 6.1 |
| Duration of diabetes, years, mean ± SD | 15.5 ± 9.0 | 13.5 ± 7.9 |
| HbA1c, %, mean ± SD | 7.6 ± 1.3 | 7.9 ± 1.4 |
| Serum potassium, mEq/L, mean ± SD | 4.35 ± 0.42 | 4.31 ± 0.43 |
| eGFR, mL/min/1.73 m2, mean ± SD | 55.7 ± 18.8 | 79.6 ± 17.3 |
| eGFR, mL/min/1.73 m2, n (%) | ||
| ≥60 | 1183 (34.7) | 3292 (88.3) |
| 45 to <60 | 1126 (33.0) | 336 (9.0) |
| 25 to <45 | 1081 (31.7) | 98 (2.6) |
| <25 | 24 (0.7) | 2 (<0.1) |
| UACR, mg/g, median (IQR) | 113 (65–187) | 730 (459–1304) |
| History of CV disease, n (%) | 1837 (53.8) | 1367 (36.7) |
| Current smoker, n (%) | 431 (12.6) | 837 (22.4) |
| Medication use at baseline, n (%) | ||
| RAS inhibitors | 3409 (99.9) | 3725 (99.9) |
| Beta blockers | 1802 (52.8) | 1618 (43.4) |
| Diuretics | 1825 (53.5) | 1562 (41.9) |
| Statins | 2561 (75.0) | 2465 (66.1) |
| Potassium supplements | 120 (3.5) | 90 (2.4) |
| Potassium-lowering agents | 28 (0.8) | 18 (0.5) |
| Glucose-lowering therapies | 3318 (97.2) | 3676 (98.6) |
| Insulin | 1791 (52.2) | 2102 (56.4) |
| Metformin | 2104 (61.6) | 2835 (76.0) |
| SGLT-2i | 244 (7.1) | 358 (9.6) |
| GLP-1RA | 263 (7.7) | 272 (7.3) |
| . | UACR at baselinea . | |
|---|---|---|
| . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) . |
| Characteristic . | (n = 3414) . | (n = 3729) . |
| Age, years, mean ± SD | 67.7 ± 8.6 | 60.6 ± 9.7 |
| Sex, n (%) Male Female | 2430 (71.2) 984 (28.8) | 2548 (68.3) 1181 (31.7) |
| Race, n (%) | ||
| White | 2532 (74.2) | 2591 (69.5) |
| Black/African | 128 (3.7) | 125 (3.4) |
| Asian | 633 (18.5) | 783 (21.0) |
| Otherb | 121 (3.5) | 230 (6.2) |
| SBP, mmHg, mean ± SD | 134.6 ± 14.4 | 137.1 ± 13.5 |
| DBP, mmHg, mean ± SD | 74.8 ± 9.7 | 78.7 ± 9.0 |
| BMI, kg/m2, mean ± SD | 31.2 ± 5.9 | 31.6 ± 6.1 |
| Duration of diabetes, years, mean ± SD | 15.5 ± 9.0 | 13.5 ± 7.9 |
| HbA1c, %, mean ± SD | 7.6 ± 1.3 | 7.9 ± 1.4 |
| Serum potassium, mEq/L, mean ± SD | 4.35 ± 0.42 | 4.31 ± 0.43 |
| eGFR, mL/min/1.73 m2, mean ± SD | 55.7 ± 18.8 | 79.6 ± 17.3 |
| eGFR, mL/min/1.73 m2, n (%) | ||
| ≥60 | 1183 (34.7) | 3292 (88.3) |
| 45 to <60 | 1126 (33.0) | 336 (9.0) |
| 25 to <45 | 1081 (31.7) | 98 (2.6) |
| <25 | 24 (0.7) | 2 (<0.1) |
| UACR, mg/g, median (IQR) | 113 (65–187) | 730 (459–1304) |
| History of CV disease, n (%) | 1837 (53.8) | 1367 (36.7) |
| Current smoker, n (%) | 431 (12.6) | 837 (22.4) |
| Medication use at baseline, n (%) | ||
| RAS inhibitors | 3409 (99.9) | 3725 (99.9) |
| Beta blockers | 1802 (52.8) | 1618 (43.4) |
| Diuretics | 1825 (53.5) | 1562 (41.9) |
| Statins | 2561 (75.0) | 2465 (66.1) |
| Potassium supplements | 120 (3.5) | 90 (2.4) |
| Potassium-lowering agents | 28 (0.8) | 18 (0.5) |
| Glucose-lowering therapies | 3318 (97.2) | 3676 (98.6) |
| Insulin | 1791 (52.2) | 2102 (56.4) |
| Metformin | 2104 (61.6) | 2835 (76.0) |
| SGLT-2i | 244 (7.1) | 358 (9.6) |
| GLP-1RA | 263 (7.7) | 272 (7.3) |
207 patients with UACR <30 mg/g and 2 patients with missing UACR were excluded from this analysis.
