-
PDF
- Split View
-
Views
-
Cite
Cite
Manuela Yepes-Calderón, Camilo G Sotomayor, Rijk O B Gans, Stefan P Berger, Henri G D Leuvenink, Dimitrios Tsikas, Ramón Rodrigo, Gerjan J Navis, Stephan J L Bakker, Post-transplantation plasma malondialdehyde is associated with cardiovascular mortality in renal transplant recipients: a prospective cohort study, Nephrology Dialysis Transplantation, Volume 35, Issue 3, March 2020, Pages 512–519, https://doi.org/10.1093/ndt/gfz288
Close - Share Icon Share
Abstract
In renal transplant recipients (RTRs), cardiovascular mortality is the most common cause of long-term renal graft loss. Oxidative stress (OS) has been associated with cardiovascular disease and is known to be enhanced in RTRs. We aimed to prospectively investigate whether the concentration of the OS biomarker malondialdehyde (MDA) is associated with long-term risk of cardiovascular mortality in a large cohort of RTRs.
The plasma MDA concentration was measured using the thiobarbituric acid reaction assay in 604 extensively phenotyped RTRs with a functioning allograft for ≥1 year. The association between MDA and cardiovascular mortality was assessed using Cox proportional hazard regression analyses in the overall cohort and within subgroups according to significant effect modifiers.
Median circulating MDA concentration at baseline was 5.38 [interquartile range (IQR) 4.31–6.45] μmol/L. During a follow-up period of 6.4 (IQR 5.6–6.8) years, 110 (18%) RTRs died, with 40% of deaths due to cardiovascular causes. MDA concentration was significantly associated with the risk for cardiovascular mortality {hazard ratio [HR] 1.31 [95% confidence interval (CI) 1.03–1.67] per 1-SD increment}, independent of adjustment for potential confounders, including renal function, immunosuppressive therapy, smoking status and blood pressure. The association between MDA concentration and the risk for cardiovascular mortality was stronger in RTRs with relatively lower plasma ascorbic acid concentrations [≤42.5 µmol/L; HR 1.79 (95% CI 1.30–2.48) per 1-SD increment] or relatively lower estimated glomerular filtration rates [≤45 mL/min/1.73 m2; HR 2.09 (95% CI 1.45–3.00) per 1-SD increment].
Circulating MDA concentration is independently associated with long-term risk for cardiovascular mortality, particularly in RTRs with relatively lower ascorbic acid concentrations or renal function. Further studies are warranted to elucidate whether OS-targeted interventions could decrease cardiovascular mortality in RTRs.
INTRODUCTION
Although renal transplantation is the best available treatment for end-stage renal disease [1, 2], improving long-term survival in renal transplant recipients (RTRs) has proven to be challenging [3]. The incidence of cardiovascular events among RTRs is at least twice that in the general population [4] and cardiovascular disease accounts for 35–50% of deaths [4], thus playing a leading role [5–7] among the main causes of premature death in RTRs.
Beyond traditional cardiovascular risk factors, attention has been focused on complex pathophysiological processes in relation to chronic renal malfunction [8], including oxidative stress (OS). OS corresponds to an imbalance between pro-oxidant species and the antioxidant defence systems, whereby the latter becomes overwhelmed. OS is increased in end-stage renal disease and further exacerbated by renal replacement therapies [9]. It has been associated with atherosclerosis, left ventricular hypertrophy and cardiorenal syndrome [8]. Several mechanisms have been proposed for the association between OS and atherosclerosis: (i) increased OS promotes enzymatic modification of circulating lipids and lipoproteins [10]; (ii) reactive oxygen species (ROS) can directly cause and enhance endothelial dysfunction [11]; (iii) OS is also known to stimulate the immune system, which in turn promotes a chronic pro-inflammatory status and (iv) OS promotes osteoblastic differentiation of vascular cells [12]. However, to date, there are no prospective studies investigating a plausible association between post-transplant OS and the risk for cardiovascular mortality in RTRs. Plasma malondialdehyde (MDA) is the principal and best investigated product of lipid peroxidation, one of the main reactions caused by enhanced OS [13], and has been widely used as an OS biomarker in several clinical settings, including in the study of kidney disease patients [14–16].
In the current study, we aimed to test prospectively the hypothesis that plasma MDA concentration is associated with the long-term risk for cardiovascular mortality in RTRs. In addition, we aimed to evaluate whether a putative association between plasma MDA concentration and the risk for cardiovascular mortality in RTRs may be modified by traditional cardiovascular risk factors and oxidative status-associated biomarkers.
MATERIALS AND METHODS
Study design
In this prospective cohort study, all adult RTRs with a functioning allograft of ≥1 year and without known or apparent systemic illnesses who visited the outpatient clinic of the University Medical Center of Groningen (The Netherlands) were suitable for inclusion. Between August 2001 and July 2003, 847 RTRs were invited for enrolment in the study, of whom 606 (72%) provided informed written consent. We excluded patients lacking plasma MDA measurements (n = 2), thus resulting in 604 RTRs, whose data are presented here. This study was approved by the institutional review board (METc 2001/039) and in accordance with the Declarations of Helsinki and Istanbul.
The endpoint of the study was cardiovascular morality. A continuous surveillance system as part of the outpatient programme and close collaboration with affiliated hospitals ensured up-to-date information on patient status. We contacted general practitioners or referring nephrologists in cases where the patient status was unknown. There was no loss to follow-up.
Patients characteristics
Relevant donor, recipient and transplant characteristics were extracted from the Groningen Renal Transplant Database, which has been previously described in detail [16]. Data on steroid dosages, use of other immunosuppressive medications and incidence of acute rejection were retrieved from individual patient files.
Measurements and definitions
Anthropometric measurements were taken with participants wearing indoor clothing, with no shoes on. Waist circumference was measured on bare skin midway between the iliac crest and the tenth rib and blood pressure was measured as the average of three measurements taken at 1-min intervals after a 6-min rest in the supine position using an automated device (Omron M4, Omron Europe, Hoofddorp, The Netherlands).
According to a strict protocol, all RTRs collected a 24-h urine sample during the day before to their visit to the outpatient clinic and all patients provided a blood sample in the morning after an 8- to 12-h overnight fasting period, which included no medication intake. Plasma MDA is considered a preferred biomarker of OS since lipids are the main target of OS and MDA is the principal and best-investigated product of lipid peroxidation [13, 17]. Also, it has been widely used as a marker of OS in several pathological states, including kidney disease [14–16]. MDA concentration was determined using the following method. Thiobarbituric acid was used to bind MDA, forming Thiobarbituric acid reaction species (TBARS), which were then extracted in a butanol layer and measured using a fluorescence spectrophotometer at 485/590 nm (FL600, Beun de Ronde, Abcoude, The Netherlands). This method has a reported intra-assay coefficient of variation of 6.0% [18]. Serum creatinine concentrations were determined by the Jaffé method (MEGA AU510, Merck Diagnostica, Darmstadt, Germany) and ascorbic acid concentrations were measured by reverse-phase liquid chromatography with fluorescence detection, as previously described in detail [19].
