-
PDF
- Split View
-
Views
-
Cite
Cite
Wim Van Biesen, Norbert Lameire, Increasing peritoneal dialysis initiation worldwide: ‘there are none so blind as those who will not see’, Nephrology Dialysis Transplantation, Volume 35, Issue 9, September 2020, Pages 1458–1461, https://doi.org/10.1093/ndt/gfaa024
Close - Share Icon Share
There is increasing concern that the uptake of peritoneal dialysis (PD) is decreasing, especially in Europe, where there is also substantial variation in PD prevalence and occurrence [1]. In an interesting contribution to this issue of Nephrology Dialysis Transplantation, Boyer et al. [2] report how the introduction of an assisted PD programme positively influenced the uptake of PD and counterbalanced the decline in its occurrence over the last decade due to a variety of reasons. Of the patients included, ~20% died during the observation period and a comparable number changed to in-centre haemodialysis (HD), leaving two of three patients on PD. Half of those remaining on PD became independent in their PD-related care.
The mean age and number of comorbidities of the patient population with end-stage kidney disease have increased over recent decades. Therefore, at first glance, home-based therapies may not seem to be a straightforward option for such patients and many physicians do not offer PD in this setting [3]. However, their frailty also means that patients in this group might benefit most from spending more time in their home environment, avoiding the burden of thrice-weekly transfer to a dialysis unit and post-dialysis hangover. Thus, assisted PD might also be useful to increase PD use in frail and comorbid patients [4]. Some groups advocate the use of (assisted) PD as a kind of transition between full and conservative care, allowing treatment to be tailored to the specific needs of the patient at specific moments in time, and the focus to be on quality of life rather than biochemical surrogates [5, 6]. Most of the obstacles to home-based treatment for this frail population can be readily overcome by providing assistance for the performance of PD. Hence the concept of assisted PD makes sense.
Boyer et al. [2] conclude that implementation of an assisted PD programme might be a way to tackle the declining uptake of PD in Europe. Whereas this statement is generally likely to be true, some unanswered questions remain. In their study, positive change was most pronounced in the years immediately after the start of the programme [2], suggesting that a pre-existing pool of potential assisted PD candidates was drained. Thus the real long-term impact might be smaller than that observed. Assisted PD can potentially prolong the success of PD as a technique, as demonstrated by the fact that two of three patients remained on PD. Other reports have also observed that increasing the occurrence of PD does not jeopardize the technique’s success and contributes to greater PD use [7–9]. However, as overall survival is lower in this patient group, often accompanied by extensive comorbidity and frailty, the impact of assisted PD on PD prevalence might be less than hoped for.
Other important questions about the impact of assisted PD on PD occurrence and prevalence include: (i) can the success of this initiative be generalized to other settings, (ii) is assisted PD a panacea to increase PD use and (iii) is it really relevant and necessary to augment the incidence or prevalence of PD?
There are several reasons why the findings of Boyer et al. [2] might not be generalizable to other settings. The initiative to increase the occurrence of PD by creating a programme for assisted PD was set in a single centre, where a strong focus on PD already existed and PD prevalence was already above the average of most regions in Europe. Patient uptake of assisted PD varies between centres, even when it is universally available [4]. It is likely that the reasons that make overall PD prevalence lower in some centres are exactly the same as those that explain why some centres would not be willing to or would not be successful in implementing an assisted PD programme [10]. The organization of healthcare in the UK and the associated reimbursement system might also have an impact [11, 12]. Public, not-for-profit centres might find it easier to rearrange their budgets so that assisted PD programmes could be supported in a structured way. In France, sufficient reimbursement has been foreseen for (private) district nurses, avoiding financial disincentives for assisted PD. In settings where reimbursement by the healthcare payer is insufficient for assisted PD, it would be difficult to implement it, as neither nephrologists, the dialysis unit nor the patients would want to suffer financial penalties.