Other = Native American, Native Hawaiian Islander, not reported and multiple.
BMI, body mass index; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HbA1c, glycated hemoglobin; IQR, interquartile range; RAS, renin–angiotensin system; SBP, systolic blood pressure; SD, standard deviation; SGLT-2i, sodium-glucose co-transporter-2 inhibitor; UACR, urine albumin-to-creatinine ratio.
Noteworthy differences in baseline characteristics between the two subgroups were likely related to the differences in mean eGFR and the recruitment cap for history of CV disease. Patients with moderately increased albuminuria were older, had lower blood pressure, longer mean duration of diabetes and were more likely to report a history of CV disease (Table 1). Patients with moderately increased albuminuria were also less likely to be current smokers. Medication use at baseline was broadly similar in both groups, although patients with severely increased albuminuria reported lower use of statins, diuretics and β-blockers, and higher use of metformin and SGLT-2is at baseline (Table 1).
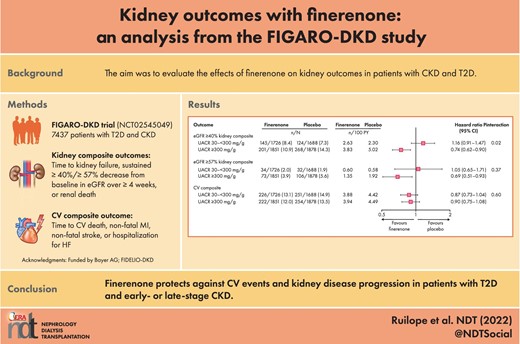
Effects of finerenone on kidney outcomes in the overall population
As previously reported, there was a 13% relative risk reduction for the eGFR ≥40% kidney composite endpoint in the overall population; however, this was not statistically significant (HR: 0.87; 95% CI: 0.76–1.01; P = .069) [10]. A sustained ≥40% decrease in eGFR from baseline was reported in 338 patients treated with finerenone and 385 patients treated with placebo (HR: 0.87; 95% CI: 0.75–1.00) (Fig. 1A). The risk of the eGFR ≥57% kidney composite endpoint was reduced with finerenone compared with placebo [108 (2.9%) versus 139 (3.8%) patients; HR: 0.77; 95% CI: 0.60–0.99; P = .041]. In the finerenone group, 90 (2.4%) patients experienced a sustained ≥57% decrease in eGFR from baseline compared with 116 (3.2%) in the placebo group (HR: 0.76; 95% CI: 0.58–1.00; P = .053) (Fig. 1B). There was a 36% relative risk reduction in ESKD with finerenone versus placebo, occurring in 32 (0.9%) patients treated with finerenone, compared with 49 (1.3%) patients treated with placebo (HR: 0.64; 95% CI: 0.41–0.995; P = .046; Fig. 1C). Based on an absolute between-group difference of 0.6% (95% CI: 0–1.1) after 42 months, the number of patients who needed to be treated with finerenone to prevent one ESKD event was 175. The cumulative incidences for kidney failure and sustained decrease in eGFR to <15 mL/min/1.73 m2 are shown in the Supplementary data, Fig. S2. Incidence of the eGFR ≥57% kidney composite endpoint by key subgroups is shown in the Supplementary data, Fig. S3. The treatment effect of finerenone appeared consistent in patients with or without a history of CV disease (Pinteraction = 0.37), by baseline glycated hemoglobin (Pinteraction = 0.56 for ≤7.5% versus >7.5%) and by baseline systolic blood pressure (Pinteraction = 0.14 for above versus below median).
Kidney outcomes in the overall population. CI, confidence interval; eGFR, estimated glomerular filtration rate.
