-
PDF
- Split View
-
Views
-
Cite
Cite
Federica Barutta, Serena Grimaldi, Roberto Gambino, Kiran Vemuri, Alexandros Makriyannis, Laura Annaratone, Vincenzo di Marzo, Graziella Bruno, Gabriella Gruden, Dual therapy targeting the endocannabinoid system prevents experimental diabetic nephropathy, Nephrology Dialysis Transplantation, Volume 32, Issue 10, October 2017, Pages 1655–1665, https://doi.org/10.1093/ndt/gfx010
Close - Share Icon Share
Abstract
The endocannabinoid system has been implicated in the pathogenesis of diabetic nephropathy (DN). We investigated the effect of combined therapy with AM6545, a ‘peripherally’ restricted cannabinoid receptor type 1 (CB1R) neutral antagonist, and AM1241, a cannabinoid receptor type 2 (CB2R) agonist, in experimental DN.
Renal function and structure, podocyte proteins and markers of both fibrosis and inflammation were studied in streptozotocin-induced diabetic mice treated for 14 weeks with vehicle, AM6545, AM1241 and AM6545–AM1241.
Single treatment with either AM6545 or AM1241 alone reduced diabetes-induced albuminuria and prevented nephrin loss both in vivo and in vitro in podocytes exposed to glycated albumin. Dual therapy performed better than monotherapies, as it abolished albuminuria, inflammation, tubular injury and markedly reduced renal fibrosis. Converging anti-inflammatory mechanisms provide an explanation for this greater efficacy as dual therapy abolished diabetes-induced renal monocyte infiltration and M1/M2 macrophage imbalance in vivo and abrogated the profibrotic effect of M1 macrophage–conditioned media on cultured mesangial cells.
‘Peripheral’ CB1R blockade is beneficial in experimental DN and this effect is synergically magnified by CB2R activation.
Diabetic nephropathy (DN) is characterized by both increased glomerular permeability to proteins and excessive mesangial matrix deposition. Intensified glycaemic control and anti-hypertensive treatment are the currently available therapeutic strategies in patients at risk and with established DN. In spite of the demonstrated efficacy of these treatments, DN is the leading cause of end-stage renal failure and there is a need to identify new therapeutic strategies [1].
The endocannabinoids (ECs) anandamide and 2-arachidonoylglycerol (2-AG) are synthesized on demand from arachidonic acid–containing phospholipid precursors and bind to G protein–coupled cannabinoid receptors called cannabinoid receptor type 1 (CB1R) and type 2 (CB2R). The endocannabinoid system (ECS) plays an important role in obesity, insulin resistance and type 2 diabetes [2, 3]. The ECS is also present in the kidney and has been recently implicated in the pathogenesis of DN [4–13]. In the diabetic kidney, expression of the two ECS receptors undergoes opposing changes wherein CB1R is upregulated while CB2R is downregulated [3, 4, 12, 13]. Moreover, treatments that counteract these abnormalities by either blocking CB1R or activating CB2R have anti-proteinuric effects in experimental DN [4, 5, 9, 11, 12]. However, these therapeutic strategies fail to ameliorate renal fibrosis in animal models of type 1 diabetes [4, 5] and the therapeutic development of drugs blocking CB1R has been halted because of the adverse neuropsychiatric side effects that are associated with brain-penetrant CB1R inverse agonists/antagonists [2].
Newly developed ‘peripherally restricted’ CB1R antagonists that are devoid of psychoactive effects, as they do not cross the blood–brain barrier, appear to retain the beneficial effects of global CB1R antagonists on obesity and insulin resistance [14, 15]. Thus there is growing interest in this class of compounds as tools to study and treat diabetes-related conditions and to translate related preclinical findings on the ECS towards clinical practice [2]. In addition, because CB1R and CB2R share the same ligands, form heterodimers [16] and have overlapping intracellular signalling [2, 17], drugs blocking/activating one ECS receptor may indirectly affect the other, potentially leading to paradox effects. Therefore, dual therapy with a peripheral CB1R antagonist and a CB2R agonist may represent a safer approach and result in further benefits beyond the potential additive properties of single drugs. To date, this approach has not yet been investigated in any disease model implicating the ECS, including DN.
Therefore, our aim was to investigate in an animal model of type 1 diabetes the efficacy of combined therapy with AM6545, a peripherally restricted CB1R neutral antagonist, and the CB2R agonist AM1241 on both functional and structural abnormalities of DN.
MATERIALS AND METHODS
Materials
All materials were from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated.
Drugs
AM6545 {5-[4-(4-cyanobut-1-ynyl)phenyl]-1-[2,4-dichlorophenyl]-4-methyl-N-[1,1-dioxo-thiomorpholino]-1H-pyrazole-3-carboxamide} was synthesized at the Center for Drug Discovery, Northeastern University (Boston, MA, USA). AM6545 exhibits a 302-fold selectivity for CB1R (Ki 1.7 ± 0.92 nM) versus CB2R (Ki 523 ± 143 nM) [18]. AM1241 <(2-iodo-5-nitrophenyl)-{1-[(1-methylpiperidin-2-yl}methyl]indol-3-yl]methanone> was purchased from Cayman (Ann Arbor, MI, USA). In vitro, AM1241 is a potent (Ki 2 nM) CBR agonist with 95–340-fold selectivity for CB2R and in vivo, AM1241 exhibits an 82-fold selectivity for CB2R (Ki 3.4 ± 0.5 nM) versus CB1R (Ki 280 ± 41 nM) [19]. For the in vivo study, drugs were dissolved in DMSO, diluted in an 18:1:1 ratio of saline:emulphor-620:DMSO.