Estimated glomerular filtration rate (eGFR) was calculated by applying the Chronic Kidney Disease Epidemiology Collaboration equation [20] and the cumulative dose of prednisolone was calculated as the sum of the maintenance dose of prednisolone until study inclusion and the dose of prednisolone or methylprednisolone required for treatment of acute rejection (a conversion factor of 1.25 was used to convert the dose of methylprednisolone to that of prednisolone).
Proteinuria was defined as urinary protein excretion of >0.5 g in 24 h. Cardiovascular disease history was considered positive if participants had a myocardial infarction, transient ischaemic attack or cerebrovascular accident.
The cause of death was obtained by linking the number of the death certificate to the primary cause of death as coded by a physician from the Central Bureau of Statistics according to the International Classification of Diseases, Ninth Revision (ICD-9) [21]. Cardiovascular death was defined as the principal cause of death being cardiovascular in nature (ICD-9 codes 410–447).
Statistical analysis
Data were analysed using SPSS software version 23.0 (IBM, Armonk, NY, USA), Stata 14.1 (StataCorp, College Station, TX, USA) and R version 3.2.3 (R Foundation, Vienna, Austria). In all analyses, a two-sided P-value <0.05 was considered significant, except for the effect modification analyses, where the significance level was P < 0.10 [22]. Continuous variables were summarized using the mean ± standard deviation (SD) for normally distributed data, median [interquartile range (IQR)] for skewed distributed variables and percentages for categorical variables. In order to determine in an integrated manner which baseline variables were independently associated with circulating MDA concentration, we performed crude linear regression analyses and then adjusted for age and sex and finally for eGFR. The stronger determinants of plasma MDA were selected by stepwise backward multivariable linear regression analysis. For inclusion and exclusion, P-values were set at 0.20 and 0.05, respectively.
To analyse whether plasma MDA concentration was independently associated with the risk for cardiovascular mortality, we performed Cox proportional hazards regression analyses. First, we performed a univariate analysis with MDA concentration as a continuous variable (Model 1). Thereafter we adjusted for age, sex, eGFR, time since transplantation and proteinuria status (Model 2). To avoid inclusion of too many variables for the number of events, further models included additive adjustments to Model 2. We performed additional adjustments for the traditional cardiovascular risk factors according to the Framingham risk score: smoking status, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic blood pressure (SBP) and antihypertensive drug use (Model 3); for cardiovascular risk factors proposed by the World Health Organization (WHO), including diabetes mellitus, obesity according to the WHO and waist circumference ≥102 cm in males and ≥88 cm in females (Model 4); for cardiovascular history, including previous cardiovascular events and use of statins (Model 5); and for immunosuppressive therapy, including cumulative prednisolone dose and donor status (Model 6). In order to account for non-cardiovascular mortality when assessing cardiovascular mortality, we performed competing risk analyses by fitting cause-specific multivariable-adjusted Cox proportional hazard models. In each of these models, the competing events are treated as censored observations [23]. Models were checked for fulfilment of the assumptions for linear regression and Cox regression and all assumptions were met. Hazard ratios (HR) are reported per 1-SD increment increase and with 95% confidence interval (CI).
Furthermore, we performed pre-specified effect modification analyses in which we tested known cardiovascular risk factors (e.g. eGFR, proteinuria, diabetes mellitus, smoking status) or variables associated with oxidative status (e.g. plasma ascorbic acid concentration). For these analyses, we used multiplicative interaction terms. In cases of significant effect modification, we proceeded with stratified prospective analyses for the concerned variable. Cut-off points for originally continuous variables used in the stratified analyses were determined, so they would allow a similar number of events in each category and statistical power would be maximized. Stratified prospective analyses were adjusted for age, sex, eGFR and SBP.
RESULTS
Baseline characteristics and association with plasma MDA concentration
A total of 604 stable RTRs were included (mean age 51 ± 12 years, 55% male, 96% Caucasian), at a median of 6.0 (IQR 2.7–11.5) years after transplantation. Baseline characteristics of the overall RTR population are shown in Table 1. The median plasma MDA concentration at baseline was 5.38 (IQR 4.31–6.45) µmol/L. Regarding graft function, the mean eGFR was 47 ± 16 mL/min/1.73 m2 and 28% of participants had proteinuria. As for known cardiovascular risk factors, 16% of patients had obesity and 52% had a waist circumference greater than the threshold proposed by the WHO. Mean SBP was 153 ± 23 mmHg and 87% of patients were on antihypertensive medication. Twenty per cent of patients were current smokers and diabetes mellitus was present in 18% of patients. Finally, 12% of patients had a previous cardiovascular event and half of the patients were receiving statins.