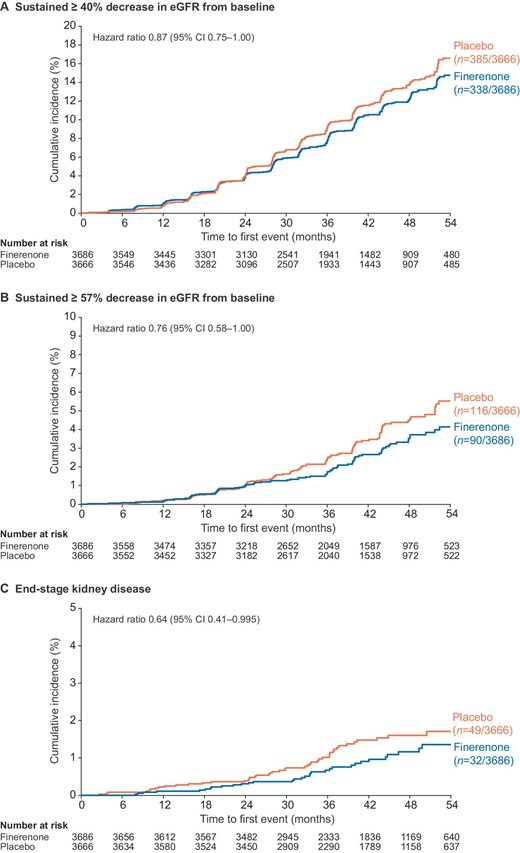
Finerenone reduced UACR compared with placebo, an effect that was maintained throughout the trial (Fig. 2A). A 32% greater reduction in the ratio of UACR from baseline to month 4 was seen with finerenone versus placebo (ratio of least-squares mean change from baseline 0.68; 95% CI: 0.65–0.70; P < .0001) [10]. Overall, patients treated with finerenone had a significantly shorter time to albuminuria regression (from severely increased to moderately increased albuminuria, or from moderately increased to normal albuminuria, each accompanied by a UACR reduction of ≥30% from baseline) over time than placebo recipients (HR: 1.82; 95% CI: 1.69–1.96; P < .0001; Fig. 2B).
(A) Change in UACR from baseline and (B) albuminuria regressiona in the overall population. aAlbuminuria regression defined as change from severely increased to moderately increased albuminuria, or moderately increased to normal albuminuria, accompanied by a UACR decrease from baseline of ≥30%. SD, standard deviation; UACR, urine albumin-to-creatinine ratio.
In the overall population, placebo-corrected change in eGFR from baseline to month 4 with finerenone was –2.24 mL/min/1.73 m2 (least-squares mean difference –3.46 versus –1.22 mL/min/1.73 m2 for finerenone and placebo, respectively; P < .0001; Supplementary data, Fig. S4). However, placebo-corrected change in chronic eGFR slope from month 4 to permanent discontinuation or the end of study with finerenone was 1.13 mL/min/1.73 m2 (least-squares mean difference –2.37 versus –3.50 mL/min/1.73 m2 for finerenone and placebo, respectively; P < .0001).
Effect of finerenone on kidney and cardiovascular outcomes in patients according to urine albumin-to-creatinine ratio status at baseline
Kidney outcomes by urine albumin-to-creatinine ratio at baseline
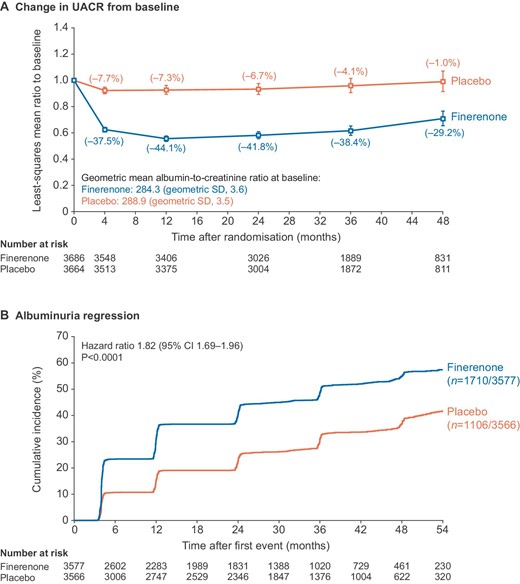
A greater improvement in the incidence rate of the eGFR ≥40% kidney composite endpoint was observed with finerenone versus placebo in patients with severely increased albuminuria at baseline than in those with moderately increased albuminuria at baseline (HR: 0.74; 95% CI: 0.62–0.90 and HR: 1.16; 95% CI: 0.91–1.47, respectively; Pinteraction = .02) (Fig. 3). Similarly, the treatment effect of finerenone versus placebo for the eGFR ≥57% kidney composite endpoint appeared more pronounced in patients with severely increased albuminuria at baseline than in those with moderately increased albuminuria at baseline (HR: 0.69; 95% CI: 0.51–0.93 and HR: 1.05; 95% CI: 0.65–1.71, respectively; Pinteraction = .37) (Fig. 3). This trend was consistent across the components of the composite kidney endpoints (Supplementary data, Fig. S5).