In vivo study
Animals and induction of diabetes
The study was approved by the Ethical Committee of the Turin University and both housing and care of laboratory animals were in accordance with Italian law (D.L.116/1992). Diabetes was induced in male C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME, USA), age 8 weeks, by streptozotocin (STZ) intraperitoneal injection, as we previously described [4].
Experimental protocol
Animals were divided into six groups: non-diabetic (ND) mice treated with either vehicle (n = 8) or AM6545 (n = 8) and diabetic mice (DM) treated with vehicle (n = 8), AM6545 (n = 8), AM1241 (n = 8) or both [AM6545 + AM1241 (n = 8)]. AM6545 and AM1241 were administered daily via intraperitoneal injection at the dosage of 10 and 3 mg/kg, respectively [5, 14]. Mice sham-injected with an 18:1:1 mixture of saline:emulphor-620:DMSO were used as controls. After 14 weeks of diabetes, mice were killed by decapitation and the kidneys were rapidly dissected and weighed. The right kidney was frozen in N2 and stored at −80°C for both mRNA and protein analysis. Half of the left kidney was fixed in 10% PBS-formalin, then paraffin-embedded for light microscopy; the remaining tissue was embedded in optimal cutting temperature compound and snap frozen in N2.
Metabolic and physiological parameters
Glycated haemoglobin, systolic blood pressure, urinary albumin concentration, creatinine clearance and urinary N-acetyl-β-D-glucosaminidase (NAG) activity were measured, as previously described [20] and detailed in the Supplementary data.
mRNA analysis
mRNA expression was analysed by real-time polymerase chain reaction (PCR) using pre-developed TaqMan reagents (Applied Biosystems, Monza, Italy), as detailed in the Supplementary data.
Histological analysis
Paraffin-embedded tissue sections were stained using periodic acid–Schiff (PAS) staining, as detailed in the Supplementary data.
Immunohistochemistry
Expression of monocytes/macrophages (F4/80), CB1R and CB2R was performed on snap-frozen/paraffin-embedded tissue sections, as detailed in the Supplementary data.
Immunofluorescence
Staining for nephrin, podocin, synaptopodin and fibronectin was performed by immunofluorescence on snap-frozen sections, as detailed in the Supplementary data.
Immunoblotting
Expression of nephrin (Progen Biotechnik, Maaßstraße, Germany), podocin and synaptopodin was measured in total protein extracts from either renal cortex or cultured podocytes by immunoblotting, as detailed in the Supplementary data.
In vitro experiments
Podocytes
Conditionally immortalized human podocytes were kindly provided by M. Saleem (University of Bristol, Bristol, UK) and cultured as previously described [21]. Differentiated podocytes were serum deprived for 24 h, then exposed to either glycated albumin (GA 200 μg/mL) or vehicle for 24 h in the presence and in the absence of AM1241 (10 μmol/L) and/or AM6545 (10 μmol/L). Appropriate dilutions of DMSO were added as vehicle controls.
Monocytes
Human monocytic leukaemia U-937 cells (ATCC) were cultured in suspension at 37°C in a humidified atmosphere of 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated foetal bovine serum (FBS), 10 mM HEPES, 1 mM sodium piruvate, 2 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin.
Macrophage-like cells (M0)
U-937 cells were seeded at a density of 5 × 105 cells/well into six-well plates with RPMI 1640 containing 5% FBS and 20 ng/mL phorbol myristate acetate (PMA) to induce differentiation in M0. After 48 h, cells were washed with PBS to remove non-adherent cells and then assessed by light microscopy.
M1 macrophages
To induce polarization towards M1 macrophages, M0 cells were exposed to both lipopolysaccharide (LPS, 100 ng/mL) and interferon γ (20 ng/mL) for 24 h [22, 23]. Following washing, cells were cultured for a further 24 h in the presence and in the absence of AM1241 (10 μmol/L) and/or AM6545 (10 μmol/L). At the end of the experimental period, total RNA was extracted, conditioned media (CM) collected, and TNF-α mRNA levels measured by real-time PCR.
Mesangial cells
Immortalized mesangial cells were established, characterized and cultured as previously described [24]. Serum-deprived cells were incubated for 48 h with CM collected from M1 macrophages exposed to the conditions described above either in the presence or in the absence of a specific TNF-α-neutralizing antibody (7 µg/mL; R&D Systems, Minneapolis, MN, USA), then fibronectin mRNA levels were measured by real-time PCR.
Statistical analysis
Data, presented as mean ± SEM, geometric mean (25th–75th percentile) or fold change over control, were analysed by ANOVA. The least significant difference test was used for posthoc comparisons. P-values <0.05 were considered statistically significant.
