-
PDF
- Split View
-
Views
-
Cite
Cite
Valentina Raglianti, Giovanni M. Rossi, Augusto Vaglio, Idiopathic retroperitoneal fibrosis: an update for nephrologists, Nephrology Dialysis Transplantation, Volume 36, Issue 10, October 2021, Pages 1773–1781, https://doi.org/10.1093/ndt/gfaa083
Close - Share Icon Share
Abstract
Idiopathic retroperitoneal fibrosis (IRF) is a rare condition characterized by the development of a peri-aortic and peri-iliac tissue showing chronic inflammatory infiltrates and pronounced fibrosis. Ureteral entrapment with consequent obstructive uropathy is one of the most common complications of IRF, which can lead to acute renal failure and, in the long term, to varying degrees of chronic kidney disease. IRF may be isolated or develop in association with autoimmune diseases (e.g. Hashimoto’s thyroiditis and psoriasis) and other fibro-inflammatory disorders (often within the spectrum of immunoglobulin G4-related disease), which suggests that it should be considered as a potentially systemic condition. IRF is an immune-mediated disease: genetic variants (e.g. human leukocyte antigen (HLA)-DRB1*03) and environmental agents (mainly exposure to asbestos and smoking) are strongly associated with an increased risk of developing the disease, while a complex network of chemokines (e.g. CXCL12 and C-C moti chemokine 11 (CCL11)) and cytokines [e.g. interleukin (IL)-6, IL-12 and IL-13] is likely to orchestrate the inflammatory response and simultaneously promote fibrosis. Glucocorticoids, alone or in combination with traditional immunosuppressants such as methotrexate and mycophenolate mofetil, are usually efficacious and promptly induce disease remission; however, up to 50% of patients relapse, thus requiring repeat immunosuppressive courses. Biologic drugs, namely rituximab, are being explored for the treatment of IRF. In addition to medical therapies, interventional procedures (mainly ureteral stenting) are required to relieve ureteral obstruction, whereas surgical ureterolysis is generally reserved to refractory cases. If appropriately treated, then the overall and renal prognosis of IRF are good, with <5% patients developing end-stage renal disease.
INTRODUCTION
Retroperitoneal fibrosis (RPF) is a rare disorder, the hallmark feature of which is a fibro-inflammatory tissue surrounding the abdominal aorta and the iliac arteries and often entrapping the ureters. RPF has an insidious onset, characterized by flank, back or abdominal pain and systemic symptoms. Acute or chronic renal insufficiency often occurs as a result of ureteral obstruction [1].
RPF can either be idiopathic or secondary. Idiopathic retroperitoneal fibrosis (IRF) is an immune-mediated disorder, which may develop alone or in association with other autoimmune diseases (e.g. autoimmune thyroiditis) [2, 3] and/or fibroinflammatory disorders, either immunoglobulin G4 (IgG4)-related or not [4]. IRF is part of a group of diseases collectively named chronic periaortitis, which also includes inflammatory abdominal aortic aneurysms and perianeurysmal retroperitoneal fibrosis, where the pathologic tissue surrounds an aneurysmal aorta, respectively, with or without ureteral involvement. These diseases have similar clinical and histologic features, although their pathogenesis and epidemiology may differ [5]. In a minority of cases, RPF is classified as secondary and has a wide array of aetiologies such as drugs, infections, malignancy and radiation therapy [1].
The epidemiology, pathophysiology, clinical features and management of IRF are herein reviewed.
EPIDEMIOLOGY
Idiopathic forms constitute 70% of all RPF cases and include both IgG4-related and -unrelated forms [6]. Few epidemiological studies providing actual data on the incidence and prevalence of the disease are available. A Finnish retrospective case–control study estimated an age-standardized incidence of 0.10 cases/100 000 person-years and a prevalence of 1.4 affected/100 000 inhabitants [7]. The disease occurs more frequently between the fifth and the seventh decade of life, and shows male predominance with a male-to-female ratio varying between 2:1 and 3:1 [8–10].
A more recent report from the Netherlands showed a higher annual incidence of 1.3 cases/100 000 inhabitants, with a higher mean age at diagnosis than previously reported [11]. It might be worth noting that elderly males are most frequently affected by IRF, as with IgG4-related disease and atherosclerosis. This has led to the hypothesis originally formulated by Mitchinson and Parums [12, 13] that an inflammatory stimulus deriving from atherosclerotic plaques, which are associated with ageing and more frequent in men, would lead in predisposed individuals to an autoimmune reaction to plaque-related antigens eventually followed by fibrosis [14]. These aspects will be expanded upon in the paragraph regarding the pathogenesis of IRF.
IDIOPATHIC VERSUS SECONDARY FORMS
About one-third of all RPF cases are secondary. Therefore, the diagnosis of IRF is one of exclusion, and secondary aetiologies need to be thoroughly excluded in the initial work-up of a patient suspected of having RPF. Secondary forms of RPF include those related to the use of certain drugs (e.g. ergot alkaloids, methysergide, beta-blockers and dopamine agonists), primary retroperitoneal cancer (e.g. lymphoma and sarcoma) or retroperitoneal metastatic disease (various carcinomas), carcinoids, trauma, radiotherapy, major abdominal surgery and infections (e.g. tuberculosis and actinomycosis) [15, 16].
RISK FACTORS
Uibu et al. [7] reported the association between occupational exposure to asbestos and increased risk of IRF. They did not investigate the potential pathogenic mechanism, which therefore remains unclear; however, they suggested that the lymphatic system may transport asbestos bodies from the lungs to the retroperitoneum. This is consistent with the finding, in patients with known history of exposure to asbestos, of asbestos bodies in various organs, including retroperitoneal organs such as the kidney and adrenal glands [17].
In a more recent study, we showed how exposure to asbestos and tobacco (combined or alone) leads to an increased risk of IRF. The exposure to cigarette smoke for >5 years was associated with an odds ratio (OR) of developing IRF of 3.15 (95% confidence interval; CI 1.4–11.11), whereas the exposure to asbestos with an OR of 4.91 (95% CI 0.78–28.02). The effect of combined exposure to tobacco and asbestos resulted in a multiplicative effect on risk, even after stratification for occupational exposure (OR = 13.50, 95% CI 4.40–51.40) and extra-occupational exposure (OR = 8.42, 95% CI 2.77–30.58) [18].