Kidney and CV composite endpoints by albuminuria at baseline. CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; UACR, urine albumin-to-creatinine ratio.
Evaluation of the chronic eGFR slope by UACR subgroups showed a slower decline among patients in the placebo group with moderately increased albuminuria compared with severely increased albuminuria (–2.1 mL/min/1.73 m2 per year and –4.8 mL/min/1.73 m2 per year, respectively). Nonetheless, finerenone slowed chronic eGFR decline compared with placebo in both subgroups (difference in least-squares means 0.70; 95% CI: 0.14–1.26; P = .01 and 1.54; 95% CI: 0.60–2.48; P = .0008, respectively) (Fig. 4A and B). Change in UACR from baseline to month 4 by UACR subgroup showed a consistent reduction with finerenone versus placebo in both albuminuria subgroups (Fig. 4C and D).
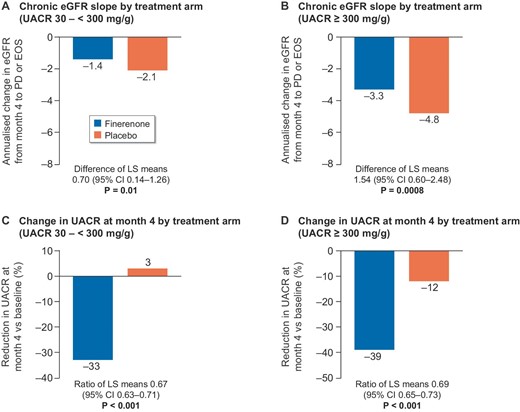
Kidney markers by albuminuria at baseline. (A) Chronic eGFR slope by treatment arm (UACR: 30 to <300 mg/g); (B) chronic eGFR slope by treatment arm (UACR ≥300 mg/g); (C) change in UACR at month 4 by treatment arm (UACR: 30 to <300 mg/g); and (D) change in UACR at month 4 by treatment arm (UACR ≥300 mg/g). Chronic slope is the annualized change from month 4 to premature discontinuation (PD) or the end-of-study (EOS) visit.
Time to progression to severely increased albuminuria in patients with moderately increased albuminuria at baseline was prolonged in patients treated with finerenone compared with placebo (HR: 0.59; 95% CI: 0.53–0.66; P < .0001, Fig. 5). Furthermore, time to albuminuria regression was shorter for the finerenone group than the placebo group, in both those patients with moderately increased albuminuria at baseline (HR: 2.18; 95% CI: 1.92–2.47; P < .0001) and those with severely increased albuminuria at baseline (HR: 1.72; 95% CI: 1.56–1.90; P < .0001).
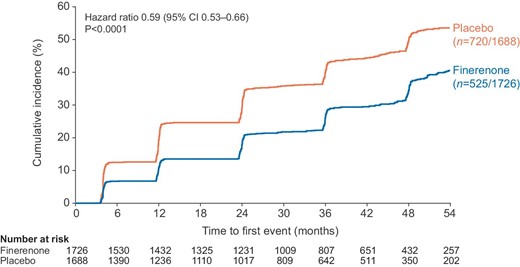
Time to progressiona from moderately to severely increased albuminuria in patients with UACR 30 to <300 mg/g at baseline. aProgression was defined as a UACR level of ≥300 mg/g that was accompanied by a ≥30% increase in UACR from baseline.
Cardiovascular outcomes by urine albumin-to-creatinine ratio at baseline
The effects of finerenone on the CV composite outcome were consistent regardless of UACR at baseline (moderately increased albuminuria: HR: 0.87; 95% CI: 0.73–1.04; severely increased albuminuria: HR: 0.90; 95% CI: 0.75–1.08; Pinteraction = .60; Fig. 3). The incidence rate of hospitalization for HF was also consistently lower with finerenone compared with placebo in patients with moderately increased albuminuria (HR: 0.73; 95% CI: 0.52–1.03) and severely increased albuminuria at baseline (HR: 0.72; 95% CI: 0.51–1.02; Pinteraction = .90).