RESULTS
Metabolic and physiological parameters
After 14 weeks of diabetes, both blood glucose and glycated haemoglobin levels were significantly higher in DM than in ND mice and were unaltered by treatment (Table 1). Although glycated haemoglobin was slightly lower in mice treated with the combined therapy, the difference was small and not statistically significant. Individual blood glucose and glycated haemoglobin levels for each mouse are reported in the Supplementary data, Figure S1. DM showed a significant decrease in body weight and a significant increase in the kidney weight:body weight (KW:BW) ratio compared with sham-injected control animals; however, no differences were observed between treated and untreated animals.
| . | Body weight (g) . | BG (mg/dL) . | Glycated Hb (%) . | KW/BW ratio . | sBP (mmHg) . |
|---|---|---|---|---|---|
| ND | 28.8 ± 0.7 | 140 ± 2 | 4.1 ± 0.1 | 5.3 ± 0.2 | 108 ± 5 |
| ND-AM6545 | 29.0 ± 0.7 | 130 ± 2 | 4.0 ± 0.0 | 5.6 ± 0.2 | 106 ± 4 |
| ND-A + A | 28.0 ± 0.3 | 130 ± 1 | 3.9 ± 0.2 | 5.5 ± 0.4 | 108 ± 2 |
| DM | 23.8 ± 0.4* | 360 ± 8* | 11.4 ± 0.6* | 7.8 ± 0.3* | 117 ± 4 |
| DM-AM6545 | 24.2 ± 0.4* | 369 ± 7* | 11.8 ± 0.4* | 7.9 ± 0.2* | 115 ± 6 |
| DM-AM1241 | 23.8 ± 0.5* | 338 ± 32* | 10.9 ± 0.8* | 7.7 ± 0.2* | 126 ± 4 |
| DM-A + A | 23.5 ± 0.5* | 361 ± 6* | 10.0 ± 0.6* | 7.8 ± 0.2* | 111 ± 3 |
| . | Body weight (g) . | BG (mg/dL) . | Glycated Hb (%) . | KW/BW ratio . | sBP (mmHg) . |
|---|---|---|---|---|---|
| ND | 28.8 ± 0.7 | 140 ± 2 | 4.1 ± 0.1 | 5.3 ± 0.2 | 108 ± 5 |
| ND-AM6545 | 29.0 ± 0.7 | 130 ± 2 | 4.0 ± 0.0 | 5.6 ± 0.2 | 106 ± 4 |
| ND-A + A | 28.0 ± 0.3 | 130 ± 1 | 3.9 ± 0.2 | 5.5 ± 0.4 | 108 ± 2 |
| DM | 23.8 ± 0.4* | 360 ± 8* | 11.4 ± 0.6* | 7.8 ± 0.3* | 117 ± 4 |
| DM-AM6545 | 24.2 ± 0.4* | 369 ± 7* | 11.8 ± 0.4* | 7.9 ± 0.2* | 115 ± 6 |
| DM-AM1241 | 23.8 ± 0.5* | 338 ± 32* | 10.9 ± 0.8* | 7.7 ± 0.2* | 126 ± 4 |
| DM-A + A | 23.5 ± 0.5* | 361 ± 6* | 10.0 ± 0.6* | 7.8 ± 0.2* | 111 ± 3 |
Data are shown as mean ± SEM.
A + A, AM6545 + AM1241; BG, blood glucose; Hb, haemoglobin; sBP, systolic blood pressure.
P < 0.05, DM groups versus ND groups.
| . | Body weight (g) . | BG (mg/dL) . | Glycated Hb (%) . | KW/BW ratio . | sBP (mmHg) . |
|---|---|---|---|---|---|
| ND | 28.8 ± 0.7 | 140 ± 2 | 4.1 ± 0.1 | 5.3 ± 0.2 | 108 ± 5 |
| ND-AM6545 | 29.0 ± 0.7 | 130 ± 2 | 4.0 ± 0.0 | 5.6 ± 0.2 | 106 ± 4 |
| ND-A + A | 28.0 ± 0.3 | 130 ± 1 | 3.9 ± 0.2 | 5.5 ± 0.4 | 108 ± 2 |
| DM | 23.8 ± 0.4* | 360 ± 8* | 11.4 ± 0.6* | 7.8 ± 0.3* | 117 ± 4 |
| DM-AM6545 | 24.2 ± 0.4* | 369 ± 7* | 11.8 ± 0.4* | 7.9 ± 0.2* | 115 ± 6 |
| DM-AM1241 | 23.8 ± 0.5* | 338 ± 32* | 10.9 ± 0.8* | 7.7 ± 0.2* | 126 ± 4 |
| DM-A + A | 23.5 ± 0.5* | 361 ± 6* | 10.0 ± 0.6* | 7.8 ± 0.2* | 111 ± 3 |
| . | Body weight (g) . | BG (mg/dL) . | Glycated Hb (%) . | KW/BW ratio . | sBP (mmHg) . |
|---|---|---|---|---|---|
| ND | 28.8 ± 0.7 | 140 ± 2 | 4.1 ± 0.1 | 5.3 ± 0.2 | 108 ± 5 |
| ND-AM6545 | 29.0 ± 0.7 | 130 ± 2 | 4.0 ± 0.0 | 5.6 ± 0.2 | 106 ± 4 |
| ND-A + A | 28.0 ± 0.3 | 130 ± 1 | 3.9 ± 0.2 | 5.5 ± 0.4 | 108 ± 2 |
| DM | 23.8 ± 0.4* | 360 ± 8* | 11.4 ± 0.6* | 7.8 ± 0.3* | 117 ± 4 |
| DM-AM6545 | 24.2 ± 0.4* | 369 ± 7* | 11.8 ± 0.4* | 7.9 ± 0.2* | 115 ± 6 |
| DM-AM1241 | 23.8 ± 0.5* | 338 ± 32* | 10.9 ± 0.8* | 7.7 ± 0.2* | 126 ± 4 |
| DM-A + A | 23.5 ± 0.5* | 361 ± 6* | 10.0 ± 0.6* | 7.8 ± 0.2* | 111 ± 3 |
Data are shown as mean ± SEM.
A + A, AM6545 + AM1241; BG, blood glucose; Hb, haemoglobin; sBP, systolic blood pressure.
P < 0.05, DM groups versus ND groups.