HISTOPATHOLOGY AND PATHOGENESIS
IRF has a multifactorial pathogenesis. Apart from the aforementioned risk factors, a genetic predisposition has been identified in the HLA-DRB1*03 allele. This variant represents an important genetic risk factor for Hashimoto’s thyroiditis, along with HLA-DR4 and HLA-DR5 [19]. Extremely rare cases of familial IRF, either alone or as part of multifocal fibroinflammatory disorders, have also been reported [20].
IRF was initially thought to represent a local inflammatory response to atherosclerotic plaques [21]. Evidence from the last decade suggests that the disease could emerge also in patients without atherosclerosis or in segments of the aorta/iliac arteries not affected by atherosclerotic plaques. We previously showed that one-third of patients with IRF also have thoracic aorta and epiaortic artery involvement, regardless of the presence of underlying atherosclerotic disease [22]. Moreover, chronic periaortitis shares features with systemic large vessel vasculitis, for example having the adventitia and the media–adventitia border as targets of the disease process [23]. Finally, histological features as detailed below and prompt response to immunosuppressants (see paragraph on treatment) support the hypothesis that IRF belongs to the spectrum of systemic inflammatory diseases.
IRF is characterized histologically by the proliferation of a fibrous and an inflammatory tissue which infiltrates, replaces and expands in the retroperitoneal soft tissue, to the point that pre-existing adipocytes may become barely recognizable, with poor demarcation between the proliferating fibroinflammatory tissue and the pre-existing retroperitoneal soft tissue. The inflammatory infiltrate can have either a perivascular or diffuse distribution and is mixed in composition, including macrophages, lymphocytes, plasma cells and occasional eosinophils with rare neutrophils (Figure 1). The lymphocytic population is mainly comprised of CD4+ T cells [24]. These cells likely orchestrate a T helper 2 (Th2)-dominant response [25]. Local interleukin (IL)-6 production stimulates B cell and fibroblast activation. B cells, which are abundant in the infiltrate, can also differentiate to plasma cells in response to IL-6 stimulation. Abundant IL-6 presence in fibrous tissue of IRF patients has been demonstrated previously [26]. Furthermore, Th2 response-related chemokines like CCL11/eotaxin are highly expressed in IRF patients and contribute to eosinophil and mast cell recruitment. Eosinophils and mast cells express C-C chemokine receptor type 3 (CCR3) (the CCL11/eotaxin receptor) and stimulate fibroblast activation, proliferation and production of collagen [27]. Collagen fibres are organized in thick and irregular bundles. The collagen fibres often surround small blood vessels and nodular inflammatory aggregates with a core of B cells and a periphery of T cells (usually CD4+), which have the features of lymphoid follicles with germinal centre reaction. This phenomenon, called ectopic lymphoneogenesis, is characteristic of chronic inflammatory and autoimmune diseases [24].
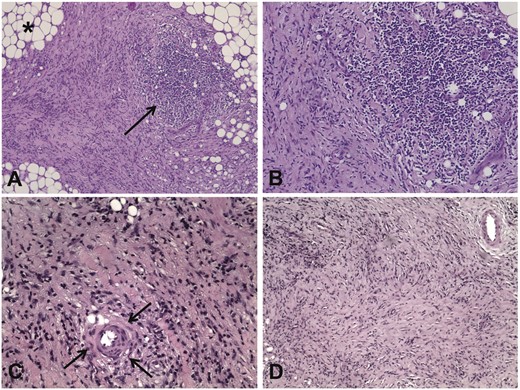
Main histological patterns in patients with IRF. (A) Low-power view of a retroperitoneal biopsy from a patient with IRF shows a fibro-inflammatory tissue replacing the normal retroperitoneal adipose tissue (asterisk); the arrow indicates a nodular aggregate of inflammatory cells, which is located within a highly fibrous tissue. Haematoxylin and eosin, ×10. (B) A higher magnification view of the histological field shown in (A) shows that the nodular aggregate is mainly comprised mononuclear inflammatory cells. Haematoxylin and eosin, ×20. (C) High-power view of a retroperitoneal biopsy from a patient with IRF shows that the thick collagen bundles are often organized around small blood vessels (arrows). Haematoxylin and eosin, ×20. (D) Low-power view of a retroperitoneal biopsy from an IRF patient with long disease duration shows mainly compact and storiform fibrosis. Haematoxylin and eosin, ×10.
Recently, a possible pathogenic role of fibrocytes has been suggested. Fibrocytes are circulating precursors of fibroblasts; they differentiate into tissue fibroblasts and myofibroblasts, and may also amplify the inflammatory response [28]. The CXCR4–CXCL12 axis could play a crucial role in fibrocyte recruitment in patients with IRF. A high level of CXCL12 and an increased number of circulating fibrocytes expressing the CXCR4 receptor has also been found in these patients [29].
CLINICAL FEATURES
Common symptoms at onset include lower back, abdominal or flank pain, and constitutional symptoms such as malaise, fever, anorexia and weight loss. Pain is frequently referred to the hip, to the groin and to the lateral regions of the leg, with nocturnal exacerbations, and typically does not modify with position [30, 31]. The pain associated with IRF is usually dull, and its intensity correlates with erythrocyte sedimentation rate (ESR) but not with the presence of hydroureteronephrosis. However, it can mimic a renal colic when ureters are involved. The diagnosis of IRF is only rarely an incidental finding in the imaging work-up of patients with a suspicion of nephrolithiasis. Other urologic manifestations include urgency, urinary frequency and dysuria. Testicular pain, varicocoele and hydrocoele due to spermatic vein encasement by IRF are also common scenarios [11]. Vascular complications including arterial obstruction and deep venous thrombosis are rare, but several cases have been reported to date, and lower limb claudication has been reported in large retrospective cohorts [32]. The relative frequency of symptoms shows overall mild variability across different cohorts (Table 1).
