-
PDF
- Split View
-
Views
-
Cite
Cite
Rosa D Wouda, Liffert Vogt, Ewout J Hoorn, Personalizing potassium management in patients on haemodialysis, Nephrology Dialysis Transplantation, Volume 36, Issue 1, January 2021, Pages 13–18, https://doi.org/10.1093/ndt/gfaa213
Close - Share Icon Share
The regulation of potassium homoeostasis changes dramatically in patients with kidney failure who are treated by haemodialysis. With the kidney largely out of the equation, haemodialysis patients rely on potassium removal during each dialysis session to prevent hyperkalaemia. In addition, the gut becomes an important accessory organ for potassium excretion [1]. Despite these adaptations, hyperkalaemia (defined as serum potassium >5.5 mmol/L) remains a common electrolyte disorder occurring in approximately 14% of haemodialysis patients [2]. Although seemingly counterintuitive, a minority of haemodialysis patients is hypokalaemic (serum potassium <3.5 mmol/L), and this is usually related to poor dietary intake [3]. The target serum potassium in haemodialysis is unknown, but one study suggests that a serum potassium between 4.6 and 5.3 mmol/L is associated with the greatest survival [3]. Of note, in patients with chronic kidney disease (CKD), this optimal serum potassium range seems to be lower (4.0–4.5 mmol/L) [4]. Furthermore , when analysing serum potassium in haemodialysis patients, it is important to factor in when it was measured (after the long or short interdialytic interval, time of day and seasonality), and to analyse serial measurements to exclude transient hyperkalemia [2, 5]. Nephrologists can manage potassium balance in haemodialysis patients in three ways, including (i) by modifying the dialysate potassium concentration, (ii) by prescribing potassium binders and (iii) by modifying dietary potassium intake (Figure 1). The reason to implement such interventions is usually driven by recurring predialysis hyperkalaemia and the related risk of cardiac arrhythmia [6]. Although the prevention of acute complications is important, another relevant question is how these interventions affect long-term outcomes in haemodialysis patients. Unfortunately, there is a scarcity of randomized controlled trials in this area. Therefore, instead, we need to rely on registries, which often provide useful insights into how real-world management influences long-term outcomes. A good example of such a registry is the French Renal Epidemiology and Information Network (REIN). In this issue, Mercadal et al. [7] use this registry to analyse the effect of prescription patterns of dialysate potassium and potassium binders on survival in over 25 000 patients who started haemodialysis in 2010–13 and were followed until the end of 2014. Using Cox proportional hazard models, the investigators show that dialysis centres that used two or three dialysate potassium concentrations had a lower mortality risk than centres that only used one formula. In addition, patients who used the potassium binder sodium or calcium polystyrene sulphonate in a dose of 4–8 or ≥8 g/day had a lower mortality risk than patients who did not use potassium binders. Conversely, patients who used potassium binders in a dose <4 g/day had a higher mortality risk. Oral potassium supplements, which were used in 6% of patients, were not associated with a survival benefit. What does this study teach us on potassium management in haemodialysis patients and what are the implications for clinical practice?
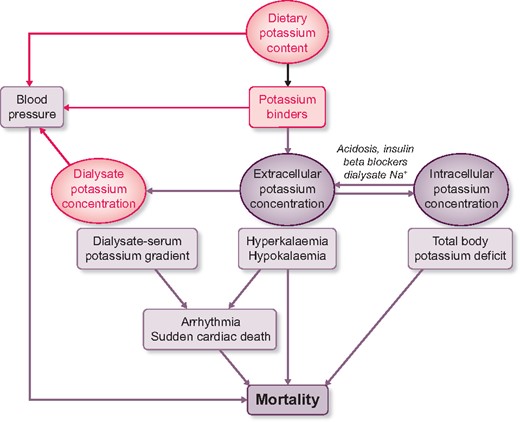
Schematic of potassium balance during haemodialysis and how it may affect mortality. The three possibilities for intervention—dietary potassium content, potassium binders and dialysate potassium—are shown in pink font. See text for further details.