Glomerular expression of CB1R and CB2R
CB1R mRNA and protein levels were significantly enhanced in the renal cortex from DM as assessed by both immunofluorescence and real-time PCR. This was not altered in DM treated with AM6545 and/or AM1241 (Figure 1A–C), making it unlikely that autoinduction was a mechanism of diabetes-induced CB1R overexpression. Neither diabetes nor drugs affected CB2R expression (Figure 1D–F).
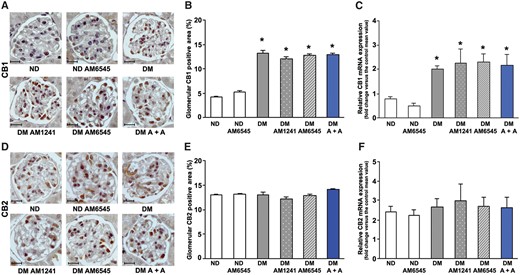
Effect of AM6545 and/or AM1241 on CB1R and CB2R expression. CB1 and CB2 mRNA and protein expression was assessed in ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group). Scale bar: 50 µm; representative immunohistochemistry images of CB1 (A) and CB2 (D) are shown and quantification of glomerular staining reported in the graphs (B, E). CB1 and CB2 mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex (C, F). *P < 0.05, DM groups versus ND groups.
Albuminuria, tubular injury and renal function
There was a significant increase in urinary albumin excretion rate (AER) in DM after 14 weeks of diabetes (Table 2). Combined treatment with AM6545 and AM1241 prevented the increase in albuminuria observed in DM, while albuminuria was only diminished (50% reduction) in DM under single treatment
| . | AER (µg/18 h) . | Creatinine clearance (mL/min) . | NAG (U/g) . |
|---|---|---|---|
| ND | 79.5 (70.7–109.3) | 0.50 ± 0.05 | 159.9 ± 3.2 |
| ND-AM6545 | 75.6 (75.6–113.7) | 0.48 ± 0.06 | 162.8 ± 14.6 |
| ND-A + A | 72.6 (56.5–100.1) | 0.59 ± 0.09 | 157.9 ± 24.6 |
| DM | 310.2 (243.8–342.0)* | 0.46 ± 0.05 | 467.3 ± 65.7* |
| DM-AM6545 | 163.8 (135.1–197.6)*,** | 0.38 ± 0.06 | 452.4 ± 30.7* |
| DM-AM1241 | 169.2 (155.5–177.5)*,** | 0.45 ± 0.08 | 461.9 ± 30.7* |
| DM-A + A | 92.2 (69.9–118.2)**,*** | 0.52 ± 0.07 | 295.0 ± 24.9*,** |
| . | AER (µg/18 h) . | Creatinine clearance (mL/min) . | NAG (U/g) . |
|---|---|---|---|
| ND | 79.5 (70.7–109.3) | 0.50 ± 0.05 | 159.9 ± 3.2 |
| ND-AM6545 | 75.6 (75.6–113.7) | 0.48 ± 0.06 | 162.8 ± 14.6 |
| ND-A + A | 72.6 (56.5–100.1) | 0.59 ± 0.09 | 157.9 ± 24.6 |
| DM | 310.2 (243.8–342.0)* | 0.46 ± 0.05 | 467.3 ± 65.7* |
| DM-AM6545 | 163.8 (135.1–197.6)*,** | 0.38 ± 0.06 | 452.4 ± 30.7* |
| DM-AM1241 | 169.2 (155.5–177.5)*,** | 0.45 ± 0.08 | 461.9 ± 30.7* |
| DM-A + A | 92.2 (69.9–118.2)**,*** | 0.52 ± 0.07 | 295.0 ± 24.9*,** |
Data are shown as mean ± SEM or geometric mean (25th–75th percentile).
A + A, AM6545 + AM1241.
P < 0.05, DM groups versus ND groups.
P < 0.05, treated DM groups versus DM.
P < 0.01, DM-A + A versus single treatments.
| . | AER (µg/18 h) . | Creatinine clearance (mL/min) . | NAG (U/g) . |
|---|---|---|---|
| ND | 79.5 (70.7–109.3) | 0.50 ± 0.05 | 159.9 ± 3.2 |
| ND-AM6545 | 75.6 (75.6–113.7) | 0.48 ± 0.06 | 162.8 ± 14.6 |
| ND-A + A | 72.6 (56.5–100.1) | 0.59 ± 0.09 | 157.9 ± 24.6 |
| DM | 310.2 (243.8–342.0)* | 0.46 ± 0.05 | 467.3 ± 65.7* |
| DM-AM6545 | 163.8 (135.1–197.6)*,** | 0.38 ± 0.06 | 452.4 ± 30.7* |
| DM-AM1241 | 169.2 (155.5–177.5)*,** | 0.45 ± 0.08 | 461.9 ± 30.7* |
| DM-A + A | 92.2 (69.9–118.2)**,*** | 0.52 ± 0.07 | 295.0 ± 24.9*,** |
| . | AER (µg/18 h) . | Creatinine clearance (mL/min) . | NAG (U/g) . |
|---|---|---|---|
| ND | 79.5 (70.7–109.3) | 0.50 ± 0.05 | 159.9 ± 3.2 |
| ND-AM6545 | 75.6 (75.6–113.7) | 0.48 ± 0.06 | 162.8 ± 14.6 |
| ND-A + A | 72.6 (56.5–100.1) | 0.59 ± 0.09 | 157.9 ± 24.6 |
| DM | 310.2 (243.8–342.0)* | 0.46 ± 0.05 | 467.3 ± 65.7* |
| DM-AM6545 | 163.8 (135.1–197.6)*,** | 0.38 ± 0.06 | 452.4 ± 30.7* |
| DM-AM1241 | 169.2 (155.5–177.5)*,** | 0.45 ± 0.08 | 461.9 ± 30.7* |
| DM-A + A | 92.2 (69.9–118.2)**,*** | 0.52 ± 0.07 | 295.0 ± 24.9*,** |
Data are shown as mean ± SEM or geometric mean (25th–75th percentile).