Safety
The incidences of any treatment-emergent adverse events (TEAEs) were generally balanced between treatment arms and similar between patients with moderately or severely increased albuminuria at baseline (Table 2). It is important to note for interpretation of the safety outcomes that the eGFR interquartile range in the moderately increased albuminuria group was 42–67 mL/min/1.73 m2, compared with 68–92 mL/min/1.73 m2 in patients with severely increased albuminuria. Consequently, hyperkalemia-related TEAEs were observed more frequently in patients with moderately increased albuminuria at baseline in both treatment arms. Nevertheless, incidence of hyperkalemia was approximately twice as frequent with finerenone than with placebo in both albuminuria subgroups (moderately increased albuminuria, 13.6% and 6.4%; severely increased albuminuria, 8.0% and 4.4%, respectively). Discontinuations due to hyperkalemia were low in all groups (<2.0% across treatment arms and UACR subgroups). Acute kidney injury adverse events were balanced between treatment groups within each albuminuria subgroup.
| Patients with treatment-emergent AEs, n (%) . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) (n = 3406) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) (n = 3727) . | ||
|---|---|---|---|---|
| . | Finerenone (n = 1724) . | Placebo (n = 1682) . | Finerenone (n = 1850) . | Placebo (n = 1877) . |
| Any AE | 1507 (87.4) | 1483 (88.2) | 1532 (82.8) | 1562 (83.2) |
| Related to study drug | 327 (19.0) | 241 (14.3) | 210 (11.4) | 161 (8.6) |
| Leading to discontinuation | 133 (7.7) | 104 (6.2) | 69 (3.7) | 72 (3.8) |
| Any SAE | 607 (35.2) | 616 (36.6) | 516 (27.9) | 571 (30.4) |
| Related to study drug | 23 (1.3) | 20 (1.2) | 10 (0.5) | 7 (0.4) |
| Leading to discontinuation | 48 (2.8) | 44 (2.6) | 20 (1.1) | 30 (1.6) |
| AE leading to death | 46 (2.7) | 49 (2.9) | 31 (1.7) | 48 (2.6) |
| Any hyperkalemia | 234 (13.6) | 108 (6.4) | 148 (8.0) | 83 (4.4) |
| Related to study drug | 142 (8.2) | 64 (3.8) | 89 (4.8) | 48 (2.6) |
| Leading to hospitalization | 14 (0.8) | 2 (0.1) | 6 (0.3) | 0 |
| Leading to permanent discontinuation | 32 (1.9) | 9 (0.5) | 12 (0.6) | 4 (0.2) |
| Leading to death | 0 | 0 | 0 | 0 |
| Acute kidney injury | 59 (3.4) | 70 (4.2) | 30 (1.6) | 26 (1.4) |
| Patients with treatment-emergent AEs, n (%) . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) (n = 3406) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) (n = 3727) . | ||
|---|---|---|---|---|
| . | Finerenone (n = 1724) . | Placebo (n = 1682) . | Finerenone (n = 1850) . | Placebo (n = 1877) . |
| Any AE | 1507 (87.4) | 1483 (88.2) | 1532 (82.8) | 1562 (83.2) |
| Related to study drug | 327 (19.0) | 241 (14.3) | 210 (11.4) | 161 (8.6) |
| Leading to discontinuation | 133 (7.7) | 104 (6.2) | 69 (3.7) | 72 (3.8) |
| Any SAE | 607 (35.2) | 616 (36.6) | 516 (27.9) | 571 (30.4) |
| Related to study drug | 23 (1.3) | 20 (1.2) | 10 (0.5) | 7 (0.4) |
| Leading to discontinuation | 48 (2.8) | 44 (2.6) | 20 (1.1) | 30 (1.6) |
| AE leading to death | 46 (2.7) | 49 (2.9) | 31 (1.7) | 48 (2.6) |
| Any hyperkalemia | 234 (13.6) | 108 (6.4) | 148 (8.0) | 83 (4.4) |
| Related to study drug | 142 (8.2) | 64 (3.8) | 89 (4.8) | 48 (2.6) |
| Leading to hospitalization | 14 (0.8) | 2 (0.1) | 6 (0.3) | 0 |
| Leading to permanent discontinuation | 32 (1.9) | 9 (0.5) | 12 (0.6) | 4 (0.2) |
| Leading to death | 0 | 0 | 0 | 0 |
| Acute kidney injury | 59 (3.4) | 70 (4.2) | 30 (1.6) | 26 (1.4) |
AE, adverse event; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SAE, serious adverse event; UACR, urine albumin-to-creatinine ratio.
