-
PDF
- Split View
-
Views
-
Cite
Cite
Anthony M Provenzano, Matthew A Sparks, The renin–angiotensin–aldosterone system update: full-court press, Nephrology Dialysis Transplantation, Volume 35, Issue 9, September 2020, Pages 1488–1490, https://doi.org/10.1093/ndt/gfz123
Close - Share Icon Share
Cardiovascular disease, blood pressure and the kidneys are inextricably linked by the renin–angiotensin–aldosterone system (RAAS). The RAAS plays a dominant role in the maintenance of basal blood pressure and the pathogenesis of hypertension, and exerts powerful effects on kidney function [1]. The last 50 years could be dubbed the RAAS years, as manipulation of this system with pharmacologic inhibition has been the mainstay of therapy for a wide variety of diseases including hypertension, kidney disease, diabetes and heart disease. We review recent advances in how the RAAS is being used in a variety of ways in these disease states. We discuss the fate of dual RAAS blockade in diabetic kidney disease, neprilysin inhibition in heart failure (HF), the use of angiotensin (Ang) II in septic shock, Ang II vaccination for hypertension, the potential use of biased agonism of the type 1 Ang receptor and the renewed interest in blocking aldosterone in hypertension (Figure 1).
The biologic exploitation of the RAAS in human disease started after the isolation of the first angiotensin-converting enzyme inhibitor (ACEi) from the venom of the Brazilian pit viper Bothrops jararaca [2]. The next decade saw numerous randomized clinical trials utilizing RAAS blockade, demonstrating efficacy in patients with hypertension, HF and diabetic kidney disease. This also ushered the advent of a variety of pharmacologic inhibitors of the RAAS including the direct renin inhibitors and the angiotensin receptor blockers (ARBs), which demonstrated similar efficacy to ACEis but with diminished side effects including cough and angioedema. Since ACEis and ARBs have different targets, it was tempting to use these together for a potential synergistic effect. However, large clinical trials utilizing dual RAAS blockade in patients with diabetic nephropathy did not demonstrate an overwhelming kidney and cardiovascular benefit. In fact, they resulted in more adverse events such as hyperkalemia. Thus, the use of dual RAAS blockade is not a current strategy used in proteinuric kidney disease [3, 4].
The RAAS plays an important role in the pathophysiology of HF with reduced ejection fraction (HFrEF) [5]. Several randomized clinical trials have demonstrated efficacy of RAAS inhibition to improve mortality and morbidity [5]. Recently, novel therapies have shown promise in patients with HFrEF. Neprilysin, an enzyme that degrades natriuretic peptides and Ang II, both of which are upregulated in patients with HF, has emerged as a key target. In nonpathologic states, natriuretic peptides increase in response to RAAS activation and counteract its downstream effects [6]. However, in HF, natriuretic peptides are dysregulated with concomitant RAAS overactivation. A key component of this system is neprilysin which is an endopeptidase that breaks down biologically active natriuretic peptides. Multiple trials have attempted to study the clinical significance of blocking neprilysin, the most significant of which is the angiotensin–neprilysin inhibition versus enalapril in HF [Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF)] trial [7]. This randomized clinical trial compared the combined neprilysin inhibitor/ARB (sacubatril/valsartan) with ACEi (enalapril). The primary endpoint was a composite of cardiovascular death or HF hospitalization [7]. The study was terminated early due to the benefit of the neprilysin inhibitor/ARB combination, providing yet another example of the importance of regulation of the RAAS and indicating that there are other targets within this system that can be of pharmacologic benefit.