A + A, AM6545 + AM1241.
P < 0.05, DM groups versus ND groups.
P < 0.05, treated DM groups versus DM.
P < 0.01, DM-A + A versus single treatments.
Expression of podocytes proteins
Diabetes-induced downregulation of nephrin, podocin and synaptopodin was abolished in mice treated with either AM6545 or AM1241, as assessed by immunofluorescence, immunoblotting and real-time PCR, providing a potential mechanism whereby AM6545 and AM1241 reduced diabetes-induced albuminuria. Combined therapy did not result in a further benefit, as a maximal effect was achieved with single treatments (Figures 2 and 3A–C).
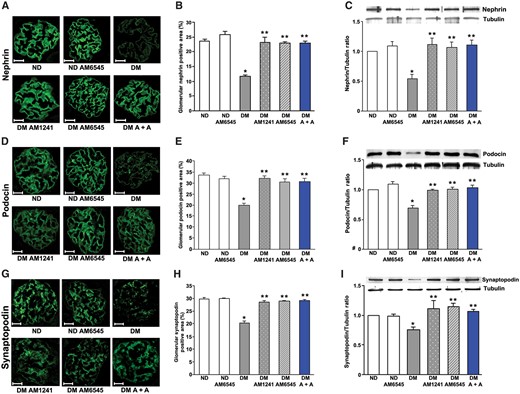
Both AM6545 and AM1241 prevented podocyte protein downregulation in diabetic mice. Nephrin, podocin and synaptopodin protein expression was assessed in ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group) by immunofluorescence and western blotting. Scale bar: 50 µm. Representative immunofluorescence images of nephrin (A), podocin (D) and synaptopodin (G) are shown and quantification of glomerular staining reported in the graphs (B, E, H). Representative immunoblots of nephrin (C), podocin (F) and synaptopodin (I) protein expression in total protein extracts from the renal cortex and corresponding results of densitometry analyses are shown in the graphs. Tubulin was used as an internal control. *P < 0.05, DM group versus ND groups; **P < 0.05, treated DM groups versus DM group.
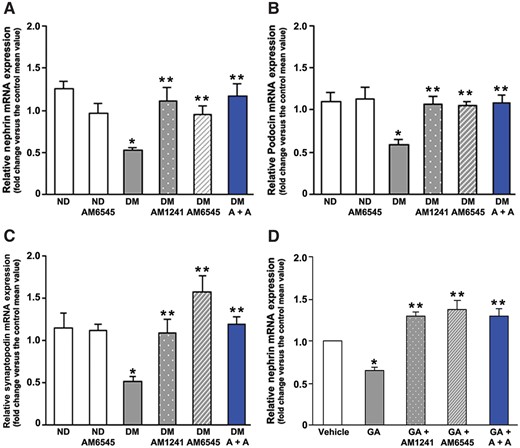
In vivo and in vitro effect of CB1R blockade and/or CB2R activation on nephrin, podocin and synaptopodin gene expression. Nephrin (A), podocin (B) and synaptopodin (C) mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex of ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group). *P < 0.05, DM group versus ND groups; **P < 0.05, treated DM groups versus DM group. (D) Cultured podocytes were exposed to glycated albumin (GA 200 μg/mL) for 24 h in the presence of AM1241 (10 μmol/L), AM6545 (10 μmol/L) or both (A + A), then total mRNA was extracted and nephrin mRNA levels measured by real-timePCR. *P < 0.05, GA versus others; **P < 0.05, vehicle versus treated groups.
Expression of nephrin in cultured podocytes
To clarify if AM6545 and AM1241 prevented nephrin downregulation by acting directly on podocytes, podocytes were exposed in vitro to GA to mimic the diabetic milieu. GA significantly reduced nephrin mRNA expression, as previously reported [25]. Treatment with AM6545 and/or AM1241 not only prevented GA-induced nephrin downregulation, but significantly increased nephrin expression compared with controls (Figure 3D).
Mesangial expansion and expression of extracellular matrix components
PAS staining revealed a mild mesangial expansion in DM that was not altered by treatment with AM1241 and was only modestly diminished by AM6545, whereas a marked reduction was observed in DM treated with the dual therapy (Figure 4A and B).
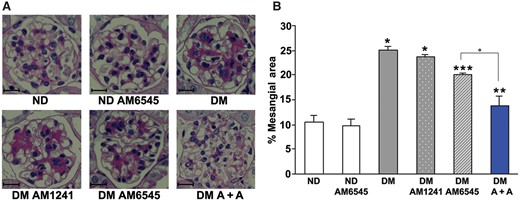
Dual therapy prevented glomerular mesangial expansion in diabetic mice. Renal cortex from ND mice and DM treated with AM6545, AM1241 or both (A + A) was studied 14 weeks after diabetes induction (n = 8 mice per group). Scale bar: 50 µm. Representative PAS staining images (×400) (A) and quantitation of the percentage of mesangial areas (B) are shown. *P < 0.05, DM groups versus ND controls; **P < 0.05, DM-AM6545 and DM-A + A versus DM; ***P < 0.05, DM-A + A versus DM-AM6545.