Main clinical manifestations, laboratory findings, treatment regimens and outcomes in different published series of patients with RPF
| References . | Moriconi et al. [33] . | Raffiotta et al. [30] . | Zhao et al. [31] . | Jadhav et al. [34] . | Labidi et al. [35] . | Brandt et al. [36] . | Kermani et al. [32] . |
|---|---|---|---|---|---|---|---|
| . | . | ||||||
| . | 37 pts, Italy . | 50 pts, Italy . | 155 pts, China . | 21 ptsa, India . | 30 ptsb, Tunisia . | 204 ptsc, Germany . | 185 pts, USA . |
| Age, mean ± SD or median (IQR), years | 55 ± 11 | 58.7 (50.9–64) | 58.1 ± 12 | 50.8 (26–74) | 50.5 (32–77) | 55.6 ± 11.9 | 57.6 ± 11.8 |
| Male, % | 76.6 | 58 | 71 | 47.62 | 80 | 68.1 | 61 |
| Clinical features at diagnosis, % | |||||||
| Abdominal or lumbar pain | 62 | 78 | 56.8 | 38.1 | 94 | 66.3 | 40 |
| Constitutional symptoms | 29.7 | 48 | 36.8 | 23.8 | 10 | 36.6 | 27 |
| Limb oedema | 5.4 | 10 | 34.2 | 19 | 13 | 16.6 | 13 |
| Ureteral involvement | 78.4 | 94 | 94.8 | 61.1 | – | 95.5 | 57 |
| Unilateral hydronephrosis | 54 | 38 | 38.1 | 52.4 | 20 | 39.7 | – |
| Bilateral hydronephrosis | 32.4 | 56 | 56.8 | 47.6 | 43.4 | 55.9 | 56 |
| Impaired renal function at diagnosis | 33 | – | 61.3 | 71.43 | 54 | – | 42 |
| Renal atrophy at diagnosis | 8.1 | 58 | 41.5 | 47.6 | 16.7 | 22.5 | 8 |
| Hypertension at diagnosis | 35.3 | 52 | – | 71.4 | 10 | 44.6 | – |
| Laboratory findings at diagnosis | |||||||
| ESR, mean ± SD or median (IQR), mm/h | – | 52 (30.8–73.8) | 45 (17–154) | 56.7 (39–87) | 50.5 (2–130) | – | 32.3 ± 29.1 |
| CRP, mean ± SD or median IQR), mg/dL | 2.24 ± 1.7 | – | 2.6 (0.83–23.1) | – | 40.5 (4–192) | – | 20.7 ± 26.4 |
| High IgG4 (>140 mg/dL), % | 50d | 17 | 38.2 | – | – | – | – |
| ANA positivity, % | 10.8 | 24 | – | – | – | – | 5 |
| eGFR, mean ± SD or median (IQR), mL/min/1.73 m2 | 77 ± 20e | 31.5 (10–66)e | – | – | – | – | – |
| Serum creatinine, mean ± SD or median (IQR), mg/dL | – | 1.9 (1–5.6) | 4.53(1.2–21.5) | 1.8 (0.9–11.2) | 3.37 (0.6–26.3) | – | 1.3 (1.1–2.1) |
| Treatments | |||||||
| Prednisone monotherapy, % | 65 | 30 | 10f | 10 | 84 | 57 | 12f |
| Prednisone + tamoxifen, % | 35 | 22 | – | 24 | 0 | 2 | – |
| Tamoxifen alone, % | 0 | 0 | – | 52 | 0 | 13 | – |
| Prednisone + MTX, % | 0 | 2 | – | 0 | 13 | 1 | – |
| Prednisone + MMF, % | 0 | 16 | – | 0 | 3 | 2 | – |
| Prednisone + AZA, % | 0 | 28 | – | 0 | 0 | 18 | – |
| None, % | 0 | 2 | 11.6 | 14 | 0 | 7 | 12 |
| Disease and renal outcomes | |||||||
| Follow-up duration, mean ± SD or median (IQR), months | 90 ± 56 | 106.8 (56.4–152.4) | 45.3 (0.1–169) | 20.4 | 53.2 | – | 48 |
| Remission, % | 70.2 | 35 | – | 70 | 76 | – | 54 |
| Relapse, % | 40.5 | 38 | 17.6 | 17.65 | 53 | – | 12 |
| Ureteral stents, % | 42 | 44 | 82 | 71.43 | 30 | 12 | 57 |
| Stenting duration, mean ± SD or median (IQR), months | 18 (8–36) | – | – | 9.8 (1.2–60) | 2 ± 1.4 | – | – |
| Nephrostomy, % | – | 16 | 17.6 | – | 3 | 1.5 | 6 |
| Ureterolysis, % | 8 | 10 | 12.3 | 100 | 6 | 24 | 28 |
| ESRD, % | 8 | 2 | 0 | – | 0 | – | 0 |
| eGFR <60 mL/min/1.73 m2, % | – | 39 | 47.4 | – | 20 | – | 32 |
| References . | Moriconi et al. [33] . | Raffiotta et al. [30] . | Zhao et al. [31] . | Jadhav et al. [34] . | Labidi et al. [35] . | Brandt et al. [36] . | Kermani et al. [32] . |
|---|---|---|---|---|---|---|---|
| . | . | ||||||
| . | 37 pts, Italy . | 50 pts, Italy . | 155 pts, China . | 21 ptsa, India . | 30 ptsb, Tunisia . | 204 ptsc, Germany . | 185 pts, USA . |
| Age, mean ± SD or median (IQR), years | 55 ± 11 | 58.7 (50.9–64) | 58.1 ± 12 | 50.8 (26–74) | 50.5 (32–77) | 55.6 ± 11.9 | 57.6 ± 11.8 |