DIALYSATE POTASSIUM
An unresolved issue is whether predialysis hyperkalaemia or a high dialysate–serum potassium gradient is the most important risk factor for adverse outcomes in haemodialysis patients (Figure 1) [5, 8]. The most commonly used dialysate potassium concentration varies widely per country with some countries using predominantly 1–2 mmol/L and other countries using 3–4 mmol/L [9]. Pun and Middleton [10] reviewed the nine retrospective studies that analysed the association between the dialysate potassium concentration and outcome, which usually was sudden cardiac death or all-cause mortality. Although five studies identified an increased risk of adverse outcomes with lower dialysate potassium (defined as <2 or <3 mmol/L), two studies found no association, and two studies found associations that favoured lower dialysate potassium [10]. Using sales data, Mercadal et al. [7] show that over time a lower dialysate potassium concentration was being used less frequently. For example, the percentage of centres using <2 mmol/L decreased from 57% to 49%, whereas the use of 3 mmol/L or ≥4 mmol/L increased from 89% to 91% and from 3% to 13%, respectively. This trend seems to echo the recent literature in which most studies linked a low dialysate potassium to an increased risk of adverse outcomes [10]. However, this was not a universal finding, because Mercadal et al. [7] also showed that the occasional use of dialysate potassium <2 mmol/L was associated with lower mortality compared with no use. This heterogeneity seems to suggest that it is impossible to select one ideal dialysate potassium concentration, and that the dialysate potassium prescription needs to be personalized, as proposed by others previously [11]. The data by Mercadal et al. [7] confirm this impression, because centres that used more dialysate potassium concentrations had lower mortality rates. A limitation of the study by Mercadal et al. was that serum potassium measurements were not included, and that therefore analysis of the dialysate–serum potassium gradient was not possible. That aside, Mercadal et al. and others have called to change the practice of ‘the regrettable routine use of a single dialysate potassium concentration’ [3, 7]. Equally regrettable is the fact that the evidence for a positive effect of a more personalized approach has not yet reached clinical practice. Redaelli et al. [12] performed a randomized cross-over trial to compare a fixed dialysate potassium concentration with a strategy during which the dialysate potassium concentration was adjusted to obtain a constant dialysate–serum potassium gradient. The latter approach reduced the arrhythmogenic effect of a fixed dialysate potassium concentration. It is important to emphasize that not only low dialysate potassium but also low calcium and magnesium contribute to this arrhythmogenic effect [10, 13]. In addition to the arrhythmogenic effect, a lower dialysate potassium concentration may also affect blood pressure during and after dialysis (Figure 1). Gabutti et al. [14] showed that the risk of intra-dialysis hypotension was inversely correlated to the potassium concentration in the dialysate. Conversely, dialysate potassium concentrations of 1 and 2 mmol/L produce ‘rebound hypertension’ 1 h after dialysis, a phenomenon that was not observed with a dialysate potassium concentration of 3 mmol/L [15]. In summary, the inclination to lower the dialysate potassium concentration in haemodialysis patients with recurring hyperkalaemia may in itself be harmful by imposing a higher dialysate–serum potassium gradient. In this era of artificial intelligence, it must be feasible to design more individualized dialysate potassium prescriptions that receive feedback from serial measurements of serum potassium. Indeed, mathematical modelling of potassium profiling has been proposed [16] and may benefit from emerging technologies using in-line monitoring of potassium with optical ion-selective microsensors [17]. Because less potassium is removed with a more constant dialysate–serum potassium gradient, this also implies that potassium management in haemodialysis patients should not solely rely on the dialysate potassium concentration.
POTASSIUM BINDERS
Potassium binders reduce serum potassium because they exchange potassium for sodium or calcium in the gastrointestinal tract and thereby limit potassium absorption (Figure 1). In the study by Mercadal et al. [7], 37% of patients used potassium binders at the start of the observation period, although this decreased over time. Another French registry study observed a much higher potassium binder prescription rate of 61% [2]. Of note, prescription may differ from actual use, as some potassium binders are poorly palatable thereby reducing adherence. In both French registries, patients were usually prescribed the potassium binder sodium polystyrene sulphonate [2, 7]. In a small randomized clinical trial in patients with CKD, sodium polystyrene sulphonate was superior to placebo and lowered serum potassium by approximately 1 mmol/L [18]. A concern regarding the use of sodium polystyrene sulphonate, however, is that it can cause colonic necrosis as a rare side-effect [19]. The gastrointestinal side-effects of sodium polystyrene sulphonate were recently studied more systematically in a population-based study from Canada and a CKD-based study from Sweden that also included patients treated with haemodialysis [20, 21]. Both studies showed that the use of sodium polystyrene sulphonate was associated with a higher risk of gastrointestinal complications, including intestinal ischaemia or thrombosis, ulceration or perforation, and resection or