Collagen staining was enhanced in DM in both the glomeruli and the interstitial area. This effect was reduced by AM6545, unaltered by AM1241 and abolished by dual treatment (Figure 5A and B). Similarly, the increase in fibronectin immunofluorescence staining induced by diabetes was only slightly attenuated by AM6545, but was prevented by combination therapy (Figure 5D and E). Analyses of collagen type 1 and fibronectin mRNA expression showed a similar trend (Figure 5C and F), although the effect of single treatment with AM6545 was greater. Taken together, these data indicate a greater effectiveness of dual treatment in preventing diabetes-induced fibrotic processes within the kidney.
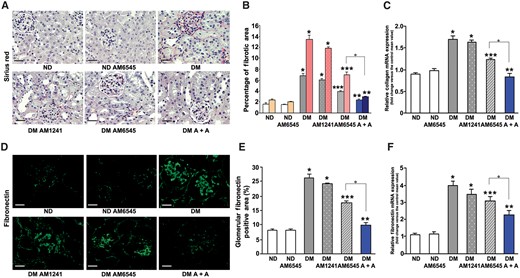
Effect of AM6545 and/or AM1241 on both collagen and fibronectin expression. Collagen and fibronectin protein and mRNA expression was assessed in ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group). (A) Representative images of picrosirius red staining and (B) quantitation of glomerular (first columns) and interstitial staining (second columns) are shown. Images in (D) show immunofluorescence staining for fibronectin. Scale bar: 100 µm. Quantification of fibronectin staining is reported in the graph (E). Collagen type I (C) and fibronectin (F) mRNA expression was measured by real-time PCR on total RNA extracted from the renal cortex and results reported in the graphs. *P < 0.05, DM groups versus ND controls; **P < 0.05, DM-AM6545 and DM-A + A versus DM; ***P < 0.05, DM-A + A versus DM-AM6545.
Monocyte infiltration
Given the key role of inflammatory processes in promoting fibrosis in DN [26–28], renal monocyte infiltration was assessed by counting the number of F4/80-positive cells in the renal cortex. The number of infiltrating monocytes/macrophages was significantly augmented in DM: there was a 6-fold increase within the glomeruli and a 12-fold increase in the tubulo-interstitium. Combined therapy abolished diabetes-induced monocyte/macrophage accrual in both the glomeruli and the interstitium, while the number of infiltrating monocytes/macrophages fell only by half in mice under single treatment and was still significantly greater than in control animals (Figure 6A–D). Collectively, these data indicate that both CB1R blockade and CB2R activation reduce diabetes-induced renal inflammation, but this is achieved much more effectively by dual treatment.
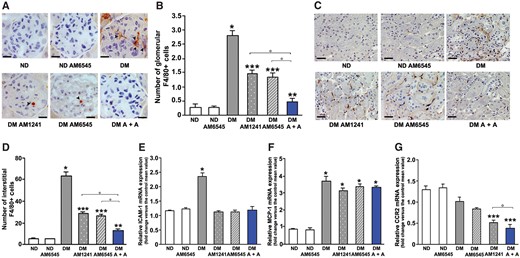
Effect of AM6545 and AM1241 on diabetes-induced inflammation. Monocyte infiltration and markers of inflammation were assessed in ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group). Representative images of both glomerular (A) and tubulo-interstitial (C) F4/80-positive staining are shown and quantitation reported in the graphs (B, D). Scale bar: 50 µm (A) and 100 µm (C). Total RNA was extracted from the renal cortex and mRNA levels of ICAM-1 (E), MCP-1 (F), CCR2 (G) measured by real time-PCR. *P < 0.05, DM groups versus ND controls; **P < 0.05, treated DM groups versus DM group; ***P < 0.05, DM under combined treatments versus DM under single treatment.
ICAM-1 and the MCP-1/CCR2 system
We then studied both ICAM-1 and the MCP-1/CCR2 system, as they play a key role in renal monocyte recruitment. Diabetes induced overexpression of both ICAM-1 and MCP-1, while expression of the MCP-1 receptor CCR2 was not significantly altered. Diabetes-induced ICAM-1 overexpression was prevented in mice treated with AM6545 and/or AM1241, showing that these compounds can interfere with monocytes entering the glomerular tuft. In addition, AM1241 downregulated CCR2 expression, likely resulting in reduced MCP-1 signalling, and this effect was retained in mice treated with dual therapy (Figure 6E–G).
M1 and M2 macrophages
In DM, markers of pro-inflammatory M1 macrophages [29], TNF-α and NOS2, were overexpressed in the renal cortex, whereas markers of anti-inflammatory M2 macrophages [29–31] arginase-1 (Arg-1) and CD163 were downregulated, indicating a shift towards a pro-inflammatory M1 phenotype. Treatment with either AM6545 or AM1241 completely suppressed diabetes-induced NOS2 overexpression, whereas TNF-α overexpression was only attenuated by single treatment, while dual therapy was required to abolish the effect (Figure 7A and B), suggesting an additive contribution of enhanced CB1R and insufficient CB2R signalling in TNF-α induction.
