-
PDF
- Split View
-
Views
-
Cite
Cite
José Jesús Broseta, Diana Rodríguez-Espinosa, José Luis Bedini, Néstor Rodríguez, Francisco Maduell, Antibody maintenance 3 months after complete messenger RNA COVID-19 vaccination in haemodialysis patients, Nephrology Dialysis Transplantation, Volume 36, Issue 12, December 2021, Pages 2340–2341, https://doi.org/10.1093/ndt/gfab272
Close - Share Icon Share
In-centre haemodialysis (HD) patients have a greater risk of sudden acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and more significant mortality associated with coronavirus disease 2019 (COVID-19) than the general population [1, 2], which made their vaccination a priority for healthcare authorities [3]. Even though real-world data on vaccine efficacy in preventing severe COVID-19 in the HD subpopulation are lacking, and antibody levels have only been indirectly associated with viral neutralization [4], several published studies have evaluated the humoral response of these patients after completing vaccination with mRNA-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech) vaccines in terms of seroconversion and intensity (i.e. antibody levels) as an efficacy surrogate, with satisfactory results. The results were similar to those observed in the general population except for patients receiving immunosuppressive medication during vaccination [5–7]. Recently there has been evidence of persistent, although declining [8], antibody positivity in healthy vaccinated individuals with both mRNA-1273 and BNT162b2 vaccines 3 and 6 months after completing vaccination [9–12]. Given the known impaired cellular and humoral responses of HD patients to other immunization programs (e.g. hepatitis B, influenza) [13, 14], there was concern regarding the durability of the SARS-CoV-2 vaccine’s effect in this population. Therefore we aimed to determine anti-spike S1 receptor-binding domain (anti-S1-RBD) immunoglobulin G (IgG) presence and degree of decline 3 months after completing vaccination.
A total of 201 patients completed vaccination in April 2021. The BNT162b2 vaccine was administered to 91 patients and the mRNA-1273 to 110 patients. The methodology and results of their humoral and cellular response and their anti-S1-RBD IgG levels are detailed in a previous paper [6]. Three months later we measured anti-S1-RBD IgG to determine the durability and decline rate in 180 patients. Twenty-one were lost to follow-up: 8 died, 10 received a kidney transplant and the remaining 3 were on vacation. One of the eight patients with an initially negative anti-S1-RBD test seroconverted and 10 of the 172 positive patients seroreverted (Figure 1A).
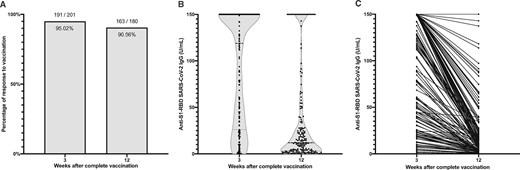
(A) Proportion of seropositive (i.e. anti-S1-RBD IgG >1 U/mL) patients 3 and 12 weeks after completing vaccination with either mRNA-1273 or BNT162b2. (B) Violin plot of anti-S1-RBD IgG levels in the population at 3 and 12 weeks post-vaccination. (C) Variation of anti-S1-RBD IgG in every individual from anti-S1-RBD IgG measurement at 3 and 12 weeks post-vaccination.
Moreover, there was a statistically significant median decline in antibody levels of 84% (interquartile range 30.5) (Figure 1B and C); however, patients who had COVID-19 before vaccination maintained higher antibody levels than those who had not been infected (162.7 and 80.4, respectively; P < 0.001). There was no association between the 3-month seroreversion rate and age, dialysis vintage or immunosuppressive medication. Only five patients were infected with SARS-CoV-2 post-vaccination. One remained asymptomatic (detected during routine reverse transcription polymerase chain reaction screening at the HD unit), one presented mild, simple upper respiratory tract symptoms (i.e. rhinorrhoea and congestion) and three were admitted to the hospital for COVID-19 pneumonia, where none died or developed severe symptoms requiring intensive care.
Few studies report that humoral response persists in all healthy adult individuals up to 6 months after vaccination with mRNA-1273 and similar preprint data 3 months after vaccination with BNT162b2 [8, 9]. However, there are no data on the persistence of anti-S1-RBD IgG levels post-vaccination in HD patients. We found in our cohort, unlike in the healthy population, that not only do antibody levels decline more, but some patients serorevert, in keeping with what has been experienced in other immunization programs, as previously stated [13, 14]. We will continue to monitor the humoral response of our HD cohort and register the incidence of SARS-CoV-2 infection to better understand the vaccine’s protective capacity in this population and determine the future need for boosters.
FUNDING
This work has received no private or public funding. The authors declare that they have no relevant financial interests.
CONFLICT OF INTEREST STATEMENT
None declared.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
Author notes
José Jesús Broseta, Diana Rodríguez-Espinosa contributed equally to this manuscript.





Comments