Baseline characteristics of the study population (N = 604) and their association with circulating MDA concentration
| Baseline characteristics . | All patients (n = 604) . | Plasma MDA, µg/L . | |||
|---|---|---|---|---|---|
| Linear regressiona . | Adjusted linear regressionb . | Adjusted linear regressionc . | Backward linear regressiond . | ||
| Std. β . | Std. β . | Std. β . | Std. β . | ||
| Plasma MDA concentration (µmol/L), median (IQR) | 5.38 (4.31‒6.45) | – | – | – | – |
| Demographic and body composition | |||||
| Age (years), mean ± SD | 51 ± 12 | 0.08* | 0.08* | 0.10** | 0.11** |
| Sex (male), n (%) | 331 (55) | 0.07* | 0.07* | 0.06* | 0.12** |
| Caucasian ethnicity, n (%) | 582 (96) | −0.003 | 0.01 | −0.003 | |
| Body mass index (kg/m2), mean ± SD | 26.04 ± 4.29 | 0.03 | 0.03 | 0.03 | |
| Body mass index ≥30 kg/m2, n (%) | 96 (16) | 0.07* | 0.07* | 0.07* | –e |
| Waist circumference (cm)f, mean ± SD | 97 ± 14 | 0.10** | 0.07* | 0.09* | 0.16** |
| Waist circumference ≥102 cm (M)/≥88 cm (F), n (%)f | 316 (52) | 0.03 | 0.02 | 0.03 | |
| Cardiovascular history | |||||
| History of cardiovascular disease, n (%)g | 75 (12) | −0.04 | −0.06* | −0.05 | |
| Systolic blood pressure (mmHg), mean ± SD | 153 ± 23 | 0.01 | −0.02 | 0.02 | |
| Diastolic blood pressure (mmHg), mean ± SD | 90 ± 10 | 0.06* | 0.07* | 0.09** | –e |
| Use of ACE inhibitors or ARBs, n (%) | 202 (33) | −0.10** | −0.11** | −0.10** | −0.14** |
| Use of β-blockers, n (%) | 374 (62) | 0.00 | −0.001 | 0.01 | |
| Use of calcium channel antagonists, n (%) | 230 (38) | 0.06* | 0.06* | 0.06* | –e |
| Use of statins, n (%) | 300 (50) | −0.04 | −0.05 | −0.04 | |
| Current smoker, n (%) | 133 (22) | −0.06* | −0.05 | −0.04 | |
| Renal allograft function | |||||
| eGFR (mL/min/1.73 m2), mean ± SD | 47 ± 16 | 0.14** | 0.15** | – | 0.24** |
| Proteinuria ≥0.5 g/24 h, n (%)h | 168 (28) | −0.09** | −0.09** | −0.06* | –e |
| Plasma urea (mmol/L), median (IQR) | 9.50 (7.20‒13.18) | −0.10** | −0.12** | −0.01 | |
| Renal transplant and immunosuppressive therapy | |||||
| Living donor, n (%) | 83 (14) | −0.08* | −0.06* | −0.07* | −0.13** |
| Time since transplantation (years), median (IQR) | 6.0 (2.7‒11.5) | −0.12** | −0.13** | −0.15** | –e |
| Cumulative prednisolone dose (g), median (IQR) | 21.35 (11.38‒37.97) | −0.14** | −0.15** | −0.16** | −0.18** |
| Sirolimus or rapamune use, n (%) | 10 (2) | 0.001 | 0.001 | 0.01 | |
| Type of calcineurin inhibitor | 0.06* | 0.07* | 0.08* | –e | |
| Ciclosporin, n (%) | 389 (64) | ||||
| Tacrolimus, n (%) | 84 (14) | ||||
| Type of proliferation inhibitor | 0.03 | 0.04 | 0.03 | ||
| Azathioprine, n (%) | 198 (33) | ||||
| Mycophenolic acid, n (%) | 249 (41) | ||||
| Acute rejection treatment, n (%) | 332 (55) | 0.08* | 0.08* | 0.06* | –e |
| Metabolic parameters | |||||
| Total cholesterol (mmol/L), median (IQR) | 5.59 (4.92‒6.19) | 0.08* | 0.08* | 0.08** | 0.09* |
| High-density lipoprotein cholesterol (mmol/L), median (IQR) | 1.05 (0.86‒1.28) | 0.03 | 0.05 | 0.02 | |
| Low-density lipoprotein cholesterol (mmol/L), median (IQR) | 3.53 (2.93‒4.12) | 0.06* | 0.06* | 0.06* | –e |
| Triglycerides (mmol/L), median (IQR) | 1.92 (1.40‒2.64) | 0.03 | 0.03 | 0.04 | |
| HbA1c (%)f, mean ± SD | 6.52 ± 1.06 | 0.04 | 0.02 | 0.05 | |
| Diabetic subjects, n (%) | 106 (18) | −0.01 | −0.02 | −0.02 | |
| OS and inflammatory parameters | |||||
| hs-CRP (mg/L), median (IQR) | 2.04 (0.79‒4.82) | 0.05 | 0.05 | 0.07* | 0.16** |
| Plasma ascorbic acid (µmol/L)i, mean ± SD | 44.49 ± 20.00 | 0.003 | 0.02 | 0.004 | |
| CML (µmol/L), median (IQR) | 1.79 (1.47‒2.09) | 0.05 | 0.05 | 0.13* | 0.18** |
| ICAM-1 (ng/L), median (IQR) | 603 (513‒722) | −0.06* | −0.07* | −0.06* | −0.14** |
| Baseline characteristics . | All patients (n = 604) . | Plasma MDA, µg/L . | |||
|---|---|---|---|---|---|
| Linear regressiona . | Adjusted linear regressionb . | Adjusted linear regressionc . | Backward linear regressiond . | ||
| Std. β . | Std. β . | Std. β . | Std. β . | ||
| Plasma MDA concentration (µmol/L), median (IQR) | 5.38 (4.31‒6.45) | – | – | – | – |
| Demographic and body composition | |||||
| Age (years), mean ± SD | 51 ± 12 | 0.08* | 0.08* | 0.10** | 0.11** |
| Sex (male), n (%) | 331 (55) | 0.07* | 0.07* | 0.06* | 0.12** |
| Caucasian ethnicity, n (%) | 582 (96) | −0.003 | 0.01 | −0.003 | |
| Body mass index (kg/m2), mean ± SD | 26.04 ± 4.29 | 0.03 | 0.03 | 0.03 | |
| Body mass index ≥30 kg/m2, n (%) | 96 (16) | 0.07* | 0.07* | 0.07* | –e |
| Waist circumference (cm)f, mean ± SD | 97 ± 14 | 0.10** | 0.07* | 0.09* | 0.16** |
| Waist circumference ≥102 cm (M)/≥88 cm (F), n (%)f | 316 (52) | 0.03 | 0.02 | 0.03 | |
| Cardiovascular history | |||||
| History of cardiovascular disease, n (%)g | 75 (12) | −0.04 | −0.06* | −0.05 | |
| Systolic blood pressure (mmHg), mean ± SD | 153 ± 23 | 0.01 | −0.02 | 0.02 | |
| Diastolic blood pressure (mmHg), mean ± SD | 90 ± 10 | 0.06* | 0.07* | 0.09** | –e |
| Use of ACE inhibitors or ARBs, n (%) | 202 (33) | −0.10** | −0.11** | −0.10** | −0.14** |
| Use of β-blockers, n (%) | 374 (62) | 0.00 | −0.001 | 0.01 | |