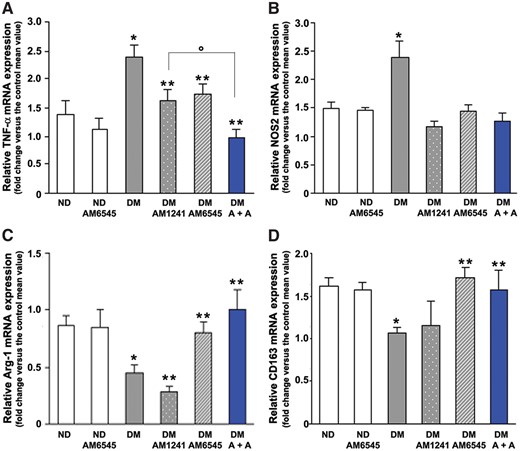
Effect of AM6545 and AM1241 on diabetes-induced macrophage phenotype. TNF-α (A), NOS2 (B), Arg-1 (C) and CD163 (D) mRNA levels were measured by real-time PCR on total RNA extracted from the renal cortex of ND mice and DM treated with AM6545, AM1241 or both (A + A) (n = 8 mice per group). *P < 0.05, DM groups versus ND controls; **P < 0.05, treated DM groups versus DM group; ***P < 0.05, DM under combined treatments versus DM under single treatment.
Diabetes-induced Arg-1 and CD163 downregulation was not affected by treatment with AM1241, but was suppressed by AM6545 either alone or in combination therapy (Figure 4C and D), indicating a predominant role of CB1R in mediating impaired M2 polarization. Quantitatively, the 4-fold increase in the M1:M2 ratio [32] that was observed in DM compared with ND mice was reduced by both AM1241 and AM6545, but was prevented only by dual therapy (ND: 1.57 ± 0.40; DM: 6.19 ± 1.11; DM-AM1241: 3.42 ± 0.83; DM-AM6545: 2.29 ± 0.37; DM-A + A: 1.37 ± 0.27; *P < 0.05 for DM versus others; DM-A + A versus DM-AM6545 and DM-AM1241).
In vitro experiments on cultured macrophages
To explore the possibility that the effect of dual therapy on the M1/M2 imbalance may provide an explanation for its greater anti-fibrotic efficacy, we performed in vitro experiments on mesangial cells, which play a predominant role in glomerulosclerosis [33]. Exposure of mesangial cells to CM from M1 macrophages induced a 2.5-fold increase in fibronectin mRNA, confirming that factors/cytokines released by M1 macrophages enhance the expression of extracellular matrix components [26]. However, when mesangial cells were incubated with CM from M1 macrophages pre-exposed to AM6545 (CMAM6545), AM1241 (CMAM1241) or both (CMA +A), fibronectin overexpression was reduced by single and abolished by combined pretreatments, indicating that dual therapy can affect the phenotype of M1 macrophages, abolishing their paracrine pro-fibrotic activity (Figure 8A). In addition, AM6545 and/or AM1241 treatment significantly lowered TNF-α expression in M1 macrophages (Figure 8B). Moreover, the increase in fibronectin expression in response to CM from M1 macrophages was abolished by a specific TNF-α neutralizing antibody (Figure 8C), suggesting TNF-α as a likely mediator of EC-modulated M1 macrophage pro-sclerotic activity.
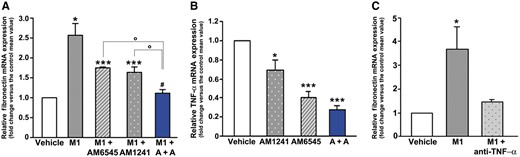
CB1R blockade and CB2R activation affected the pro-fibrotic phenotype of M1 macrophages. (A) Mesangial cells were exposed for 48 h to CM from M1 macrophages pre-exposed for 24 h to vehicle (CM), AM6545 (CMAM6545), AM1241 (CMAM1241) or both (CMA+A), then fibronectin mRNA levels were measured by real-time PCR. *P < 0.05, M1, M1-AM6545, M1-AM1241 versus vehicle; **P < 0.05, M1-AM6545, M1-AM1241 versus M1; ***P < 0.05, M1-A + A versus M1-AM6545 and M1-AM1241. (B) Total RNA was extracted from M1 macrophages and TNF-α mRNA levels measured by real-time PCR. *P < 0.05, AM1241, AM6545, A + A versus vehicle; ***P < 0.05, AM6545 and A + A versus AM1241. (C) Fibronectin mRNA expression in mesangial cells incubated with CM from M1 macrophages in the presence and in the absence of a neutralizing TNF-α antibody. *P < 0.05, M1 versus others.
DISCUSSION
In the present study, we have provided evidence that combined therapy with AM6545, a novel peripheral CB1R antagonist, and AM1241, a CB2R agonist, prevents diabetes-induced renal abnormalities, both functional and structural.
In DM, dual therapy with AM6545 and AM1241 abolished albuminuria and thus performed better than monotherapies, which reduced it by only 50%, in line with previous reports in the same animal model [4, 5]. This also indicates that peripheral and global CB1R inhibition is equally effective at preventing proteinuria in experimental type 1 diabetes. JD5037, another peripheral CB1R inverse agonist, has also been shown to diminish albuminuria in obese hypertensive ZDF rats [12]; however, in this model, beneficial effects may be secondary to amelioration of hypertension and metabolism [2, 34].
Both nephrin and podocin are important in preventing glomerular protein leakage, and their loss has been implicated in the pathogenesis of albuminuria in various renal diseases, including DN [33, 35]. AM6545 and/or AM1241 prevented nephrin and podocin downregulation in vivo and abolished nephrin loss in podocytes exposed to glycated albumin to mimic a diabetic milieu, suggesting a direct effect on podocytes that is consistent with the predominant expression of CB1R/CB2R by this cell type [4, 5, 11, 12]. Our finding, together with previous results showing that CB1R mediates both high-glucose– and angiotensin II–induced nephrin downregulation [12], supports the hypothesis that CB1R signalling is a final common pathway of multiple diabetes-related insults leading to both podocyte protein loss and proteinuria. Our data also demonstrate a previously unrecognized direct effect of CB2R signalling on nephrin expression that might be relevant beyond DN. Moreover, the observation that AM6545 and AM1241 enhanced nephrin expression beyond control levels supports the notion that the balance in ECS receptor signalling is a crucial determinant of podocyte phenotype.
