-
PDF
- Split View
-
Views
-
Cite
Cite
Tobias Felix Kröpelin, Dick de Zeeuw, Frank Arjan Holtkamp, David Kenneth Packham, Hiddo J. L. Heerspink, Individual long-term albuminuria exposure during angiotensin receptor blocker therapy is the optimal predictor for renal outcome, Nephrology Dialysis Transplantation, Volume 31, Issue 9, September 2016, Pages 1471–1477, https://doi.org/10.1093/ndt/gfv429
Close - Share Icon Share
Abstract
Albuminuria reduction due to angiotensin receptor blockers (ARBs) predicts subsequent renoprotection. Relating the initial albuminuria reduction to subsequent renoprotection assumes that the initial ARB-induced albuminuria reduction remains stable during follow-up. The aim of this study was to assess individual albuminuria fluctuations after the initial ARB response and to determine whether taking individual albuminuria fluctuations into account improves renal outcome prediction.
Patients with diabetes and nephropathy treated with losartan or irbesartan in the RENAAL and IDNT trials were included. Patients with >30% reduction in albuminuria 3 months after ARB initiation were stratified by the subsequent change in albuminuria until Month 12 in enhanced responders (>50% albuminuria reduction), sustained responders (between 20 and 50% reduction), and response escapers (<20% reduction). Predictive performance of the individual albuminuria exposure until Month 3 was compared with the exposure over the first 12 months using receiver operating characteristics (ROC) curves.
Following ARB initiation, 388 (36.3%) patients showed an >30% reduction in albuminuria. Among these patients, the albuminuria level further decreased in 174 (44.8%), remained stable in 123 (31.7%), and increased in 91 (23.5%) patients. Similar albuminuria fluctuations were observed in patients with <30% albuminuria reduction. Renal risk prediction improved when using the albuminuria exposure during the first 12 months versus the initial Month 3 change [ROC difference: 0.78 (95% CI 0.75–0.82) versus 0.68 (0.64–0.72); P < 0.0001].
Following the initial response to ARBs, a large within-patient albuminuria variability is observed. Hence, incorporating multiple albuminuria measurements over time in risk algorithms may be more appropriate to monitor treatment effects and quantify renal risk.
INTRODUCTION
Albuminuria has been shown to be a good predictor and risk marker for renal morbidity and mortality [1–5]. The causality of this association is extensively debated, the cons stating that albuminuria increase is a consequence of renal/cardiovascular disease [6], the pros stating that albuminuria leads to tissue damage [7]. This debate could be supported by data that show or deny that albuminuria lowering is associated with and predictive of renal disease protection, and in particular that the degree of renal protection is fully explained by the albuminuria reduction. This is not only important for the research into the mechanism, but also important in aiding the treatment of a patient, since one can estimate by the initial effects of the treatment (first couple of weeks/months) what will likely happen in the long run.
However, relating the lowering of a risk factor to subsequent renoprotection relies on the assumption that the initial reduction of the risk factor remains stable during prolonged follow-up. Blood pressure reduction in the first couple of weeks during antihypertensive treatment is indeed related to the degree of renoprotection. However, many patients can show a subsequent further decrease or increase in blood pressure, the latter being due to either drug effect escape or progression of the underlying disease. These blood pressure changes during treatment are important for accurately predicting the true effect of an antihypertensive on subsequent cardiovascular outcome [8].
Given these considerations, the aim of this study was to assess whether taking into account the fluctuations in albuminuria within individuals (e.g. the exposure to albuminuria over time) after the initial response to angiotensin receptor blockers would be a more accurate and precise predictor of renal outcome.