| Use of calcium channel antagonists, n (%) | 230 (38) | 0.06* | 0.06* | 0.06* | –e |
| Use of statins, n (%) | 300 (50) | −0.04 | −0.05 | −0.04 | |
| Current smoker, n (%) | 133 (22) | −0.06* | −0.05 | −0.04 | |
| Renal allograft function | |||||
| eGFR (mL/min/1.73 m2), mean ± SD | 47 ± 16 | 0.14** | 0.15** | – | 0.24** |
| Proteinuria ≥0.5 g/24 h, n (%)h | 168 (28) | −0.09** | −0.09** | −0.06* | –e |
| Plasma urea (mmol/L), median (IQR) | 9.50 (7.20‒13.18) | −0.10** | −0.12** | −0.01 | |
| Renal transplant and immunosuppressive therapy | |||||
| Living donor, n (%) | 83 (14) | −0.08* | −0.06* | −0.07* | −0.13** |
| Time since transplantation (years), median (IQR) | 6.0 (2.7‒11.5) | −0.12** | −0.13** | −0.15** | –e |
| Cumulative prednisolone dose (g), median (IQR) | 21.35 (11.38‒37.97) | −0.14** | −0.15** | −0.16** | −0.18** |
| Sirolimus or rapamune use, n (%) | 10 (2) | 0.001 | 0.001 | 0.01 | |
| Type of calcineurin inhibitor | 0.06* | 0.07* | 0.08* | –e | |
| Ciclosporin, n (%) | 389 (64) | ||||
| Tacrolimus, n (%) | 84 (14) | ||||
| Type of proliferation inhibitor | 0.03 | 0.04 | 0.03 | ||
| Azathioprine, n (%) | 198 (33) | ||||
| Mycophenolic acid, n (%) | 249 (41) | ||||
| Acute rejection treatment, n (%) | 332 (55) | 0.08* | 0.08* | 0.06* | –e |
| Metabolic parameters | |||||
| Total cholesterol (mmol/L), median (IQR) | 5.59 (4.92‒6.19) | 0.08* | 0.08* | 0.08** | 0.09* |
| High-density lipoprotein cholesterol (mmol/L), median (IQR) | 1.05 (0.86‒1.28) | 0.03 | 0.05 | 0.02 | |
| Low-density lipoprotein cholesterol (mmol/L), median (IQR) | 3.53 (2.93‒4.12) | 0.06* | 0.06* | 0.06* | –e |
| Triglycerides (mmol/L), median (IQR) | 1.92 (1.40‒2.64) | 0.03 | 0.03 | 0.04 | |
| HbA1c (%)f, mean ± SD | 6.52 ± 1.06 | 0.04 | 0.02 | 0.05 | |
| Diabetic subjects, n (%) | 106 (18) | −0.01 | −0.02 | −0.02 | |
| OS and inflammatory parameters | |||||
| hs-CRP (mg/L), median (IQR) | 2.04 (0.79‒4.82) | 0.05 | 0.05 | 0.07* | 0.16** |
| Plasma ascorbic acid (µmol/L)i, mean ± SD | 44.49 ± 20.00 | 0.003 | 0.02 | 0.004 | |
| CML (µmol/L), median (IQR) | 1.79 (1.47‒2.09) | 0.05 | 0.05 | 0.13* | 0.18** |
| ICAM-1 (ng/L), median (IQR) | 603 (513‒722) | −0.06* | −0.07* | −0.06* | −0.14** |
P < 0.20; **P < 0.05.
Crude linear regression analysis.
Linear regression analysis adjusted for age and sex.
Linear regression analysis adjusted for age, sex, and eGFR.
Stepwise backward linear regression analysis; for inclusion and exclusion in this analysis, P-values were set at 0.2 and 0.05, respectively.
Excluded from the final model.
Data available in 603 patients.
Data available in 600 patients.
Data available in 602 patients.
Data available in 596 patients.
HbA1c, glycated haemoglobin; CML, Nε-(carboxymethyl)lysine; ICAM-1, intercellular adhesion molecule-1.
Baseline characteristics of the study population (N = 604) and their association with circulating MDA concentration
| Baseline characteristics . | All patients (n = 604) . | Plasma MDA, µg/L . | |||
|---|---|---|---|---|---|
| Linear regressiona . | Adjusted linear regressionb . | Adjusted linear regressionc . | Backward linear regressiond . | ||
| Std. β . | Std. β . | Std. β . | Std. β . | ||
| Plasma MDA concentration (µmol/L), median (IQR) | 5.38 (4.31‒6.45) | – | – | – | – |
| Demographic and body composition | |||||
| Age (years), mean ± SD | 51 ± 12 | 0.08* | 0.08* | 0.10** | 0.11** |
| Sex (male), n (%) | 331 (55) | 0.07* | 0.07* | 0.06* | 0.12** |
| Caucasian ethnicity, n (%) | 582 (96) | −0.003 | 0.01 | −0.003 | |
| Body mass index (kg/m2), mean ± SD | 26.04 ± 4.29 | 0.03 | 0.03 | 0.03 | |
| Body mass index ≥30 kg/m2, n (%) | 96 (16) | 0.07* | 0.07* | 0.07* | –e |
| Waist circumference (cm)f, mean ± SD | 97 ± 14 | 0.10** | 0.07* | 0.09* | 0.16** |
| Waist circumference ≥102 cm (M)/≥88 cm (F), n (%)f | 316 (52) | 0.03 | 0.02 | 0.03 | |
| Cardiovascular history | |||||
| History of cardiovascular disease, n (%)g | 75 (12) | −0.04 | −0.06* | −0.05 | |
| Systolic blood pressure (mmHg), mean ± SD | 153 ± 23 | 0.01 | −0.02 | 0.02 | |
| Diastolic blood pressure (mmHg), mean ± SD | 90 ± 10 | 0.06* | 0.07* | 0.09** | –e |
| Use of ACE inhibitors or ARBs, n (%) | 202 (33) | −0.10** | −0.11** | −0.10** | −0.14** |
| Use of β-blockers, n (%) | 374 (62) | 0.00 | −0.001 | 0.01 | |
| Use of calcium channel antagonists, n (%) | 230 (38) | 0.06* | 0.06* | 0.06* | –e |
| Use of statins, n (%) | 300 (50) | −0.04 | −0.05 | −0.04 | |
| Current smoker, n (%) | 133 (22) | −0.06* | −0.05 | −0.04 | |
| Renal allograft function | |||||
| eGFR (mL/min/1.73 m2), mean ± SD | 47 ± 16 | 0.14** | 0.15** | – | 0.24** |
| Proteinuria ≥0.5 g/24 h, n (%)h | 168 (28) | −0.09** | −0.09** | −0.06* | –e |
| Plasma urea (mmol/L), median (IQR) | 9.50 (7.20‒13.18) | −0.10** | −0.12** | −0.01 | |
| Renal transplant and immunosuppressive therapy | |||||
| Living donor, n (%) | 83 (14) | −0.08* | −0.06* | −0.07* | −0.13** |
| Time since transplantation (years), median (IQR) | 6.0 (2.7‒11.5) | −0.12** | −0.13** | −0.15** | –e |