Structural analysis showed abolishment of mesangial expansion in mice under double treatment in parallel with complete reversal of glomerular fibronectin upregulation and collagen deposition in both the mesangial and the interstitial area. By contrast, treatment with AM1241 was ineffective and AM6545 only partially reduced these abnormalities. Furthermore, dual therapy showed a protective effect on the tubulo-interstitium that was not observed in DM under single treatments. These findings are of relevance, as glomerulosclerosis and tubulo-interstitial injury are major determinants of renal dysfunction and DN progression [33]. Moreover, they support the hypothesis that dual therapy has beneficial effects beyond the additive effect of single treatments.
In contrast with our previous results where there was no effect of CB1R inhibition with AM251 on renal fibrosis in STZ-induced diabetes [4], treatment with AM6545 alone reduced expression of extracellular matrix components and modestly ameliorated the renal structural injury. The reason for this discrepancy is unclear; however, the effect of the CB1R neutral antagonist AM6545 may differ from that of the inverse agonist AM251 [36]. Moreover, the possibility of an AM6545 off-target effect cannot be excluded. Consistent with our present data, an anti-fibrotic effect of CB1R blockade has been shown in mice with unilateral ureteral obstruction (UUO) [13] and in the kidney of type 2 diabetes mellitus [7, 9].
Mounting evidence highlights the important role of renal monocyte/macrophage infiltration in the pathogenesis of DN [25, 37]. Moreover, recent investigations suggest that the phenotype of recruited macrophages is of paramount importance. In our study, DM showed both an enhanced renal macrophage accrual and a shift towards inflammatory M1 macrophages. These effects were only attenuated by single treatments, but abrogated by dual therapy. Complementary anti-inflammatory effects of AM6545 and AM1241 may explain this greater efficacy. AM6545 had no effect on the MCP-1/CCR2 system, while AM1241 downregulated CCR2, thus reducing responsiveness to MCP-1. This suggests that the pro-inflammatory effects of MCP-1, which trigger and fuel inflammation in DM treated with AM6545, may be neutralized by co-administration of AM1241. On the other hand, both compounds reduced markers of pro-inflammatory M1 macrophages, but only AM6545 prevented diabetes-induced downregulation of markers of anti-inflammatory M2 macrophages, which are known to play a key role in resolving inflammation and promoting repair [29–31].
The mechanism whereby dual therapy abolished renal fibrosis is unknown. Macrophages are likely involved, as they can release factors that enhance mesangial cell expression of extracellular matrix components [26, 37]. In keeping with the hypothesis that dual therapy more effectively suppressed fibrotic processes because of a greater anti-inflammatory activity, our in vitro experiments showed that M1 macrophages enhanced mesangial cell fibronectin expression via a paracrine mechanism mediated by TNF-α and that this effect was diminished by M1 pre-exposure to either AM6545 or AM1241 and abolished by dual therapy. Alternatively, epithelial–mesenchymal transition may also be involved, as CBR1 is expressed by renal myofibroblasts and CB1R blockade prevents fibrosis in the UUO model [13]. However, in contrast with DN, CB2R is overexpressed in UUO and CB2R activation fails to amplify the effect of CB1R blockade [13], suggesting that pathophysiological mechanisms operating in the two models are not entirely overlapping.
In conclusion, these in vitro and in vivo findings may have important implications for DN in humans. Both CB1R overexpression and CB2R downregulation have been proven in renal biopsies from patients with DN [5, 13]. Moreover, a CB1R polymorphism has been associated with renal disease in diabetic patients [38]. Previous studies in experimental diabetes have shown that single treatment with either CB1R inverse agonists/antagonists or CB2R agonists can ameliorate experimental DN [4, 5, 9–12]. However, this is the first study exploring the effect of a combined treatment in DN. The data reported herein showing a greater efficacy of dual therapy may prompt further studies applying this novel therapeutic approach to DN and other pathological conditions characterized by an underlying CBR1/CB2R imbalance, thus opening new avenues of research. Moreover, our study provides evidence that AM6545 ameliorates DN in experimental type 1 diabetes, supporting the hypothesis that peripheral restricted CB1R antagonists may hold promise for future potential therapeutic applications in DN. In view of a possible clinical use, it is noteworthy that a non-brain-penetrant CB1R antagonist can also revert established DN abnormalities in a model of type 2 diabetes [12]. Additional studies are required to establish if the addition of dual ECS-targeting treatment to current DN therapy will result in further benefit.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
ACKNOWLEDGEMENTS
This work was supported by the European Foundation for the Study of Diabetes (EASD), the Compagnia di San Paolo and the Italian Society of Diabetes (SID).
CONFLICT OF INTEREST STATEMENT
None declared.
References
- anti-inflammatory agents
- diabetes mellitus
- diabetic nephropathy
- inflammation
- renal function
- albumins
- diabetes mellitus, type 2
- fibrosis
- culture media, conditioned
- kidney glomerulus
- macrophages
- monocytes
- psychotherapy, multiple
- rna, messenger
- streptozocin
- kidney
- mice
- agonists
- antagonists
- endocannabinoids
- mesangial cells
- podocytes
- cannabinoid receptors
- albuminuria





Comments