METHODS
Patients and study design
For the present study we combined data from the Reduction of Endpoints in Non-Insulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) trial and the Irbesartan Type II Diabetic Nephropathy Trial (IDNT). The detailed design, rationale and study outcome for these trials have been previously published [9–12]. Both trials investigated the efficacy of an ARB (irbesartan in the IDNT, losartan in the RENAAL trial) on renal outcomes in subjects with type 2 diabetes and nephropathy. In addition, the IDNT included a calcium channel blocker (amlodipine) treatment arm. For our study, only the active treatment arms (irbesartan, losartan) were included. Inclusion criteria in the IDNT and RENAAL trial were similar, with only minor differences. Patients ages 30–70 years with type 2 diabetes, hypertension and nephropathy were eligible for both trials. Serum creatinine levels ranged between 1.0 and 3.0 mg/dL. All subjects had proteinuria, defined as 24-h urinary protein excretion >900 mg in the IDNT, whereas for the RENAAL trial a urinary albumin:creatinine ratio (UACR) >300 mg/g based on a single first morning void or a 24-h urinary protein excretion >500 mg/day was required. In both trials the glomerular filtration rate (GFR) was not measured; the estimated GFR (eGFR) for each patient was determined using the modified Modification of Diet in Renal Disease (MDRD) Study formula [13]. Exclusion criteria for both trials were type 1 diabetes or non-diabetic renal disease. Patients in the RENAAL trial were randomly allocated to treatment with losartan 100 mg/day or matched placebo, whereas patients in the IDNT were randomly allocated to treatment with irbesartan 300 mg/day, amlodipine 10 mg/day or matched placebo. The trials were designed to keep the dose of the ARBs stable during follow-up. Additional antihypertensive agents [but not angiotensin-converting enzyme inhibitors (ACEis) or ARBs in the RENAAL trial and ACEis, ARBs or calcium channel blockers in the IDNT) were allowed during the trial to achieve the target blood pressure.
Albuminuria changes over time
In both trials, urinary albumin and urinary creatinine, and thereby the albumin:creatinine ratio was measured at baseline, 3 months, 6 months and every 6 months thereafter. Urinary albumin and creatinine were measured in a central laboratory in both trials. The initial change in albuminuria was calculated from baseline to Month 3. Subsequently, individual variations in albuminuria were ascertained from Month 3 to Month 12.
Clinical endpoints
A composite of end-stage renal disease (ESRD), defined as chronic dialysis or renal transplantation, or a confirmed doubling of serum creatinine from baseline was used as the primary renal endpoint for this study. In an additional analysis, the predictive performance of the total exposure to albuminuria for all-cause mortality and hospitalization for heart failure was assessed. The hospitalization for heart failure endpoint was chosen since the original RENAAL trial and IDNT showed that losartan and irbesartan had a beneficial effect on this endpoint. In both trials all clinical endpoints were adjudicated by an independent endpoint committee using rigorous guidelines and definitions. Outcome ascertainment started from the date of the 12-month albuminuria measurement until the end of both trials.
Statistical analyses and responder definition
All patients receiving ARB treatment with available albuminuria data at baseline and Month 3 and at least one measurement for Month 6 or Month 12 were included in the analysis. The impact of excluding patients with missing albuminuria data was assessed in sensitivity analyses. To this end, within-individual regression methods were used to impute missing albuminuria measurements between Month 3 and Month 12.
We stratified the population according to their initial treatment response in albuminuria levels from baseline to Month 3. Responders showed an initial reduction in albuminuria from baseline of at least 30% [14]. Non-responders had a reduction <30%. This threshold was chosen based on prior work [15, 16]. Subsequently, we further stratified the responder and non-responder groups into three strata based on the change in albuminuria from Month 3 to 12. Sustained albuminuria responders showed average albuminuria reduction between 20 and 50%, enhanced albuminuria responders showed a further reduction to >50% and those who escaped the response showed a reduction <20% relative to baseline. Non-responders were also stratified in three groups. Sustained albuminuria non-responders showed an albuminuria change between −30 and 30%, enhanced non-responders showed a further increase to >30% and those who switched to a response showed a reduction in albuminuria >30% relative to baseline. Baseline characteristics of the population stratified by response patterns were summarized by means and standard deviations for continuous data and counts and percentages for categorical data. The risk of renal outcomes in the three strata of non-responders and responders was determined using Cox proportional hazard regression using the sustained responders or sustained non-responders as the reference group. Patients who reached a primary renal event during the first 12 months of the trial were excluded. The Cox proportional hazard models were adjusted for age, gender, race, baseline eGFR, albuminuria, systolic and diastolic blood pressure, haemoglobin and HbA1c and changes in systolic and diastolic blood pressure and serum potassium from Month 3 to 12. These covariates were chosen based on prior work [17]. Baseline albuminuria was log-transformed for regression analyses to take into account the skewed distribution.