Vasopressors are a mainstay of therapy for patients with circulatory collapse. Evolutionarily, this is the entire reason for the existence of the RAAS. Thus, it is logical that Ang II itself could be a therapeutic option in patients with low blood pressure and septic shock. Circulatory collapse syndromes related to peripheral/distributive shock are some of the most difficult to treat, with very high mortality especially when acute kidney injury (AKI) is present and renal replacement therapy (RRT) is required. Is it possible that Ang II could have beneficial effects on kidney function during septic shock? The mechanism for such an effect would be through efferent arteriolar vasoconstriction leading to increased intraglomerular pressure and preservation of the filtration pressure. The Angiotensin II for the Treatment of High-Output Shock (ATHOS) and ATHOS-3 trials sought to answer this question [8, 9]. The larger of these two studies was ATHOS-3, which looked at 344 patients and randomized them to receive either Ang II or placebo on a background of standard care. The primary endpoint was an increase in mean arterial pressure (MAP) by 10 mmHg after 3 h on the infusion or to a MAP of 75 mmHg. This was greater in the Ang II group, as was the cardiovascular Sequential Organ Failure Assessment (SOFA) score, but not the total SOFA score [9, 10]. Multiple limitations were noted in the trial including the lack of fluid balance, lactate and peripheral capillary oxygen saturation (SpO2) comparisons between the groups. A post hoc analysis of patients with AKI requiring RRT at study drug initiation in the ATHOS-3 trial suggested greater RRT liberation in the Ang II group [11]. Therefore, this suggests that Ang II could become a potential pressor choice in patients with septic shock. More studies are needed to determine its efficacy in improving overall survival and benefit in kidney outcomes.
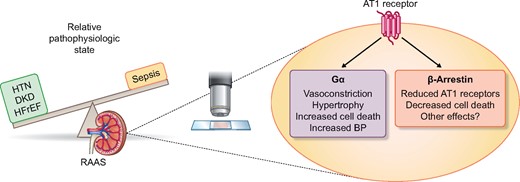
The RAAS is being used in a variety of ways from blocking its effect in states of relative excess in hypertension (HTN), diabetic kidney disease (DKD), and heart failure with reduced ejection fraction (HFrEF) to supplementing with exogenous angiotensin II during shock and sepsis. Progress is also advancing on how intracellular signaling can be manipulated by utilizing biased agonist of the type 1 angiotensin receptor.
Given the systemic effects of RAAS on multiple disease states, global and permanent reduction in the form of a vaccine to temper the effect of the RAAS may be beneficial. Another potential benefit would be negating the need for daily oral lifetime therapy, thus improving medication compliance and potentially diminishing healthcare costs [12]. This strategy has been employed in a variety of animal models of hypertension [12]. Short-term human studies have also shown that vaccines that affect RAAS components can result in diminished blood pressure in patients with hypertension. However, the long-term effects are not yet known, and importantly, the physiologic need for RAAS activation in states of volume depletion do necessitate caution in the broad application of this strategy.
There have also been attempts to modulate the type 1 Ang (AT1) receptor signaling pathway. The AT1 receptor signals as a prototypical G-protein-coupled receptor (GPCR) and mobilizes either the Gi/o or G12/13 G-protein families. Recently, just as in other GPCRs, it has been shown that the AT1 receptor can signal through a G-independent β-arrestin pathway. Many of the downstream signaling pathways from β-arrestin agonism are thought to be beneficial, whereas G-protein stimulation is maladaptive. β-arrestin signaling allows for receptor desensitization and internalization and leads to independent signaling through the extracellular signal-regulated kinase pathway [13]. Attempts to block the G-protein and yet stimulate the β-arrestin pathway simultaneously (termed biased agonism) are currently being investigated as novel therapeutic targets in a wide range of cardiovascular diseases.
The last 5 years have seen a renewed interest in aldosterone and the mineralocorticoid receptor as a therapeutic target in hypertension. The PATHWAY-2 trial demonstrated that spironolactone was more efficacious in terms of blood pressure lowering in patients with resistant hypertension compared with bisoprolol or doxazosin [14], thus demonstrating that RAAS inhibition even in resistant hypertension is efficacious.
The RAAS remains an important physiologic target for both further study and therapies. From its history and through its more recent advances, the RAAS remains an important area of investigation to help improve the burden of not only hypertension but also hypotension, HF, fibrosis and inflammation. Recently, the Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy trial demonstrated impressive kidney and cardiovascular effects of sodium-glucose cotransporter-2 (SGLT2) inhibitors in patients with diabetic kidney disease. These patients were also on concomitant RAAS inhibitors [15]. As SGLT2 inhibitors have known effects on the afferent arteriole and RAAS inhibitors the efferent arteriole, it is possible that the combination has a synergistic effect. Even though research on the RAAS has been intensely investigated over the last half a century, there continue to be new discoveries and progress made.
CONFLICT OF INTEREST STATEMENT
None declared.





Comments