| Male, % | 76.6 | 58 | 71 | 47.62 | 80 | 68.1 | 61 |
| Clinical features at diagnosis, % | |||||||
| Abdominal or lumbar pain | 62 | 78 | 56.8 | 38.1 | 94 | 66.3 | 40 |
| Constitutional symptoms | 29.7 | 48 | 36.8 | 23.8 | 10 | 36.6 | 27 |
| Limb oedema | 5.4 | 10 | 34.2 | 19 | 13 | 16.6 | 13 |
| Ureteral involvement | 78.4 | 94 | 94.8 | 61.1 | – | 95.5 | 57 |
| Unilateral hydronephrosis | 54 | 38 | 38.1 | 52.4 | 20 | 39.7 | – |
| Bilateral hydronephrosis | 32.4 | 56 | 56.8 | 47.6 | 43.4 | 55.9 | 56 |
| Impaired renal function at diagnosis | 33 | – | 61.3 | 71.43 | 54 | – | 42 |
| Renal atrophy at diagnosis | 8.1 | 58 | 41.5 | 47.6 | 16.7 | 22.5 | 8 |
| Hypertension at diagnosis | 35.3 | 52 | – | 71.4 | 10 | 44.6 | – |
| Laboratory findings at diagnosis | |||||||
| ESR, mean ± SD or median (IQR), mm/h | – | 52 (30.8–73.8) | 45 (17–154) | 56.7 (39–87) | 50.5 (2–130) | – | 32.3 ± 29.1 |
| CRP, mean ± SD or median IQR), mg/dL | 2.24 ± 1.7 | – | 2.6 (0.83–23.1) | – | 40.5 (4–192) | – | 20.7 ± 26.4 |
| High IgG4 (>140 mg/dL), % | 50d | 17 | 38.2 | – | – | – | – |
| ANA positivity, % | 10.8 | 24 | – | – | – | – | 5 |
| eGFR, mean ± SD or median (IQR), mL/min/1.73 m2 | 77 ± 20e | 31.5 (10–66)e | – | – | – | – | – |
| Serum creatinine, mean ± SD or median (IQR), mg/dL | – | 1.9 (1–5.6) | 4.53(1.2–21.5) | 1.8 (0.9–11.2) | 3.37 (0.6–26.3) | – | 1.3 (1.1–2.1) |
| Treatments | |||||||
| Prednisone monotherapy, % | 65 | 30 | 10f | 10 | 84 | 57 | 12f |
| Prednisone + tamoxifen, % | 35 | 22 | – | 24 | 0 | 2 | – |
| Tamoxifen alone, % | 0 | 0 | – | 52 | 0 | 13 | – |
| Prednisone + MTX, % | 0 | 2 | – | 0 | 13 | 1 | – |
| Prednisone + MMF, % | 0 | 16 | – | 0 | 3 | 2 | – |
| Prednisone + AZA, % | 0 | 28 | – | 0 | 0 | 18 | – |
| None, % | 0 | 2 | 11.6 | 14 | 0 | 7 | 12 |
| Disease and renal outcomes | |||||||
| Follow-up duration, mean ± SD or median (IQR), months | 90 ± 56 | 106.8 (56.4–152.4) | 45.3 (0.1–169) | 20.4 | 53.2 | – | 48 |
| Remission, % | 70.2 | 35 | – | 70 | 76 | – | 54 |
| Relapse, % | 40.5 | 38 | 17.6 | 17.65 | 53 | – | 12 |
| Ureteral stents, % | 42 | 44 | 82 | 71.43 | 30 | 12 | 57 |
| Stenting duration, mean ± SD or median (IQR), months | 18 (8–36) | – | – | 9.8 (1.2–60) | 2 ± 1.4 | – | – |
| Nephrostomy, % | – | 16 | 17.6 | – | 3 | 1.5 | 6 |
| Ureterolysis, % | 8 | 10 | 12.3 | 100 | 6 | 24 | 28 |
| ESRD, % | 8 | 2 | 0 | – | 0 | – | 0 |
| eGFR <60 mL/min/1.73 m2, % | – | 39 | 47.4 | – | 20 | – | 32 |
The studies included in the table were identified by searching the English-language literature using PubMed limited to the 2009–19 period. We only selected studies that provided outcome data of the patients and included >20 patients.
24% had secondary RPF (secondary to radiation in three cases, while two patients had RPF with non-caseating granulomatous inflammation).
17% had secondary RPF (secondary to abdominal trauma in two cases, abdominal surgery in one, tuberculosis in one and sclerotic pancreatitis in one).
9% had secondary RPF (secondary to malignancy in 11, to abdominal surgery in 14, to ergot derivatives in 4; three patients reported diverticulitis and one pancreatitis; in four patients radiation therapy was likely causative; one case had tuberculosis).
Data are available for only 20 patients.
Used the Chronic Kidney Disease Epidemiology Collaboration creatinine equation to estimated glomerular filtration rate (eGFR).
In these studies, it is not clearly reported whether other immunosuppressive therapies were used alone or in combination with prednisone.
pts, patients; IQR, interquartile range.