Assisted care can be organized in different ways [6] regarding the type of modality supported [ambulatory PD or continuous ambulatory PD (CAPD) or both], the type and extent of assistance provided (only logistical help, only nursing care and supervision, the actual connection of the patient to the bag or cycler or a combination of these) and the types of healthcare workers involved. In the study by Boyer et al. [2], assistance was only provided by healthcare assistants for practical issues, such as building up the cycler machine and transporting bags into the house, but not for the actual connection of the patient to the machine; in addition, there was no option for assisted CAPD. As such, this form of assisted PD would be unsuitable for patients with visual, dexterity or coordination problems. It also presumes that the patient is able to manage a cycler alone during the night. This setup might thus have a negative impact on the number of potentially eligible patients and greater gains could be expected in settings where more extensive assistance is provided. However, more extensive help regarding the type of healthcare worker and the amount of time involved would increase the cost of assisted PD, possibly up to the level of in-centre HD. The success of the technique might also be different in a population of patients unable to connect themselves, as comorbidities would likely be higher. The willingness of patients to accept assisted PD and of staff to perform it depends upon the balance between expected and provided service. This balance is partially influenced by reimbursement and a willingness to put patient comfort first.
The second question is even more relevant: is introducing assisted PD a sufficient measure to increase PD uptake? In the previous paragraph, we indicated that the willingness and capacity to implement assisted PD is probably linked to the willingness and capacity to perform PD in general [10]. Thus we need to explore the true reasons why PD uptake might remain below expected levels in Europe (Figure 1). During several recent focus groups, a EuroPD-led group of PD enthusiasts identified potential barriers to PD growth. More specifically, they identified the potential interconnections between these barriers, indicating that it is unlikely that a single intervention will be sufficient for change. Further discussion is ongoing and results will be presented during the International Society for Peritoneal Dialysis/EuroPD meeting in Glasgow in May 2020. It is hard to accept that these barriers are based on differences in medical outcomes, as most studies indicate that outcomes of patients on PD and HD are comparable, especially in the first few years after starting treatment [13]. Even differences in case mix cannot explain regional differences in PD uptake, as similar outcomes for patients on PD versus HD remain after stratification for the most important comorbidities [13]. Some have formulated a hypothesis that financial incentives might play a role [14]. For example, uptake of PD has surged in the USA after a change in the reimbursement system in favour of PD, but uptake still remains lower than expected, at ~10% [15]. In many other countries, such as Germany and Belgium, increasing reimbursement for PD did not result in increased uptake of PD [16]. Of note, to improve PD occurrence and prevalence, Kaiser Permanente started a multidisciplinary PD initiative that included patient and caregiver education, education and support tools for healthcare professionals, streamlined system-level processes, monitoring and continuous quality improvement [8]. As a result, PD occurrence increased from 15.2% in 2008 to 33.8% in 2018 where this initiative was in place, and contrasts with the rather small increase observed in the rest of the USA. While it can be debated whether the motivation for this initiative is driven by underlying financial motives, it does at least indicate that improving reimbursement per se is not sufficient to increase PD use. Willingness, a holistic vision and the right organizational structure are also needed, as are present in a large integrated healthcare system such as Kaiser Permanente. It is often claimed that PD is cheaper than HD; however, this statement is strongly dependent on the organization of care and logistics. Some countries, like Hong Kong and Taiwan, have introduced a PD-first policy, as in their settings it is a reasonable approach to ensure that renal replacement therapy is available to as many people who need it as possible. However, there is no clear relationship between the differing costs of HD versus PD and the prevalence of PD patients [12]. Thus, developing a PD-friendly ‘policy’ and creating ‘commitment’ seems to be more important than financial arrangements [17]. The observation that >80% of patients who initiated PD in the Kaiser Permanente programme were still on PD 1 year after initiation, with a stable mortality rate similar to that of patients on HD [8], fits reports from other groups who have successfully increased PD occurrence [2, 7, 9]. This undermines the excuse of many centres that they do not have the right patient mix and that forcing patients to undergo PD would result in a surge in technique failure [18].
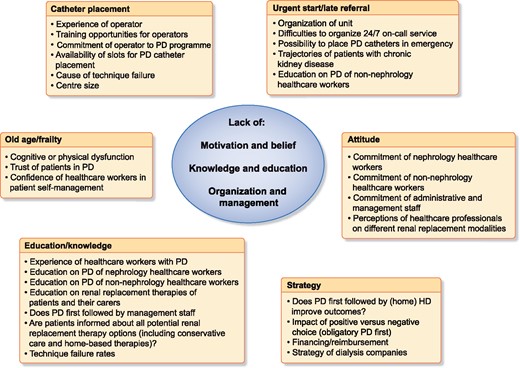
Barriers and obstacles to increasing PD use reported as unidimensional barriers, aggregated into categories, show underlying common hidden drivers for low PD use.