ostomy [20, 21]. Although the incidence rate for these serious complications was still low, both studies provided a clear signal for caution [20, 21]. In the previous 5 years, two novel potassium binders have been introduced in nephrology and cardiology, including patiromer and sodium zirconium cyclosilicate. Both potassium binders have recently also been studied in haemodialysis patients. A retrospective cohort study showed that patiromer effectively reduced serum potassium in haemodialysis patients, with an average decrease in serum potassium of 0.5 mmol/L [22]. In a randomized, double-blind and placebo-controlled clinical trial, sodium zirconium cyclosilicate reduced the incidence of predialysis hyperkalaemia [23]. More specifically, 41% of patients had a predialysis serum potassium of 4.0–5.0 mmol/L during at least three of four haemodialysis treatments after the long interdialytic interval [23]. Although the use of patiromer and sodium zirconium cyclosilicate appears to be safe in clinical trial settings, post-marketing surveillance should monitor for gastrointestinal side-effects. Another consideration for all potassium binders is that they will increase absorption of the electrolyte for which potassium is exchanged (sodium or calcium), which could potentially contribute to sodium overload or vascular calcification [24]. Thus, both the older and newer potassium binders may help to manage hyperkalaemia in haemodialysis patients, but the question is how this affects long-term outcomes. A paradoxical finding in the study by Mercadal et al. [7] was that prescription of higher doses of potassium binders was associated with lower mortality, whereas lower dosing was associated with higher mortality. The authors acknowledge that these associations are likely explained by factors other than potassium control. An important alternative explanation could be that patients receiving higher doses of potassium binders more often consume a potassium-rich diet, which in itself is associated with better survival [25]. Another interesting observation by another study was that patiromer reduced blood pressure in patients with CKD, hyperkalaemia and the use of renin–angiotensin system inhibitors [26]. Although this study requires confirmation, an antihypertensive effect of potassium binders might be explained by lowering of plasma aldosterone [26]. If this is a dose-dependent class effect of potassium binders, this could also add to the explanation of why higher doses of potassium binders associate with lower mortality (Figure 1).
DIETARY POTASSIUM INTAKE
Rather than binding potassium after dietary intake, a more direct strategy against hyperkalaemia could be to prescribe a low potassium diet (Figure 1). In patients with a tendency to develop hyperkalaemia, a dietary potassium intake of <3 g/day (<77 mmol/day) is recommended [27]. Of note, the general population and patients with CKD already consume a relatively low potassium diet [25, 28, 29]. A recent systematic review and meta-analysis compared the effects of lower and higher dietary potassium intake in patients with CKD (including patients treated with dialysis) on serum potassium and mortality [30]. This review found that a potassium-restricted diet (1295 mg/day) lowered serum potassium by 0.22 mmol/L compared with an unrestricted—but still low potassium—diet (1570 mg/day). Furthermore, a low potassium diet (1670 mg/day) was associated with a 40% reduction in mortality hazard compared with higher dietary potassium intake (4414 mg/day). However, the evidence to support these effects was classified as very low-quality evidence [30]. A small randomized controlled trial analysed the effect of dietary potassium restriction for 2 years on nerve function in patients with CKD Stage G3 or G4 [31]. The intervention caused modest but significant reductions in dietary potassium intake (3272 versus 3874 g/day) and serum potassium (4.6 versus 4.8 mmol/L), which was sufficient to improve the total neuropathy score (the primary outcome). This study illustrates that dietary potassium restriction may have benefits beyond lowering serum potassium, although the study had several limitations [31]. At the same time, emerging evidence indicates that higher dietary potassium intake may be beneficial, even for patients with CKD [28]. To date, 11 cohort studies analysed the association between urinary potassium excretion (as a proxy for dietary intake) and kidney outcomes in patients with CKD [32]. Although the majority of these studies showed that a higher urinary potassium excretion was associated with a lower risk of adverse kidney outcomes or mortality, this was not a consistent finding. To address the possibility of a causal link between dietary potassium depletion and kidney outcomes, we are currently conducting a randomized, double-blind and placebo-controlled trial with potassium supplementation in patients with CKD [29]. Higher dietary potassium intake has been shown to reduce blood pressure and the risk of stroke in subjects without CKD and prevents kidney damage in experimental models of CKD [28]. Because a high potassium diet usually consists of fruits and vegetables, potassium-independent factors such as low animal protein, high-fiber content and an alkaline diet may also play a role. Indeed, a randomized clinical trial showed that both alkali treatment and fruits and vegetables can reduce kidney injury in patients with CKD [33]. To take advantage of these effects, the need for clinical trials comparing a potassium-restricted diet with the combination of a potassium-liberalized diet and potassium binders in patients treated with haemodialysis has recently been emphasized [34].