Main clinical manifestations, laboratory findings, treatment regimens and outcomes in different published series of patients with RPF
| References . | Moriconi et al. [33] . | Raffiotta et al. [30] . | Zhao et al. [31] . | Jadhav et al. [34] . | Labidi et al. [35] . | Brandt et al. [36] . | Kermani et al. [32] . |
|---|---|---|---|---|---|---|---|
| . | . | ||||||
| . | 37 pts, Italy . | 50 pts, Italy . | 155 pts, China . | 21 ptsa, India . | 30 ptsb, Tunisia . | 204 ptsc, Germany . | 185 pts, USA . |
| Age, mean ± SD or median (IQR), years | 55 ± 11 | 58.7 (50.9–64) | 58.1 ± 12 | 50.8 (26–74) | 50.5 (32–77) | 55.6 ± 11.9 | 57.6 ± 11.8 |
| Male, % | 76.6 | 58 | 71 | 47.62 | 80 | 68.1 | 61 |
| Clinical features at diagnosis, % | |||||||
| Abdominal or lumbar pain | 62 | 78 | 56.8 | 38.1 | 94 | 66.3 | 40 |
| Constitutional symptoms | 29.7 | 48 | 36.8 | 23.8 | 10 | 36.6 | 27 |
| Limb oedema | 5.4 | 10 | 34.2 | 19 | 13 | 16.6 | 13 |
| Ureteral involvement | 78.4 | 94 | 94.8 | 61.1 | – | 95.5 | 57 |
| Unilateral hydronephrosis | 54 | 38 | 38.1 | 52.4 | 20 | 39.7 | – |
| Bilateral hydronephrosis | 32.4 | 56 | 56.8 | 47.6 | 43.4 | 55.9 | 56 |
| Impaired renal function at diagnosis | 33 | – | 61.3 | 71.43 | 54 | – | 42 |
| Renal atrophy at diagnosis | 8.1 | 58 | 41.5 | 47.6 | 16.7 | 22.5 | 8 |
| Hypertension at diagnosis | 35.3 | 52 | – | 71.4 | 10 | 44.6 | – |
| Laboratory findings at diagnosis | |||||||
| ESR, mean ± SD or median (IQR), mm/h | – | 52 (30.8–73.8) | 45 (17–154) | 56.7 (39–87) | 50.5 (2–130) | – | 32.3 ± 29.1 |
| CRP, mean ± SD or median IQR), mg/dL | 2.24 ± 1.7 | – | 2.6 (0.83–23.1) | – | 40.5 (4–192) | – | 20.7 ± 26.4 |
| High IgG4 (>140 mg/dL), % | 50d | 17 | 38.2 | – | – | – | – |
| ANA positivity, % | 10.8 | 24 | – | – | – | – | 5 |
| eGFR, mean ± SD or median (IQR), mL/min/1.73 m2 | 77 ± 20e | 31.5 (10–66)e | – | – | – | – | – |
| Serum creatinine, mean ± SD or median (IQR), mg/dL | – | 1.9 (1–5.6) | 4.53(1.2–21.5) | 1.8 (0.9–11.2) | 3.37 (0.6–26.3) | – | 1.3 (1.1–2.1) |
| Treatments | |||||||
| Prednisone monotherapy, % | 65 | 30 | 10f | 10 | 84 | 57 | 12f |
| Prednisone + tamoxifen, % | 35 | 22 | – | 24 | 0 | 2 | – |
| Tamoxifen alone, % | 0 | 0 | – | 52 | 0 | 13 | – |
| Prednisone + MTX, % | 0 | 2 | – | 0 | 13 | 1 | – |
| Prednisone + MMF, % | 0 | 16 | – | 0 | 3 | 2 | – |
| Prednisone + AZA, % | 0 | 28 | – | 0 | 0 | 18 | – |
| None, % | 0 | 2 | 11.6 | 14 | 0 | 7 | 12 |
| Disease and renal outcomes | |||||||
| Follow-up duration, mean ± SD or median (IQR), months | 90 ± 56 | 106.8 (56.4–152.4) | 45.3 (0.1–169) | 20.4 | 53.2 | – | 48 |
| Remission, % | 70.2 | 35 | – | 70 | 76 | – | 54 |
| Relapse, % | 40.5 | 38 | 17.6 | 17.65 | 53 | – | 12 |
| Ureteral stents, % | 42 | 44 | 82 | 71.43 | 30 | 12 | 57 |
| Stenting duration, mean ± SD or median (IQR), months | 18 (8–36) | – | – | 9.8 (1.2–60) | 2 ± 1.4 | – | – |
| Nephrostomy, % | – | 16 | 17.6 | – | 3 | 1.5 | 6 |
| Ureterolysis, % | 8 | 10 | 12.3 | 100 | 6 | 24 | 28 |
| ESRD, % | 8 | 2 | 0 | – | 0 | – | 0 |
| eGFR <60 mL/min/1.73 m2, % | – | 39 | 47.4 | – | 20 | – | 32 |
| References . | Moriconi et al. [33] . | Raffiotta et al. [30] . | Zhao et al. [31] . | Jadhav et al. [34] . | Labidi et al. [35] . | Brandt et al. [36] . | Kermani et al. [32] . |
|---|---|---|---|---|---|---|---|
| . | . | ||||||
| . | 37 pts, Italy . | 50 pts, Italy . | 155 pts, China . | 21 ptsa, India . | 30 ptsb, Tunisia . | 204 ptsc, Germany . | 185 pts, USA . |
| Age, mean ± SD or median (IQR), years | 55 ± 11 | 58.7 (50.9–64) | 58.1 ± 12 | 50.8 (26–74) | 50.5 (32–77) | 55.6 ± 11.9 | 57.6 ± 11.8 |
| Male, % | 76.6 | 58 | 71 | 47.62 | 80 | 68.1 | 61 |
| Clinical features at diagnosis, % | |||||||
| Abdominal or lumbar pain | 62 | 78 | 56.8 | 38.1 | 94 | 66.3 | 40 |
| Constitutional symptoms | 29.7 | 48 | 36.8 | 23.8 | 10 | 36.6 | 27 |
| Limb oedema | 5.4 | 10 | 34.2 | 19 | 13 | 16.6 | 13 |
| Ureteral involvement | 78.4 | 94 | 94.8 | 61.1 | – | 95.5 | 57 |
| Unilateral hydronephrosis | 54 | 38 | 38.1 | 52.4 | 20 | 39.7 | – |
| Bilateral hydronephrosis | 32.4 | 56 | 56.8 | 47.6 | 43.4 | 55.9 | 56 |
| Impaired renal function at diagnosis | 33 | – | 61.3 | 71.43 | 54 | – | 42 |
| Renal atrophy at diagnosis | 8.1 | 58 | 41.5 | 47.6 | 16.7 | 22.5 | 8 |
| Hypertension at diagnosis | 35.3 | 52 | – | 71.4 | 10 | 44.6 | – |
| Laboratory findings at diagnosis | |||||||
| ESR, mean ± SD or median (IQR), mm/h | – | 52 (30.8–73.8) | 45 (17–154) | 56.7 (39–87) | 50.5 (2–130) | – | 32.3 ± 29.1 |
| CRP, mean ± SD or median IQR), mg/dL | 2.24 ± 1.7 | – | 2.6 (0.83–23.1) | – | 40.5 (4–192) | – | 20.7 ± 26.4 |
| High IgG4 (>140 mg/dL), % | 50d | 17 | 38.2 | – | – | – | – |
| ANA positivity, % | 10.8 | 24 | – | – | – | – | 5 |
| eGFR, mean ± SD or median (IQR), mL/min/1.73 m2 | 77 ± 20e | 31.5 (10–66)e | – | – | – | – | – |
| Serum creatinine, mean ± SD or median (IQR), mg/dL | – | 1.9 (1–5.6) | 4.53(1.2–21.5) | 1.8 (0.9–11.2) | 3.37 (0.6–26.3) | – | 1.3 (1.1–2.1) |
| Treatments | |||||||
| Prednisone monotherapy, % | 65 | 30 | 10f | 10 | 84 | 57 | 12f |
| Prednisone + tamoxifen, % | 35 | 22 | – | 24 | 0 | 2 | – |
| Tamoxifen alone, % | 0 | 0 | – | 52 | 0 | 13 | – |
| Prednisone + MTX, % | 0 | 2 | – | 0 | 13 | 1 | – |
| Prednisone + MMF, % | 0 | 16 | – | 0 | 3 | 2 | – |