| Cumulative prednisolone dose (g), median (IQR) | 21.35 (11.38‒37.97) | −0.14** | −0.15** | −0.16** | −0.18** |
| Sirolimus or rapamune use, n (%) | 10 (2) | 0.001 | 0.001 | 0.01 | |
| Type of calcineurin inhibitor | 0.06* | 0.07* | 0.08* | –e | |
| Ciclosporin, n (%) | 389 (64) | ||||
| Tacrolimus, n (%) | 84 (14) | ||||
| Type of proliferation inhibitor | 0.03 | 0.04 | 0.03 | ||
| Azathioprine, n (%) | 198 (33) | ||||
| Mycophenolic acid, n (%) | 249 (41) | ||||
| Acute rejection treatment, n (%) | 332 (55) | 0.08* | 0.08* | 0.06* | –e |
| Metabolic parameters | |||||
| Total cholesterol (mmol/L), median (IQR) | 5.59 (4.92‒6.19) | 0.08* | 0.08* | 0.08** | 0.09* |
| High-density lipoprotein cholesterol (mmol/L), median (IQR) | 1.05 (0.86‒1.28) | 0.03 | 0.05 | 0.02 | |
| Low-density lipoprotein cholesterol (mmol/L), median (IQR) | 3.53 (2.93‒4.12) | 0.06* | 0.06* | 0.06* | –e |
| Triglycerides (mmol/L), median (IQR) | 1.92 (1.40‒2.64) | 0.03 | 0.03 | 0.04 | |
| HbA1c (%)f, mean ± SD | 6.52 ± 1.06 | 0.04 | 0.02 | 0.05 | |
| Diabetic subjects, n (%) | 106 (18) | −0.01 | −0.02 | −0.02 | |
| OS and inflammatory parameters | |||||
| hs-CRP (mg/L), median (IQR) | 2.04 (0.79‒4.82) | 0.05 | 0.05 | 0.07* | 0.16** |
| Plasma ascorbic acid (µmol/L)i, mean ± SD | 44.49 ± 20.00 | 0.003 | 0.02 | 0.004 | |
| CML (µmol/L), median (IQR) | 1.79 (1.47‒2.09) | 0.05 | 0.05 | 0.13* | 0.18** |
| ICAM-1 (ng/L), median (IQR) | 603 (513‒722) | −0.06* | −0.07* | −0.06* | −0.14** |
| Baseline characteristics . | All patients (n = 604) . | Plasma MDA, µg/L . | |||
|---|---|---|---|---|---|
| Linear regressiona . | Adjusted linear regressionb . | Adjusted linear regressionc . | Backward linear regressiond . | ||
| Std. β . | Std. β . | Std. β . | Std. β . | ||
| Plasma MDA concentration (µmol/L), median (IQR) | 5.38 (4.31‒6.45) | – | – | – | – |
| Demographic and body composition | |||||
| Age (years), mean ± SD | 51 ± 12 | 0.08* | 0.08* | 0.10** | 0.11** |
| Sex (male), n (%) | 331 (55) | 0.07* | 0.07* | 0.06* | 0.12** |
| Caucasian ethnicity, n (%) | 582 (96) | −0.003 | 0.01 | −0.003 | |
| Body mass index (kg/m2), mean ± SD | 26.04 ± 4.29 | 0.03 | 0.03 | 0.03 | |
| Body mass index ≥30 kg/m2, n (%) | 96 (16) | 0.07* | 0.07* | 0.07* | –e |
| Waist circumference (cm)f, mean ± SD | 97 ± 14 | 0.10** | 0.07* | 0.09* | 0.16** |
| Waist circumference ≥102 cm (M)/≥88 cm (F), n (%)f | 316 (52) | 0.03 | 0.02 | 0.03 | |
| Cardiovascular history | |||||
| History of cardiovascular disease, n (%)g | 75 (12) | −0.04 | −0.06* | −0.05 | |
| Systolic blood pressure (mmHg), mean ± SD | 153 ± 23 | 0.01 | −0.02 | 0.02 | |
| Diastolic blood pressure (mmHg), mean ± SD | 90 ± 10 | 0.06* | 0.07* | 0.09** | –e |
| Use of ACE inhibitors or ARBs, n (%) | 202 (33) | −0.10** | −0.11** | −0.10** | −0.14** |
| Use of β-blockers, n (%) | 374 (62) | 0.00 | −0.001 | 0.01 | |
| Use of calcium channel antagonists, n (%) | 230 (38) | 0.06* | 0.06* | 0.06* | –e |
| Use of statins, n (%) | 300 (50) | −0.04 | −0.05 | −0.04 | |
| Current smoker, n (%) | 133 (22) | −0.06* | −0.05 | −0.04 | |
| Renal allograft function | |||||
| eGFR (mL/min/1.73 m2), mean ± SD | 47 ± 16 | 0.14** | 0.15** | – | 0.24** |
| Proteinuria ≥0.5 g/24 h, n (%)h | 168 (28) | −0.09** | −0.09** | −0.06* | –e |
| Plasma urea (mmol/L), median (IQR) | 9.50 (7.20‒13.18) | −0.10** | −0.12** | −0.01 | |
| Renal transplant and immunosuppressive therapy | |||||
| Living donor, n (%) | 83 (14) | −0.08* | −0.06* | −0.07* | −0.13** |
| Time since transplantation (years), median (IQR) | 6.0 (2.7‒11.5) | −0.12** | −0.13** | −0.15** | –e |
| Cumulative prednisolone dose (g), median (IQR) | 21.35 (11.38‒37.97) | −0.14** | −0.15** | −0.16** | −0.18** |
| Sirolimus or rapamune use, n (%) | 10 (2) | 0.001 | 0.001 | 0.01 | |
| Type of calcineurin inhibitor | 0.06* | 0.07* | 0.08* | –e | |
| Ciclosporin, n (%) | 389 (64) | ||||
| Tacrolimus, n (%) | 84 (14) | ||||
| Type of proliferation inhibitor | 0.03 | 0.04 | 0.03 | ||
| Azathioprine, n (%) | 198 (33) | ||||
| Mycophenolic acid, n (%) | 249 (41) | ||||
| Acute rejection treatment, n (%) | 332 (55) | 0.08* | 0.08* | 0.06* | –e |
| Metabolic parameters | |||||
| Total cholesterol (mmol/L), median (IQR) | 5.59 (4.92‒6.19) | 0.08* | 0.08* | 0.08** | 0.09* |
| High-density lipoprotein cholesterol (mmol/L), median (IQR) | 1.05 (0.86‒1.28) | 0.03 | 0.05 | 0.02 | |
| Low-density lipoprotein cholesterol (mmol/L), median (IQR) | 3.53 (2.93‒4.12) | 0.06* | 0.06* | 0.06* | –e |
| Triglycerides (mmol/L), median (IQR) | 1.92 (1.40‒2.64) | 0.03 | 0.03 | 0.04 | |
| HbA1c (%)f, mean ± SD | 6.52 ± 1.06 | 0.04 | 0.02 | 0.05 | |
| Diabetic subjects, n (%) | 106 (18) | −0.01 | −0.02 | −0.02 | |
| OS and inflammatory parameters | |||||
| hs-CRP (mg/L), median (IQR) | 2.04 (0.79‒4.82) | 0.05 | 0.05 | 0.07* | 0.16** |
| Plasma ascorbic acid (µmol/L)i, mean ± SD | 44.49 ± 20.00 | 0.003 | 0.02 | 0.004 | |
| CML (µmol/L), median (IQR) | 1.79 (1.47‒2.09) | 0.05 | 0.05 | 0.13* | 0.18** |
| ICAM-1 (ng/L), median (IQR) | 603 (513‒722) | −0.06* | −0.07* | −0.06* | −0.14** |
P < 0.20; **P < 0.05.