| Patients with treatment-emergent AEs, n (%) . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) (n = 3406) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) (n = 3727) . | ||
|---|---|---|---|---|
| . | Finerenone (n = 1724) . | Placebo (n = 1682) . | Finerenone (n = 1850) . | Placebo (n = 1877) . |
| Any AE | 1507 (87.4) | 1483 (88.2) | 1532 (82.8) | 1562 (83.2) |
| Related to study drug | 327 (19.0) | 241 (14.3) | 210 (11.4) | 161 (8.6) |
| Leading to discontinuation | 133 (7.7) | 104 (6.2) | 69 (3.7) | 72 (3.8) |
| Any SAE | 607 (35.2) | 616 (36.6) | 516 (27.9) | 571 (30.4) |
| Related to study drug | 23 (1.3) | 20 (1.2) | 10 (0.5) | 7 (0.4) |
| Leading to discontinuation | 48 (2.8) | 44 (2.6) | 20 (1.1) | 30 (1.6) |
| AE leading to death | 46 (2.7) | 49 (2.9) | 31 (1.7) | 48 (2.6) |
| Any hyperkalemia | 234 (13.6) | 108 (6.4) | 148 (8.0) | 83 (4.4) |
| Related to study drug | 142 (8.2) | 64 (3.8) | 89 (4.8) | 48 (2.6) |
| Leading to hospitalization | 14 (0.8) | 2 (0.1) | 6 (0.3) | 0 |
| Leading to permanent discontinuation | 32 (1.9) | 9 (0.5) | 12 (0.6) | 4 (0.2) |
| Leading to death | 0 | 0 | 0 | 0 |
| Acute kidney injury | 59 (3.4) | 70 (4.2) | 30 (1.6) | 26 (1.4) |
| Patients with treatment-emergent AEs, n (%) . | Moderately increased albuminuria (UACR: 30 to <300 mg/g) (baseline eGFR IQR 42–67 mL/min/1.73 m2) (n = 3406) . | Severely increased albuminuria (UACR ≥300 mg/g) (baseline eGFR IQR 68–92 mL/min/1.73 m2) (n = 3727) . | ||
|---|---|---|---|---|
| . | Finerenone (n = 1724) . | Placebo (n = 1682) . | Finerenone (n = 1850) . | Placebo (n = 1877) . |
| Any AE | 1507 (87.4) | 1483 (88.2) | 1532 (82.8) | 1562 (83.2) |
| Related to study drug | 327 (19.0) | 241 (14.3) | 210 (11.4) | 161 (8.6) |
| Leading to discontinuation | 133 (7.7) | 104 (6.2) | 69 (3.7) | 72 (3.8) |
| Any SAE | 607 (35.2) | 616 (36.6) | 516 (27.9) | 571 (30.4) |
| Related to study drug | 23 (1.3) | 20 (1.2) | 10 (0.5) | 7 (0.4) |
| Leading to discontinuation | 48 (2.8) | 44 (2.6) | 20 (1.1) | 30 (1.6) |
| AE leading to death | 46 (2.7) | 49 (2.9) | 31 (1.7) | 48 (2.6) |
| Any hyperkalemia | 234 (13.6) | 108 (6.4) | 148 (8.0) | 83 (4.4) |
| Related to study drug | 142 (8.2) | 64 (3.8) | 89 (4.8) | 48 (2.6) |
| Leading to hospitalization | 14 (0.8) | 2 (0.1) | 6 (0.3) | 0 |
| Leading to permanent discontinuation | 32 (1.9) | 9 (0.5) | 12 (0.6) | 4 (0.2) |
| Leading to death | 0 | 0 | 0 | 0 |
| Acute kidney injury | 59 (3.4) | 70 (4.2) | 30 (1.6) | 26 (1.4) |
AE, adverse event; eGFR, estimated glomerular filtration rate; IQR, interquartile range; SAE, serious adverse event; UACR, urine albumin-to-creatinine ratio.