| Prednisone + AZA, % | 0 | 28 | – | 0 | 0 | 18 | – |
| None, % | 0 | 2 | 11.6 | 14 | 0 | 7 | 12 |
| Disease and renal outcomes | |||||||
| Follow-up duration, mean ± SD or median (IQR), months | 90 ± 56 | 106.8 (56.4–152.4) | 45.3 (0.1–169) | 20.4 | 53.2 | – | 48 |
| Remission, % | 70.2 | 35 | – | 70 | 76 | – | 54 |
| Relapse, % | 40.5 | 38 | 17.6 | 17.65 | 53 | – | 12 |
| Ureteral stents, % | 42 | 44 | 82 | 71.43 | 30 | 12 | 57 |
| Stenting duration, mean ± SD or median (IQR), months | 18 (8–36) | – | – | 9.8 (1.2–60) | 2 ± 1.4 | – | – |
| Nephrostomy, % | – | 16 | 17.6 | – | 3 | 1.5 | 6 |
| Ureterolysis, % | 8 | 10 | 12.3 | 100 | 6 | 24 | 28 |
| ESRD, % | 8 | 2 | 0 | – | 0 | – | 0 |
| eGFR <60 mL/min/1.73 m2, % | – | 39 | 47.4 | – | 20 | – | 32 |
The studies included in the table were identified by searching the English-language literature using PubMed limited to the 2009–19 period. We only selected studies that provided outcome data of the patients and included >20 patients.
24% had secondary RPF (secondary to radiation in three cases, while two patients had RPF with non-caseating granulomatous inflammation).
17% had secondary RPF (secondary to abdominal trauma in two cases, abdominal surgery in one, tuberculosis in one and sclerotic pancreatitis in one).
9% had secondary RPF (secondary to malignancy in 11, to abdominal surgery in 14, to ergot derivatives in 4; three patients reported diverticulitis and one pancreatitis; in four patients radiation therapy was likely causative; one case had tuberculosis).
Data are available for only 20 patients.
Used the Chronic Kidney Disease Epidemiology Collaboration creatinine equation to estimated glomerular filtration rate (eGFR).
In these studies, it is not clearly reported whether other immunosuppressive therapies were used alone or in combination with prednisone.
pts, patients; IQR, interquartile range.
IRF can present in association with other systemic autoimmune diseases, such as systemic lupus erythematosus [37], rheumatoid arthritis [38], and small- and medium-sized vessel vasculitides (e.g. granulomatosis with polyangiitis and polyarteritis nodosa) [39], or can be found associated with organ-specific autoimmune disorders such as autoimmune thyroiditis [40, 41].
RENAL INVOLVEMENT
Ureteral involvement by IRF often results in obstructive uropathy. The ureteral involvement can be unilateral or bilateral, but patients with unilateral disease often develop contralateral involvement from weeks to months after diagnosis [32, 34]. When both ureters are encased by the fibrotic tissue, acute kidney injury (AKI) may ensue. Moreover, at diagnosis, 8–30% of patients present with renal atrophy. It is debated whether this is due to long lasting obstructive uropathy or is the result of renal artery stenosis [24]. Indeed, IRF may encase the renal vascular peduncle and compress renal veins or arteries. Renal artery involvement may manifest as new-onset hypertension or worsening of pre-existing hypertension in up to 30% of the patients [8].
LABORATORY FINDINGS
At presentation, most patients have elevated ESR or C-reactive protein (CRP) levels. Anti-nuclear antibody (ANA) positivity was observed in 25–60% of IRF patients [8, 42]. The presence of other autoantibodies was also described: 31% of patients showed anti-thyroid antibodies, 14% are positive for rheumatoid factor and 10% showed positive perinuclear or cytoplasmic anti-neutrophil cytoplasmic antibodies [43].
Serum IgG4 levels are usually tested at the time of diagnosis in patients with IRF, although they are of little value in the differentiation between IRF and other inflammatory or neoplastic disorders. In a recent study on 113 IRF patients, we found that 21% of them had high IgG4 levels at diagnosis (>135 mg/dL); IRF patients with high IgG4 had similar clinical presentation and outcomes (response to therapy, relapse) as compared with those who had normal IgG4. However, high-IgG4 patients more frequently had extra-retroperitoneal fibro-inflammatory lesions belonging to the spectrum of IgG4-RD (e.g. sclerosing pancreatitis and mediastinal fibrosis) [44]. Therefore, the finding of high serum IgG4 in IRF patients should prompt the search for other potentially involved sites. This view was supported by several studies that were recently reviewed [4].
IMAGING STUDIES AND DIAGNOSIS
There is no agreement about the use of imaging to diagnose IRF. Some computed tomography (CT)/magnetic resonance imaging (MRI) features are typical of IRF; however, they are not pathognomonic. Therefore, in order to reach a diagnosis, an integrative approach taking into account both imaging and clinical presentation is needed.
The typical CT/MRI findings of IRF, as opposed to secondary RPF (e.g. to malignancy), include: a single/confluent mass surrounding anterolaterally, but not significantly displacing, a non-aneurysmal distal abdominal aorta and/or proximal common iliac arteries (Figure 2); additionally, IRF tends to deviate the ureters medially (as opposed to malignant RPF, that displaces them laterally) and does not infiltrate the muscle or the bone. Finally, IRF usually develops below the origin of the renal arteries, as opposed to malignant forms, which usually extend above this level [45, 46].