Crude linear regression analysis.
Linear regression analysis adjusted for age and sex.
Linear regression analysis adjusted for age, sex, and eGFR.
Stepwise backward linear regression analysis; for inclusion and exclusion in this analysis, P-values were set at 0.2 and 0.05, respectively.
Excluded from the final model.
Data available in 603 patients.
Data available in 600 patients.
Data available in 602 patients.
Data available in 596 patients.
HbA1c, glycated haemoglobin; CML, Nε-(carboxymethyl)lysine; ICAM-1, intercellular adhesion molecule-1.
In crude linear regression analyses, plasma MDA concentration was significantly and directly associated with waist circumference [standardized β coefficient (Std β) = 0.10; P = 0.01] and inversely associated with the use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) (Std β = −0.10; P = 0.01). Measurements of renal function, such as plasma urea concentration (Std β = −0.10; P = 0.02), eGFR (Std β = 0.14; P < 0.01) and proteinuria (Std β = −0.09; P = 0.03), were also significantly associated with plasma MDA concentration. Among transplant-related characteristics, time since transplantation (Std β = −0.12; P < 0.01) and cumulative prednisolone dose (Std β = −0.14; P < 0.01) were also both significantly and inversely associated with plasma MDA concentration. After adjustment for age and sex, waist circumference was no longer significantly associated with circulating MDA concentration. Posterior adjustment for renal function revealed direct significant association between circulating MDA concentration and age (Std β = 0.10; P = 0.02), diastolic blood pressure (Std β = 0.09; P = 0.03) and total cholesterol (Std β = 0.08; P = 0.04), whereas proteinuria was no longer significantly associated. A final model obtained by linear regression with backward selection (α = 0.05; β = 0.20) found sex, waist circumference, use of ACE inhibitors/ARBs, eGFR, donor type (living or deceased), cumulative prednisolone dose, total cholesterol, high-sensitivity C-reactive protein (hs-CRP), Nε-(carboxymethyl)lysine and intercellular adhesion molecule-1 as the stronger determinants of circulating MDA concentration (Table 1).
Prospective analyses
During a median follow-up of 6.4 (IQR 5.6–6.8) years, 110 (18%) RTRs died, with 44 (40%) deaths due to cardiovascular causes. Prospective analyses showed that plasma MDA concentration was directly associated with the risk for cardiovascular mortality [HR 1.31 (95% CI 1.03‒1.67) per 1-SD increment; P = 0.03]. This association was independent of adjustment for potential confounders, with, for example, an HR of 1.39 (95% CI 1.05‒1.83) per 1-SD increment after adjustment for age, sex, eGFR, time since transplantation and proteinuria status. Further adjustment for the cardiovascular risk factors listed in the Framingham score and those proposed by the WHO, patients cardiovascular history and immunosuppressive therapy did not materially alter the association (Table 2). Competing risk analyses showed that MDA concentration, although consistently associated with cardiovascular mortality, was not associated with the competing event non-cardiovascular mortality, and posterior adjustments did not alter this result (Table 2).
Multivariable-adjusted associations between plasma MDA concentration and cardiovascular mortality risk in 604 RTRs
| Models . | HR per 1-SD increment . | 95% CI . | P-value . |
|---|---|---|---|
| Cardiovascular mortality | |||
| Model 1 | 1.31 | 1.03‒1.67 | 0.03 |
| Model 2 | 1.39 | 1.05‒1.83 | 0.02 |
| Model 3 | 1.43 | 1.11‒1.85 | 0.01 |
| Model 4 | 1.39 | 1.04‒1.85 | 0.02 |
| Model 5 | 1.40 | 1.06‒1.85 | 0.02 |
| Model 6 | 1.41 | 1.06‒1.88 | 0.02 |
| Non-cardiovascular mortality | |||
| Model 1 | 1.13 | 0.90‒1.41 | 0.31 |
| Model 2 | 1.14 | 0.88‒1.49 | 0.33 |
| Model 3 | 1.18 | 0.92‒1.52 | 0.19 |
| Model 4 | 1.12 | 0.89‒1.51 | 0.28 |
| Model 5 | 1.12 | 0.86‒1.45 | 0.42 |
| Model 6 | 1.16 | 0.89‒1.52 | 0.27 |
| Models . | HR per 1-SD increment . | 95% CI . | P-value . |
|---|---|---|---|
| Cardiovascular mortality | |||
| Model 1 | 1.31 | 1.03‒1.67 | 0.03 |
| Model 2 | 1.39 | 1.05‒1.83 | 0.02 |
| Model 3 | 1.43 | 1.11‒1.85 | 0.01 |
| Model 4 | 1.39 | 1.04‒1.85 | 0.02 |
| Model 5 | 1.40 | 1.06‒1.85 | 0.02 |
| Model 6 | 1.41 | 1.06‒1.88 | 0.02 |
| Non-cardiovascular mortality | |||
| Model 1 | 1.13 | 0.90‒1.41 | 0.31 |
| Model 2 | 1.14 | 0.88‒1.49 | 0.33 |
| Model 3 | 1.18 | 0.92‒1.52 | 0.19 |
| Model 4 | 1.12 | 0.89‒1.51 | 0.28 |
| Model 5 | 1.12 | 0.86‒1.45 | 0.42 |
| Model 6 | 1.16 | 0.89‒1.52 | 0.27 |
In total, 110 (18%) patients died, of which 44 (40%) deaths were due to cardiovascular causes. Model 1: crude model. Model 2: Model 1 + adjustment for age, sex, time since transplantation, eGFR and proteinuria status. Model 3: Model 2 + adjustment for cardiovascular risk factors included in the Framingham score. Model 4: Model 2 + adjustment for cardiovascular risk factors proposed by the WHO. Model 5: Model 2 + adjustment for cardiovascular history. Model 6: Model 2 + adjustment for type of donor and immunosuppressive therapy.