In order to assess whether individual albuminuria exposure during ARB treatment is a better predictor of renal outcome compared with the initial change in albuminuria, we assessed and compared the area under the ROCs curve of the initial albuminuria exposure up to Month 3 with the individual albuminuria exposure over the first 12 months. All statistical analyses were performed using SAS 9.3, with a two-sided P-value <0.05 indicating statistical significance.
RESULTS
A total of 1070 (80.5%) of the 1330 patients in the RENAAL trial and IDNT assigned to ARB treatment had baseline and up to 12 months albuminuria data available. Albuminuria levels within individuals varied considerably during ARB treatment [coefficient of variation (CV) 35.6% from Month 3 to 12]. Of the 1070 included ARB-treated patients, a total of 388 (36.3%) had a >30% reduction in albuminuria at Month 3 (responders). Geometric mean albuminuria reduction in these patients was 51.4% (95% CI 54.2–49.9). Among responder patients, the albuminuria level further decreased in 174 (44.8%) patients, remained stable in 123 (31.7%) and increased in 91 (23.5%) (Figure 1A). Among the 682 (63.7% from total included population) non-responder patients (<30% albuminuria reduction at Month 3), the albuminuria level increased by 32.1% at Month 3. Of the non-responder patients, the albuminuria level further increased in 212 (31.1%), remained stable in 287 (42.1%), and decreased in 183 (26.8%; Figure 1B) patients. Baseline characteristics according to the albuminuria response pattern are presented in Table 1. Baseline characteristics were comparable across the categories in both responder and non-responder patients except that albuminuria in enhanced non-responders was lower compared with the other subgroups (all P > 0.05). Among initial albuminuria responders, blood pressure did not change from Month 3 to Month 12 in enhanced responders and moderately increased in response escapers (Table 1). Among initial non-responders, blood pressure showed the largest reduction in subjects who switched to a response (Table 1). Between Month 3 and Month 12, a diuretic or calcium channel blocker was initiated or discontinued in a few patients and was not different across the three groups. In addition, 24-h sodium excretion (as a measure of dietary sodium intake) was similar across all subgroups (Table 1).
Baseline characteristics stratified by initial treatment response and subsequent albuminuria changes during follow-up, presented as mean and standard deviation for continuous data and as counts and percentage for categorical data
| . | Initial responders (N = 388) . | Initial non-responders (N = 682) . | ||||
|---|---|---|---|---|---|---|
| Enhanced response . | Sustained response . | Escape response . | Enhanced non-response . | Sustained non-responder . | Switch to response . | |