TOTAL BODY POTASSIUM
This editorial so far has focused on the effects of potassium interventions on the predialysis serum potassium concentration and the dialysate–serum potassium gradient. However, potassium is primarily an intracellular cation, where it plays a crucial role in cellular function. Therefore, it is also important to consider how interventions change intracellular potassium concentration and total body potassium, and how this relates to outcomes (Figure 1). Measurement of intracellular electrolyte concentrations and whole-body counting of naturally radioactive potassium can be used to provide insight into the ‘black box’ of the intracellular compartment. Unexpectedly, such measurements reveal that patients with CKD or patients on haemodialysis often have a total body potassium deficit, despite their tendency to develop hyperkalaemia (Figure 2) [35, 36]. For example, the intracellular potassium concentration and the resting membrane potential were lower in muscle cells of patients with CKD compared with healthy subjects [37]. The initiation of haemodialysis normalized intracellular potassium, but did not improve the resting membrane potential. Similarly, total body potassium is up to 10% lower in haemodialysis patients compared with control subjects [35, 36] (Figure 2). When patients transit from predialysis care to haemodialysis, total body potassium was restored in some patients, whereas in others it decreased [38]. Preliminary data suggest that total body potassium depletion is also associated with increased mortality (Figure 1) [35]. Because more than 60% of potassium removal derives from the intracellular compartment [10], dialysis may contribute to reducing total body potassium. In this regard, the combination of a low potassium diet and a low dialysate potassium concentration may exhaust intracellular potassium stores. Of note, hyperkalaemia and total body potassium deficiency may co-exist if factors prevent the entry of potassium into cells. Such factors are not uncommon in haemodialysis patients and include metabolic acidosis, insulin resistance and the use of beta-blockers (Figure 1). Of interest, a high dialysate sodium also contributes to the interdialytic increase in serum potassium because hypertonicity causes a shift of potassium out of cells [39]. Conversely, one could postulate that a higher dialysate potassium may improve sodium removal during haemodialysis and contributes to better blood pressure control. To this end, we are currently conducting a cross-over study to compare the effects of a dialysate potassium of 4.0 mmol/L with 2.0 mmol/L on blood pressure, volume and intracellular sodium and potassium.
![Comparison of total body potassium (TBK, ratio of measured versus expected normal) between haemodialysis patients and controls (patients with essential hypertension). Data are based on Williams et al. [36]. Measured TBK in controls and haemodialysis patients were 3390 and 2810 mmol, respectively. Time on dialysis varied from <1 to >5 years. TBK was measured before dialysis. Values are mean ± standard error.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/36/1/10.1093_ndt_gfaa213/1/m_gfaa213f2.jpeg?Expires=1747851060&Signature=VbYcyQdNPQNmnisxvfASVRcCkdIXe1uFcPWPdPA-TneDVOiYyQSKnBiF143D7pRwJVx3QlpCE5JHnVKT8sDgXCYQqN4AlhwyDVXTRlEV8q4O5lqix9lu69zYO0wT6jb9n~OX9Z7Ien2~hRxzNPRgGBbund1HplhJfsbEtteXpzp7DsMaVtu2yBIEl2ZfdN5lH6G-nFZSiyiJCJ64ORpZaVITc-NQ3QN1MyxeTKZMHJx~xSzUXm~np7TrVPgrXP~ljKsP-Gp--dpkOec3G-BwXyiDhaSy88VsmaDg1a8rBTbJl~L8kP6n9VPJA5g~hFJiOlazs-GDaDV3qPP0uhyB1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Comparison of total body potassium (TBK, ratio of measured versus expected normal) between haemodialysis patients and controls (patients with essential hypertension). Data are based on Williams et al. [36]. Measured TBK in controls and haemodialysis patients were 3390 and 2810 mmol, respectively. Time on dialysis varied from <1 to >5 years. TBK was measured before dialysis. Values are mean ± standard error.
In conclusion, to improve potassium management and potentially long-term outcomes in haemodialysis, we believe it is important to integrate all factors that determine potassium balance and apply a personalized approach that is dynamic and relies on more frequent serum potassium measurements and ideally also on total body potassium.
FUNDING
The authors are supported by a consortium grant from the Dutch Kidney Foundation (CP16.01).
CONFLICT OF INTEREST STATEMENT
None declared.
(See related article by Mercadal et al. Prescription patterns of dialysate potassium and potassium binders and survival on haemodialysis—the French Renal Epidemiology and Information Network registry. Nephrol Dial Transplant 2021; 36: 151--159)





Comments