Multivariable-adjusted associations between plasma MDA concentration and cardiovascular mortality risk in 604 RTRs
| Models . | HR per 1-SD increment . | 95% CI . | P-value . |
|---|---|---|---|
| Cardiovascular mortality | |||
| Model 1 | 1.31 | 1.03‒1.67 | 0.03 |
| Model 2 | 1.39 | 1.05‒1.83 | 0.02 |
| Model 3 | 1.43 | 1.11‒1.85 | 0.01 |
| Model 4 | 1.39 | 1.04‒1.85 | 0.02 |
| Model 5 | 1.40 | 1.06‒1.85 | 0.02 |
| Model 6 | 1.41 | 1.06‒1.88 | 0.02 |
| Non-cardiovascular mortality | |||
| Model 1 | 1.13 | 0.90‒1.41 | 0.31 |
| Model 2 | 1.14 | 0.88‒1.49 | 0.33 |
| Model 3 | 1.18 | 0.92‒1.52 | 0.19 |
| Model 4 | 1.12 | 0.89‒1.51 | 0.28 |
| Model 5 | 1.12 | 0.86‒1.45 | 0.42 |
| Model 6 | 1.16 | 0.89‒1.52 | 0.27 |
| Models . | HR per 1-SD increment . | 95% CI . | P-value . |
|---|---|---|---|
| Cardiovascular mortality | |||
| Model 1 | 1.31 | 1.03‒1.67 | 0.03 |
| Model 2 | 1.39 | 1.05‒1.83 | 0.02 |
| Model 3 | 1.43 | 1.11‒1.85 | 0.01 |
| Model 4 | 1.39 | 1.04‒1.85 | 0.02 |
| Model 5 | 1.40 | 1.06‒1.85 | 0.02 |
| Model 6 | 1.41 | 1.06‒1.88 | 0.02 |
| Non-cardiovascular mortality | |||
| Model 1 | 1.13 | 0.90‒1.41 | 0.31 |
| Model 2 | 1.14 | 0.88‒1.49 | 0.33 |
| Model 3 | 1.18 | 0.92‒1.52 | 0.19 |
| Model 4 | 1.12 | 0.89‒1.51 | 0.28 |
| Model 5 | 1.12 | 0.86‒1.45 | 0.42 |
| Model 6 | 1.16 | 0.89‒1.52 | 0.27 |
In total, 110 (18%) patients died, of which 44 (40%) deaths were due to cardiovascular causes. Model 1: crude model. Model 2: Model 1 + adjustment for age, sex, time since transplantation, eGFR and proteinuria status. Model 3: Model 2 + adjustment for cardiovascular risk factors included in the Framingham score. Model 4: Model 2 + adjustment for cardiovascular risk factors proposed by the WHO. Model 5: Model 2 + adjustment for cardiovascular history. Model 6: Model 2 + adjustment for type of donor and immunosuppressive therapy.
Effect modification analyses
In effect modification analyses, we found that the association between MDA concentration and the risk for cardiovascular mortality was significantly modified by plasma ascorbic acid concentration and eGFR (Pinteraction < 0.001 and Pinteraction = 0.09, respectively). Other variables such as use of ACE inhibitors/ARBs, plasma urea concentration and cumulative prednisolone dose were not significant effect modifiers (Supplementary Table S1). Next we performed stratified prospective analyses by subgroups of RTRs according to categories of plasma ascorbic acid concentration (≤ or >42.5 µmol/L) and eGFR (≤ or >45 mL/min/1.73 m2). Cut-off points were selected so there was a similar number of events in each subgroup.
The positive association between plasma MDA concentration and the risk for cardiovascular mortality was stronger among patients with relatively lower concentrations of ascorbic acid [HR 1.79 (95% CI 1.30‒2.48) per 1-SD increase; P < 0.001] compared with the overall population. Moreover, among RTRs with relatively higher ascorbic acid concentrations, the association between plasma MDA concentration and the risk for cardiovascular mortality was no longer significant [HR 0.85 (95% CI 0.52‒1.40) per 1-SD increase; P = 0.53]. Likewise, the positive association between plasma MDA concentration and the risk for cardiovascular mortality was stronger among patients with relatively lower eGFR [HR 2.09 (95% CI 1.45‒3.00) per 1-SD increase; P < 0.001] compared with the overall RTR population (Figure 1).
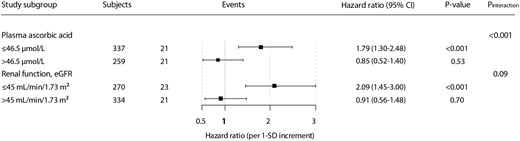
Stratified analysis of the association of MDA concentration with cardiovascular mortality, according to significant effect modifiers, in RTRs. HRs and 95% CIs were calculated with adjustment for age, sex, eGFR and SBP.
In sensitivity analyses we explored the effect modification of plasma ascorbic acid concentration by using the cut-off point for plasma ascorbic acid depletion (≤ or >28 µmol/L) [19]. In agreement with previous results, within the vitamin C–depleted subgroup of RTRs, the hazard ratios for the association of plasma MDA concentration with the risk for cardiovascular mortality were stronger [HR 2.58 (95% CI 1.63‒4.01) per 1-SD increment; P < 0.001] compared with the overall analyses, as well as stronger compared with the previous vitamin C–stratified analyses. Also, in the non-vitamin C–depleted subgroup of RTRs, no significant association was found between circulating MDA concentration and cardiovascular mortality risk (Supplementary Table S2).
DISCUSSION
This study showed that relatively higher plasma MDA concentrations are independently associated with a higher risk for cardiovascular mortality in RTRs. We also found that this association was modified by circulating ascorbic acid concentration, with a stronger association between MDA concentration and cardiovascular mortality risk among patients with relatively lower concentrations of ascorbic acid. Graft function measured in terms of eGFR was also a significant effect modifier, with a stronger association among patients with Kidney Disease: Improving Global Outcomes G3b, G4 and G5 stages of chronic kidney disease. Our findings are in agreement with several studies showing an association between OS and the pathophysiology of several cardiovascular diseases [8, 10–12]. However, to the best of our knowledge, this is the first study assessing the relationship between plasma MDA concentration and the risk for cardiovascular mortality in the post-renal transplantation setting.