| Cases, n (%)a | 174 (44.8) | 123 (31.7) | 91 (23.5) | 212 (31.1) | 287 (42.1) | 183 (26.8) |
| Age (years) | 60.6 (7.6) | 59.0 (8.1) | 58.6 (7.3) | 58.8 (7.7) | 59.1 (7.1) | 60.6 (7.6) |
| Gender, n male (%)b | 108 (62.1) | 78 (63.4) | 59 (64.8) | 150 (70.8) | 195 (67.9) | 129 (70.49) |
| Race, n (%)b | ||||||
| White | 101 (58.1) | 70 (56.9) | 101 (46.2) | 120 (56.6) | 168 (64.8) | 103 (56.3) |
| Black | 22 (12.6) | 13 (10.6) | 22 (19.8) | 44 (20.8) | 35 (12.2) | 37 (20.2) |
| Hispanic | 16 (9.2) | 18 (14.6) | 16 (18.7) | 30 (14.2) | 27 (9.4) | 21 (11.5) |
| Asian | 31 (17.8) | 18 (14.6) | 31 (11.0) | 13 (6.1) | 32 (11.2) | 19 (140.4) |
| Other | 4 (2.3) | 4 (3.3) | 4 (4.4) | 5 (2.4) | 7 (2.4) | 3 (1.6) |
| BMI (kg/m2) | 30.5 (6.5) | 30.2 (6.6) | 29.7 (6.3) | 30.2 (5.9) | 30.4 (5.9) | 30.5 (6.1) |
| Smoker, n (%) | 26 (14.9) | 27 (22.0) | 12 (13.2) | 39 (18.4) | 58 (20.3) | 35 (19.2) |
| Systolic BP (mmHg) | 157.8 (19.8) | 151.7 (19.8) | 151.3 (20.2) | 150.0 (18.6) | 153.8 (18.3) | 156.0 (20.6) |
| Diastolic BP (mmHg) | 82.6 (10.9) | 84.6 (11.5) | 84.0 (11.5) | 83.7 (10.4) | 84.5 (10.1) | 84.5 (11.8) |
| eGFR (mL/min/1.73 m2) | 40.9 (12.6) | 43.1 (14.1) | 44.1 (16.6) | 46.5 (16.0) | 43.8 (16.0) | 41.9 (14.6) |
| Haemoglobin (g/dL) | 12.9 (1.8) | 12.8 (1.7) | 12.9 (1.8) | 12.8 (1.8) | 12.8 (1.9) | 12.7 (1.9) |
| HbA1c (%) | 8.2 (1.6) | 8.7 (1.7) | 8.4 (1.7) | 8.5 (1.8) | 8.3 (1.7) | 8.2 (1.5) |
| Total cholesterol (mg/dL) | 217 (52) | 231 (58) | 222 (54) | 222 (49) | 229 (65) | 221 (55) |
| ACR (mg/g)c | 1713 (1683) | 1854 (1701) | 1558 (1417) | 1185 (1014) | 2153 (1780) | 1852 (1501) |
| Sodium excretion (mmol/24 h)d | 199 (107) | 167 (78) | 179 (84) | 184 (90) | 181 (86) | 172 (82) |
| Follow-up: absolute change from Month 3 to Month 12 | ||||||
| Systolic BP (mmHg)† | −0.1 (11.4) | 0.8 (12.1) | 3.7 (12.2) | −0.4 (10.3) | −1.2 (12.0) | −5.0 (13.5) |
| Diastolic BP (mmHg)† | −0.2 (6.3) | −0.2 (6.6) | 1.4 (5.9) | −0.8 (5.9) | −1.1 (6.6) | −2.6 (6.7) |
| Potassium† | 0.03 (0.45) | 0.07 (0.39) | −0.03 (0.43) | 0.05 (0.39) | 0.03 (0.42) | 0.03 (0.41) |
| . | Initial responders (N = 388) . | Initial non-responders (N = 682) . | ||||
|---|---|---|---|---|---|---|
| Enhanced response . | Sustained response . | Escape response . | Enhanced non-response . | Sustained non-responder . | Switch to response . | |
| Cases, n (%)a | 174 (44.8) | 123 (31.7) | 91 (23.5) | 212 (31.1) | 287 (42.1) | 183 (26.8) |
| Age (years) | 60.6 (7.6) | 59.0 (8.1) | 58.6 (7.3) | 58.8 (7.7) | 59.1 (7.1) | 60.6 (7.6) |
| Gender, n male (%)b | 108 (62.1) | 78 (63.4) | 59 (64.8) | 150 (70.8) | 195 (67.9) | 129 (70.49) |
| Race, n (%)b | ||||||
| White | 101 (58.1) | 70 (56.9) | 101 (46.2) | 120 (56.6) | 168 (64.8) | 103 (56.3) |
| Black | 22 (12.6) | 13 (10.6) | 22 (19.8) | 44 (20.8) | 35 (12.2) | 37 (20.2) |
| Hispanic | 16 (9.2) | 18 (14.6) | 16 (18.7) | 30 (14.2) | 27 (9.4) | 21 (11.5) |