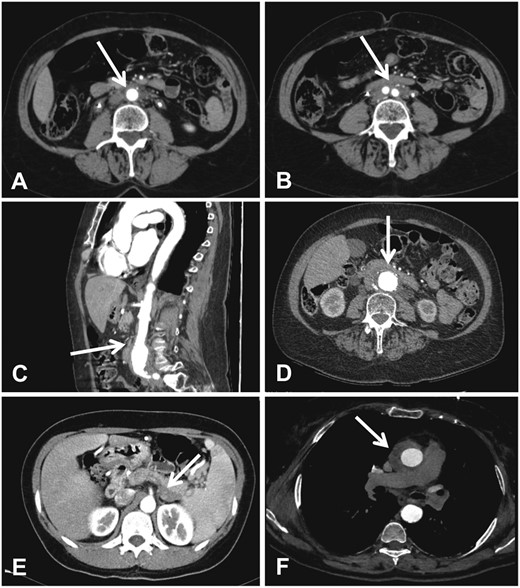
Main CT findings in patients with IRF. (A and B) Contrast-enhanced CT (axial views) of the abdomen in a patient with IRF shows the typical peri-aortic (A) and peri-iliac (B) (arrows) muscle-isodense tissue. (C and D) Contrast-enhanced CT (sagittal view in C, axial view in D) of the abdomen in a patient with perianeuruysmal retroperitoneal fibrosis shows that the muscle-isodense tissue surrounds an aneurysmal and highly calcified abdominal aorta. (E) Contrast-enhanced CT of the abdomen in a patient with IgG4-related autoimmune pancreatitis shows a retroperitoneal hypodense tissue adjacent to the pancreatic tail (arrow). (F) Contrast-enhanced CT of the chest in a patient IRF associated with thoracic periaortitis shows an isodense tissue around the ascending thoracic aorta (arrow).
On CT scans, fibrosis is muscle-isodense, with corresponding iso-hypointensity on T1- to T2-weighted MRI. However, high signal intensity can be observed on T2-weighted images, reflecting oedema or active inflammation. These features are shared by secondary RPF. However, malignant processes tend to have an inhomogeneous appearance on T2-weighted images. Both CT and MRI contrast-enhanced scans show paucity of enhancement in the parenchymal phase, with a characteristic delayed-phase enhancement; however, in more active stages of the disease, a significant enhancement is appreciated, reflecting the degree of inflammatory changes associated with fibrosis [47].
The aforementioned imaging features, indeed, exclude malignant retroperitoneal masses with only moderate certainty. In a study conducted by Cohan et al. on 68 patients with clinical suspicion of RPF, among 41 with typical presentation, one (2%) had malignancy (adenocarcinoma of unknown origin). The authors suggested that a proper diagnosis of IRF could be made in this subset of patients with an ‘IRF-typical’ peri-aortic mass, and biopsy could be avoided [48]. Based on the results of different studies and on our own experience, when the retroperitoneal mass presents with the aforementioned typical features, a diagnosis of IRF can be reached without a biopsy. At this point of the diagnosis flowchart, the use of positron emission tomography (PET)-CT plays an important role (Figure 3). The use of PET-CT allows identification of extra-retroperitoneal sites, to which the disease may be associated (e.g. IgG4-RD or thoracic aorta involvement) [49], or malignancies [50]. It is a matter of debate whether the extent of uptake can predict response to therapy. Previously, it has been reported that the response to therapy should only be expected in patients with high metabolic activity (i.e. SUVmax >4) [51]; however, according to our experience and other studies [52], negative or mildly positive [18F]-Fluorodeoxyglucose-PET is not always associated with lack of response, although the response rate is lower in patients with a low degree of metabolic activity [53]. In any case, PET-CT is useful in monitoring disease activity after treatment and thus may be of help in deciding whether treatment should be discontinued or not [54].
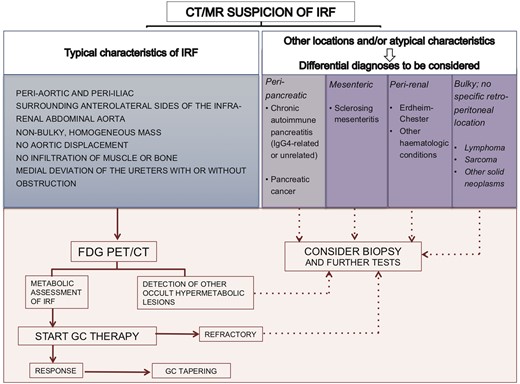
Diagnostic algorithm for idiopathic versus other forms of retroperitoneal fibrosis.
A certain degree of overlap between the benign and malignant retroperitoneal forms must be considered. A condition that must be differentiated from IRF is Erdheim–Chester disease, in which the characteristic finding of the perirenal fibrotic tissue (‘hairy kidney’) is often found; when this condition is suspected, the search for other involved sites (e.g. long bones, heart and central nervous system) is mandatory [55]. Metastatic solid or haematopoietic neoplasms may also show periaortic soft tissue infiltration, but they often displace the abdominal aorta, infiltrate the muscles, the bone or other structures and confluent lymphadenopathy is frequent [56]. In all these cases of atypical presentation, biopsy should be performed to achieve a diagnosis. Biopsy can be either CT-guided, or be performed using a laparoscopic or an open surgical approach, with the latter two being usually preferred when the tissue is not thick enough to allow a percutaneous approach.
TREATMENT
The treatment goal in IRF is obtaining remission, i.e. preventing or resolving obstructive uropathy or other compressive complications and switching off symptoms. Relapses do indeed occur, offering the rationale for prolonged clinical surveillance and maintenance therapy in most patients. If left untreated, then IRF can evolve into chronic kidney disease (CKD) and progress to end-stage renal disease (ESRD). When there is AKI and/or at least moderate obstructive uropathy on imaging, ureteral drainage is the priority (using ureteral stents or nephrostomies), followed by medical therapy [57–59]. A surgical approach should be reserved for patients who are refractory to the latter [60]. The surgical approach consists in ureterolysis by open exploration or laparoscopy. Different techniques are available, including ureteral wrapping with omental fat and intraperitoneal transposition [61, 62]. We believe that the best solution is ureterolysis plus omental fat wrapping of the ureters. This technique allows obtaining of an effective barrier from the fibrous tissue and permits the revascularization of the ureters [63]. On the other hand, when ureteral involvement is absent or mild, i.e. it does not cause renal function impairment and/or hydronephrosis is mild, a pharmacological approach is often sufficient [64, 65].