ROS are important mediators of cellular damage, with lipid peroxidation as a major manifestation of ROS-induced OS [13]. Circulating MDA concentration is one of the most commonly used biomarkers for this phenomenon [24]. Also, MDA itself is a toxic molecule, since it is highly reactive against a variety of biomolecules, including nucleic acids and proteins, and often leads to irreversible damage to important cellular structures [13]. An increase in OS, and consequently in MDA concentration, is known to be persistent through end-stage renal disease, in part due to decreased urinary clearance and also in part due to the chronic pro-inflammatory state that is characteristic of end-stage renal disease [25]. Post-renal transplantation, this imbalance is only incompletely corrected [26], in part because maintenance immunosuppressive drugs stimulate the production of oxygen radicals and lipid peroxidation [27, 28].
OS is associated with several pathophysiological mechanisms that explain the high incidence of cardiovascular events in renal patients [29]. Increased OS promotes endothelial dysfunction by decreasing the bioavailability of nitric oxide [10] and disturbs the regulatory function of HDL in atherosclerosis through promotion of HDL oxidation [30]. OS also increases calcium deposition in atherosclerotic plaques, as it acts as a positive signal for osteoblastic differentiation of vascular cells [12]. MDA also reacts with primary amines, thereby modifying the apoB fractions of oxidized low-density lipoprotein (LDL), which makes oxidized LDL a target for uptake by macrophages [13]. Consistent with this evidence, clinical studies in end-stage renal disease showed a correlation between higher circulating MDA concentrations and the severity of coronary artery disease [31]. Furthermore, OS has been associated with left ventricular hypertrophy and stiffening through increased cardiac fibroblastic differentiation, with MDA promoting intermolecular collagen cross-linking [8, 13]. MDA also inhibits the contractile function of cardiac myocytes and enhances the cardiorenal syndrome by activating inflammatory nuclear factor κB pathways [11, 32]. Extrapolating these data, we found an association between circulating MDA concentration and the risk for cardiovascular mortality in RTRs, which provides the first longitudinal evidence in the post-renal transplantation clinical setting.
In this study, plasma ascorbic acid concentration and renal function were both found to be significant and consistent effect modifiers. Vitamin C is an important antioxidant in biological fluids, including plasma [33], and one of the most important antioxidant nutrients, obtained exclusively from dietary sources [34]. Thus it is not surprising that the overall association between plasma MDA concentration and cardiovascular mortality risk was particularly strong among RTRs with relatively lower plasma ascorbic acid concentrations or those who were vitamin C–depleted. More interesting is the fact that no significant association was found among RTRs with relatively higher plasma ascorbic acid concentrations or those who were not vitamin C–depleted, which raises the question of whether vitamin C–directed interventions could be of use as a strategy for decreasing cardiovascular mortality. Further support to our findings comes from recent evidence showing that ascorbic acid depletion is independently associated with all-cause mortality in RTRs [19].
As for the effect modification of eGFR, a large body of evidence confers a causative role to OS in the underlying mechanisms leading to renal disease. Several inducers of kidney damage have been shown to induce renal OS [35–38] and kidney tissue is known to be highly vulnerable to OS damage because it is abundant in long-chain polyunsaturated fatty acids [39]. It has been hypothesized that in the kidney exists a feedback cycle whereby OS is increased by renal impairment and increased OS, which in turn, further impairs renal function [8, 40, 41].
Therapeutic reduction of OS is a matter of interest and it can be achieved by lowering exposure to environmental oxidative molecules, by decreasing the generation of OS through stabilization of mitochondrial function and by increasing the levels of endogenous and exogenous antioxidants (e.g. vitamin C, vitamin E and N-acetylcysteine), with the latter being the most widely used approach [42–44]. It should be noted that a Cochrane review conducted in 2012 could not conclude a clear benefit of antioxidant supplementation therapies in reducing cardiovascular mortality in patients with kidney disease, although this was attributed to suboptimal quality of available studies and a lack of sufficiently powered studies [45, 46]. Our results, including the effect modification of plasma concentration of ascorbic acid, underline the need for a better understanding of the impact of OS on high cardiovascular risk and premature cardiovascular mortality after renal transplantation. Our findings also highlight the need for further studies to explore the potential role of antioxidants in reducing the long-standing burden of cardiovascular mortality in the RTR population.
The current study comprises a large cohort of stable and extensively phenotyped RTRs, which allowed adjustment for several potential confounders. Furthermore, all study subjects were closely monitored through the vigilance system in place at our outpatient clinic and affiliated hospitals, thus ensuring complete information on patient status was collected, with no loss to follow-up. On the other hand, we acknowledge certain weaknesses of the study. First, this is a single-centre study with a study population almost entirely composed of white subjects, which calls for prudence when extrapolating our findings to other populations with regard to different ethnicities. Second, like most epidemiological studies, the current study used a single baseline measurement to predict outcomes, which adversely impacts the predictive properties of variables associated with outcomes. If intra-individual variability of predictive biomarkers using repeated measurements is taken into account, this results in strengthening of predictive properties, particularly in the case of markers with high intra-individual variability [47–49]. Third, although we used the most common method for determining MDA concentration, the thiobarbituric acid reaction assay is not exclusively specific for MDA and can detect other lipid peroxidation aldehydes. Next, as with any observational study, our study cannot unequivocally distinguish whether there is a causal link between MDA concentration and cardiovascular mortality risk or whether MDA is merely a marker of higher risk for cardiovascular mortality. However, currently available evidence regarding MDA biology supports a pathophysiological link between MDA and cardiovascular disease [29]. Finally, as with any observational study, unmeasured confounding may still occur, despite adjusting for a substantial number of potentially confounding variables in our analyses.
In conclusion, in stable RTRs, plasma MDA concentration is independently associated with long-term risk for cardiovascular mortality. This is the first study providing data that prospectively associate OS with a long-term survival endpoint in the post-renal transplantation setting. This evidence provides a rationale for further studies to explore potential OS-targeted therapeutic/nutritional treatments aimed to improve long-term cardiovascular outcomes in RTRs.
FUNDING
This study is based on the TransplantLines Insulin Resistance and Inflammation (TxL-IRI) Biobank and Cohort Study Database, which was funded by the Dutch Kidney Foundation (grant C00.1877). C.G.S. is supported by a doctorate studies grant from the Comisión Nacional de Investigación Científica y Tecnológica (F 72190118).
AUTHORS’ CONTRIBUTIONS
G.J.N. and S.J.L.B. designed the study. H.G.D.L. and D.T. performed the measurements and provided the data. M.Y.-C., C.G.S. and S.J.L.B. analysed the data. R.O.B.G., S.P.B. and R.R. provided critical review, advice and consultation throughout the writing of the manuscript. M.Y.-C. and C.G.S. provided the figures. M.Y.-C., C.G.S. and S.J.L.B. drafted and revised the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
Author notes
Manuela Yepes-Calderón and Camilo G. Sotomayor contributed equally to this work.





Comments