DISCUSSION
In the primary analysis of FIGARO-DKD, no significant between-group difference was observed for the first composite kidney endpoint, including a ≥40% decrease in eGFR from baseline [10]. However, a reduction in risk with finerenone treatment was observed for the kidney composite endpoint including a sustained ≥57% decrease in eGFR. A greater risk reduction in these composite kidney endpoints was observed with finerenone in patients with severely increased albuminuria than in patients with moderately increased albuminuria at baseline. Nevertheless, a reduction in chronic eGFR slope was observed with finerenone compared with placebo in both UACR subgroups, as well as a lower likelihood of albuminuria progression in patients with moderately increased albuminuria, indicating that both subgroups of patients are likely to receive kidney benefits from finerenone in the long term. CV outcomes including hospitalization for heart failure were consistently improved with finerenone irrespective of baseline albuminuria.
The results for the eGFR ≥57% kidney composite endpoint are important because it is an established and widely used surrogate endpoint for kidney failure in studies of CKD; in addition, the eGFR ≥57% kidney composite endpoint has higher specificity with kidney failure than the sustained ≥40% decrease in eGFR from baseline for the evaluation of drugs that lead to an initial and transitory eGFR decline [12, 13]. In a comparable HF analysis from the EMPEROR-Preserved trial of empagliflozin in patients with heart failure and preserved ejection fraction, eGFR mean slope change per year was –1.25 and –2.62 mL/min/1.73 m2 with empagliflozin and placebo, respectively (difference 1.36; 95% CI: 1.06–0.66; P < .001). However, the incidence rate of the composite kidney outcome (including a sustained ≥40% decrease in eGFR) was similar between treatment arms (3.6% and 3.7% of patients treated with empagliflozin and placebo, respectively; HR: 0.95; 95% CI: 0.73–1.24) [14]. Reduction in ESKD is a particularly clinically meaningful outcome because of the considerable associated mortality and morbidity as well as the negative impact of ESKD on patients’ quality of life and the healthcare cost burdens associated with its management [15–17]. Analysis of UACR over time showed that finerenone lowered the ratio of UACR from baseline by 32% versus placebo at month 4, an effect that persisted throughout the trial and translated into a shortened time to albuminuria regression with finerenone.
In patients with CKD and T2D, the effect of finerenone on kidney outcomes appeared to be more pronounced for severely increased albuminuria than for moderately increased albuminuria at baseline. Despite this, finerenone slowed the rate of chronic eGFR decline versus placebo in patients in both UACR subgroups; however, because the rate of eGFR decline was slower in patients with moderately increased albuminuria, longer follow-up periods may be required to observe a reduction in risk of kidney failure and sustained eGFR decline outcomes in these patients. In patients with moderately increased albuminuria, finerenone also slowed the rate of albuminuria progression, supporting the idea that finerenone can be expected to improve kidney outcomes over the long term in these patients. Previous research has shown that changes in albuminuria are associated with subsequent risk of outcomes such as ESKD and sustained ≥57% decrease in eGFR across a range of cohorts [4, 18–20]. The kidney benefits finerenone in patients with higher levels of albuminuria may indicate more glomerular inflammation and injury in these patients. Overactivation of the mineralocorticoid receptor and aldosterone upregulation may be contributors to this underlying kidney damage [21], and finerenone is hypothesized to reduce inflammation and fibrosis in the kidney via mineralocorticoid receptor blockade [22]. Preclinical data have demonstrated that finerenone reduces proinflammatory markers and profibrotic markers in the kidney and the heart [23] and prevents kidney glomerular and tubular damage [24]. The treatment benefit with finerenone on the eGFR ≥57% kidney composite endpoint being independent of baseline glycemic control or blood pressure might also suggest a complementary mechanism to SGLT-2is and RAS inhibitors, which are recommended for patients with T2D and CKD, and act primarily via glycemic and/or hemodynamic mechanisms [25, 26].
Finerenone had a similar magnitude of effect on CV outcomes in patients with moderately and severely increased albuminuria at baseline; these included hospitalization for HF, which was the main driver of the finerenone benefit for the CV composite primary endpoint in the FIGARO-DKD primary analysis. These findings highlight the CV benefits in patients with both early- and late-stage CKD and support the need for albuminuria screening in patients with T2D to identify patients with CKD at earlier stages to reduce their CV risk.