Other hurdles that pop up regularly are centre size, the problem of late referral and the management of peritoneal access. Smaller centres seem to have greater technique failure and lower PD occurrence rates. In the Initiative for Patient Outcomes in Dialysis-PD study, both centre size and the proportion of patients on PD/HD were associated with technique success [19]. This illustrates the point that next to experience, a commitment to PD is essential to successfully increase its uptake. Centre size might also be directly linked to the two other barriers. It is conceivable that in small centres, there is less expertise in managing PD catheter problems and that, as a consequence, there are fewer sufficiently skilled personnel to provide acute PD access for late referrals on a 24/7 basis. As for assisted PD, the creation of a dedicated programme for late referrals [20, 21] and the option of PD catheters being inserted at the bedside by a nephrologist [22] have both been demonstrated to increase PD occurrence and prevalence. However, as for assisted PD, the fact that some centres develop such urgent start programmes is a token of their commitment to offer PD to as many patients as possible, and it remains uncertain whether forcing centres to develop such programmes would actually be effective. In addition, the problems of late referral and catheter placement are indirectly linked, as described below.
Last but not least, is it reasonable for a ‘increase in PD uptake’ to be seen as a relevant goal or quality indicator by itself? In the interests of shared decision-making [23], what is truly important is that patients have free choice regarding their treatment modality. The first prerequisite for this is that all modalities are available and can be chosen. Thus it is necessary that centres offer PD in all its different formats. Expanding the range of available options, for example, by introducing assisted PD, will allow more people to choose the option that best fits their preferences, expectations and needs.
It is also essential that there is sufficient experience with all different modalities to ensure truly free choice. Thus the second prerequisite is that patients are ‘informed’ in an objective way about the different modalities available. A dedicated pre-dialysis or low-clearance clinic that works smoothly is essential to ensure that patients receive information in a structured, balanced way that helps them make the right decision [24]. There is evidence that the quality of educational material and assistance provided for decision-making is often suboptimal [25], and most surveys have revealed that a substantial proportion of patients do not remember having been informed about the different options available [26]. Some interesting initiatives for improvement have been undertaken [27]. Healthcare workers themselves need to be confident that they can manage different therapies for individual patients. Such confidence most likely depends upon adequate formal training and education [28] on the one hand and clinical experience on the other. For many healthcare workers, these prerequisites are fulfilled in a suboptimal way [28]. Training in PD is, for most trainees in nephrology, limited in scope and time, which makes them less confident regarding the management of more complex patients [29] and increases the tendency to solve PD-related problems by transferring patients to HD. In small centres, exposure to PD patients is low, so there is limited opportunity to increase one’s own experience or ask advice from someone else. In this way, a vicious downward spiral is created. Collaboration protocols between centres might be a good solution here, but this might prove difficult in a setting of privatized healthcare.
In conclusion, the decreasing trend of PD in Europe is of concern, not because of PD per se, but because it is an indicator of two worrying underlying factors. First is the fact that patients still do not have true free choice regarding renal replacement therapy that suits their preferences and expectations and which they can fit around their lives. Second, some healthcare organizations and nephrology units remain reluctant to provide truly patient-centred care. A culture of placing patient preference and quality of life first should be fostered if we truly want to improve patient care. In centres with such a culture, additional modalities such as assisted PD can open up the options for patients, even when they have serious comorbidities. In centres that only pay lip service to such patient-centred care, it is unlikely that assisted PD programmes will thrive. Programmes promoting home-based therapies can only be successful if they are accompanied by a true shift in mentality. Some of the measures suggested in this editorial are neither difficult nor costly to implement, provided one is willing to seek out and address the true underlying barriers.
CONFLICT OF INTEREST STATEMENT
W.V.B. reports grants from Fresenius Medical Care, Baxter Healthcare and Nippro during the conduct of the study.
(See related article by Boyer et al. Impact of the implementation of an assisted peritoneal dialysis service on peritoneal dialysis initiation. Nephrol Dial Transplant 2020; 35: 1595--1601)
REFERENCES
ERA-EDTA Registry Annual Report 2017. Amsterdam: Department of Medical Informatics, Amsterdam University Medical Center,
Boyer A, Solis-Trapala I, Tabinor M et al.





Comments