Glucocorticoids (GCs) are considered the cornerstone of therapy. The treatment regimen we routinely adopt is mainly based on the results of an open-label, randomized trial of prednisone against tamoxifen for the maintenance of remission in patients with IRF who achieved remission with a 1-month 1 mg/kg prednisone daily regimen [66]. Patients were randomized to either continue prednisone (0.5 mg/kg in the first month, followed by 0.25 mg/kg the second and third and then tapered off during the following 5 months) or switch to a fixed dose of tamoxifen (0.5 mg/kg daily for 8 months). The primary endpoint of the study was relapse, defined as one or more of: recurrence of hydronephrosis, increase of >20% in the size of the retroperitoneal tissue, or IRF-attributable symptoms together with an increase of at least 50% in CRP or ESR values. The beneficial effects of prednisone were dramatic: by the end of the 8 months, 6% in the prednisone and 39% in the tamoxifen arm had had relapses; all patients who suffered from relapses on tamoxifen were switched to prednisone and obtained remission. Patients were followed for an additional 18 months. At 26 months, patients in the prednisone arm had a 17% relapse rate as compared with 50% of the tamoxifen group, but there were significantly more GC-induced toxic side effects, including Cushing syndrome, weight gain and hyperlipidaemia. Overall, as many as 90% respond to prednisone alone [67]. We currently start patients on oral prednisone 1 mg/kg/day for 1 month. If there is clinical response, then the dose is tapered to 10 mg/day in the following 3 months, followed by a slow tapering for another 6 months until drug cessation [68].
The use of tamoxifen in IRF derives from the treatment of desmoid tumours, also characterized by locally invasive fibroblast proliferation, albeit with a different pathophysiology. Several studies have shown the efficacy of tamoxifen in IRF, alone or in association with steroids [36, 69–72]. A long-term follow-up study of tamoxifen monotherapy showed a 65% response, defined as a composite outcome of: subjective clinical improvement by 6 weeks, stable or decreasing periaortic mass by 4 months, definite decreasing mass by 8 months and resolution of ureteral obstruction allowing stent removal [69]. In light of the proven inferiority to prednisone in the maintenance of remission, in our practice, we reserve the use of tamoxifen for patients with contraindications to GCs.
The use of other immunosuppressive agents, including cyclophosphamide [73, 74], azathioprine (AZA) [75], methotrexate (MTX) [76], mycophenolate mofetil (MMF) [77, 78] and biological agents such as rituximab [79], tocilizumab [26] and infliximab [80] have been reported as a valuable option mostly in case reports, cases series and small studies. However, as with tamoxifen, strong evidence from controlled trials is lacking, and it is debatable whether the place of these drugs in the treatment of IRF mainly relies on their GC-sparing effect or as GC replacement in patients with contraindications to GCs or intolerable GC toxicity, or if they actually have a superior or additional role to that of GCs. Our current approach is to reserve these agents for patients who cannot tolerate GCs or who are likely to suffer from significant GC-related toxicity (overweight or obese patients, with or without diabetes), or who failed to achieve a response with GC monotherapy. Given the lack of evidence, there is no preferred or suggested second- or third-line of choice; based on our experience with MMF and MTX, we usually adopt either of these drugs in the aforementioned scenarios.
OUTCOME: FOCUS ON RENAL PROGNOSIS
In the different studies in the literature, prognostic factors or predictors of disease recurrence have not been clearly identified. This is partly attributable to the fact that the management and diagnostic and therapeutic monitoring of IRF are not standardized. However, prognosis is generally good. In patients who respond to steroid therapy, the mortality rate does not exceed 10% although this figure should be interpreted in light of the different follow-up durations of the published studies [81]. A relapse rate of ∼10–50% is reported after treatment interruption; Table 1 reports the main outcomes of IRF patients in different retrospective studies [33, 75]. CKD is certainly a major complication of IRF although its frequency is still uncertain [35]; fortunately, ESRD is quite rare, with a frequency <5% in most series (Table 1). The risk factors for developing CKD were evaluated in a study by Gallais et al., who identified age at onset, presence of diabetes and initial creatinine values as predicting factors of CKD [82]. While haemodialysis is usually considered to be a feasible renal replacement therapy in IRF patients, data on kidney transplantation in IRF are scarce; therefore, this option should be discussed on a case by case basis.
Urinary complications experienced by IRF patients during their disease course include infections, bleeding and lower urinary tract symptoms such as urgency, pollakiuria and discomfort, which are often facilitated by the presence of indwelling ureteral stents. When such symptoms are present, stent removal (followed by frequent urinary tract ultrasound to promptly detect relapsing hydronephrosis) should be attempted. In case of refractory ureteral obstruction, surgical ureterolysis should be considered.
CONCLUSIONS
IRF is a rare condition the most common complication of which is obstructive uropathy. Acute renal failure is a frequent presentation of the disease; CKD of varying severity may occur as a result of persistent ureteral obstruction. However, if adequately treated with interventional procedures (e.g. ureteral stenting) and medical therapy with GCs and immunosuppressants, renal prognosis is usually good and ESRD is rare. The association between IRF and other autoimmune or fibro-inflammatory disorders (often within the spectrum of IgG4-RD) is not uncommon; therefore, the disease should always be managed as a potentially systemic condition. Further studies are needed to better understand the pathogenesis of the disease and to define optimal treatment approaches.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- aorta
- stents
- mycophenolate mofetil
- immunosuppressive agents
- kidney failure, chronic
- asbestos
- chemokines
- renal failure, acute
- fibrosis
- glucocorticoids
- autoimmune diseases
- autoimmunity
- hydronephrosis
- retroperitoneal fibrosis
- surgical procedures, operative
- ureteral obstruction
- kidney
- ureter
- urinary tract obstruction
- rituximab
- inflammatory response
- infiltrates
- immunoglobulin g4
- ureterolysis
- medical management
- disease remission
- nephrologists





Comments