Treatment-emergent adverse events were generally well balanced between patients in the finerenone and placebo treatment arms. The incidences of TEAEs leading to discontinuation of the study and serious TEAEs were higher in patients with moderately increased albuminuria compared with patients with severely increased albuminuria at baseline, likely reflective of the lower mean eGFR of the former subgroup. Although total hyperkalemia events were increased with finerenone versus placebo, the incidence of clinically meaningful events was low in both treatment arms, with few hospitalizations or study drug discontinuations due to hyperkalemia, and no hyperkalemia-related deaths. More hyperkalemia-related TEAEs occurred with moderately increased than severely increased albuminuria; however, this observation is consistent with expectations because decreasing eGFR is an established risk factor for hyperkalemia [27]. In addition to the fact that only short-term data on UACR are available with steroidal MRAs, unlike the long-term follow-up in FIGARO-DKD [28, 29], finerenone also appears to have good tolerability in comparison with the steroidal MRAs based on their respective hyperkalemia profiles. In the phase II ARTS-HF study, finerenone treatment led to less hyperkalemia than spironolactone over 4 weeks in patients with chronic HF with reduced ejection fraction and moderate CKD [30]. Finerenone also does not appear to be associated with any sexual side effects, unlike spironolactone [22].
With this analysis, special consideration had to be given for the inclusion criteria of FIGARO-DKD, which meant that patients with moderately increased albuminuria had lower eGFR values than those patients with severely increased albuminuria (Supplementary data, Fig. S1) [10]. Although most analyses were prespecified, confirmatory testing was only applied to the CV and eGFR ≥40% kidney composite endpoints, due to the hierarchical testing strategy, which stopped at the first nonsignificant result. Therefore, the analyses described here are mostly exploratory and hypothesis-generating in nature.
Overall, the results of this analysis suggest that finerenone offers an important advance in treatment for patients with CKD and T2D with either moderately or severely increased albuminuria to protect against kidney disease progression and CV events. The findings also emphasize the need for albuminuria screening in patients with T2D because early initiation of treatment can reduce the risk of CV events and progression of albuminuria in patients with moderately increased albuminuria.
ACKNOWLEDGEMENTS
We are indebted to the patients who have participated in this trial, the FIGARO-DKD trial investigators, and the study centers that supported the trial. The FIGARO-DKD study and subanalyses were funded by Bayer AG. Medical writing assistance was provided by Emilie Topley, on behalf of Chameleon Communications International, and was funded by Bayer AG. This work was supported by Bayer AG, who funded the FIGARO-DKD study and the reported analysis.
AUTHORS’ CONTRIBUTIONS
The Executive Committee designed the study in conjunction with the sponsor. Luis Ruilope and George Bakris wrote the first draft of the report. All authors were involved in data analysis and interpretation, and in drafting and critically revising the report. All authors had access to study results and the first and corresponding author assume responsibility for the integrity and accuracy of the data reported. All authors reviewed and approved the final submitted version of the report.
CONFLICT OF INTEREST STATEMENT
L.M.R. reported receipt of consultancy fees from Bayer. B.P. reported consultant fees for AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Proton Intel, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida and Vifor/Relypsa; he has stock options for KBP Biosciences, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, Proton Intel, Sarfez, scPharmaceuticals, SQ Innovation, Tricida, Vifor/Relypsa; he also holds a patent for site-specific delivery of eplerenone to the myocardium (US patent #9 931 412) and a provisional patent for histone-acetylation-modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045 784). S.D.A. has received research support from Abbott Vascular and Vifor International, and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier and Vifor Pharma. P.R. reported personal fees from Bayer during the conduct of the study; he has received research support and personal fees from AstraZeneca and Novo Nordisk, and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi and Vifor; all fees are given to Steno Diabetes Center Copenhagen. C.P.K. reported that he is a consultant for Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Behring, GSK, Rockwell and Vifor. R.P.-F. reported honoraria fees from Akebia, AstraZeneca, Bayer and Boehringer Ingelheim, and investigator-initiated trial support from Fresenius Medical Care. P.P. reported that he is a consultant for Bayer. A.J., A.L. and N.M. are full-time employees of Bayer AG, Germany. M.F.S. is a full-time employee of Bayer AG, Germany. He is also a shareholder in AstraZeneca, Bayer, Eli Lilly and Novo Nordisk. G.L.B. reported research funding, paid to the University of Chicago Medicine, from Bayer during the conduct of the study; he also reported research funding, paid to the University of Chicago Medicine, from Novo Nordisk and Vascular Dynamics; he acted as a consultant and received personal fees from for Alnylam, Merck and Relypsa; he is an editor of the American Journal of Nephrology, Nephrology and Hypertension, and section editor of UpToDate; and is an associate editor of Diabetes Care and Hypertension Research.
FUNDING
This work was supported by Bayer AG, who funded the FIGARO-DKD study and the reported analysis.





Comments