| Asian | 31 (17.8) | 18 (14.6) | 31 (11.0) | 13 (6.1) | 32 (11.2) | 19 (140.4) |
| Other | 4 (2.3) | 4 (3.3) | 4 (4.4) | 5 (2.4) | 7 (2.4) | 3 (1.6) |
| BMI (kg/m2) | 30.5 (6.5) | 30.2 (6.6) | 29.7 (6.3) | 30.2 (5.9) | 30.4 (5.9) | 30.5 (6.1) |
| Smoker, n (%) | 26 (14.9) | 27 (22.0) | 12 (13.2) | 39 (18.4) | 58 (20.3) | 35 (19.2) |
| Systolic BP (mmHg) | 157.8 (19.8) | 151.7 (19.8) | 151.3 (20.2) | 150.0 (18.6) | 153.8 (18.3) | 156.0 (20.6) |
| Diastolic BP (mmHg) | 82.6 (10.9) | 84.6 (11.5) | 84.0 (11.5) | 83.7 (10.4) | 84.5 (10.1) | 84.5 (11.8) |
| eGFR (mL/min/1.73 m2) | 40.9 (12.6) | 43.1 (14.1) | 44.1 (16.6) | 46.5 (16.0) | 43.8 (16.0) | 41.9 (14.6) |
| Haemoglobin (g/dL) | 12.9 (1.8) | 12.8 (1.7) | 12.9 (1.8) | 12.8 (1.8) | 12.8 (1.9) | 12.7 (1.9) |
| HbA1c (%) | 8.2 (1.6) | 8.7 (1.7) | 8.4 (1.7) | 8.5 (1.8) | 8.3 (1.7) | 8.2 (1.5) |
| Total cholesterol (mg/dL) | 217 (52) | 231 (58) | 222 (54) | 222 (49) | 229 (65) | 221 (55) |
| ACR (mg/g)c | 1713 (1683) | 1854 (1701) | 1558 (1417) | 1185 (1014) | 2153 (1780) | 1852 (1501) |
| Sodium excretion (mmol/24 h)d | 199 (107) | 167 (78) | 179 (84) | 184 (90) | 181 (86) | 172 (82) |
| Follow-up: absolute change from Month 3 to Month 12 | ||||||
| Systolic BP (mmHg)† | −0.1 (11.4) | 0.8 (12.1) | 3.7 (12.2) | −0.4 (10.3) | −1.2 (12.0) | −5.0 (13.5) |
| Diastolic BP (mmHg)† | −0.2 (6.3) | −0.2 (6.6) | 1.4 (5.9) | −0.8 (5.9) | −1.1 (6.6) | −2.6 (6.7) |
| Potassium† | 0.03 (0.45) | 0.07 (0.39) | −0.03 (0.43) | 0.05 (0.39) | 0.03 (0.42) | 0.03 (0.41) |
aPercent of the initial response strata.
bPercent of the total angiotensin receptor blocker population.
cACR, albumin:creatinine ratio reported as geometric mean.
dData on 24-h sodium excretion were available in 389 (36%) subjects. Characteristics of these subjects did not differ from the overall cohort.
†P for trend >0.5.
Baseline characteristics stratified by initial treatment response and subsequent albuminuria changes during follow-up, presented as mean and standard deviation for continuous data and as counts and percentage for categorical data
| . | Initial responders (N = 388) . | Initial non-responders (N = 682) . | ||||
|---|---|---|---|---|---|---|
| Enhanced response . | Sustained response . | Escape response . | Enhanced non-response . | Sustained non-responder . | Switch to response . | |
| Cases, n (%)a | 174 (44.8) | 123 (31.7) | 91 (23.5) | 212 (31.1) | 287 (42.1) | 183 (26.8) |
| Age (years) | 60.6 (7.6) | 59.0 (8.1) | 58.6 (7.3) | 58.8 (7.7) | 59.1 (7.1) | 60.6 (7.6) |
| Gender, n male (%)b | 108 (62.1) | 78 (63.4) | 59 (64.8) | 150 (70.8) | 195 (67.9) | 129 (70.49) |
| Race, n (%)b | ||||||
| White | 101 (58.1) | 70 (56.9) | 101 (46.2) | 120 (56.6) | 168 (64.8) | 103 (56.3) |
| Black | 22 (12.6) | 13 (10.6) | 22 (19.8) | 44 (20.8) | 35 (12.2) | 37 (20.2) |
| Hispanic | 16 (9.2) | 18 (14.6) | 16 (18.7) | 30 (14.2) | 27 (9.4) | 21 (11.5) |
| Asian | 31 (17.8) | 18 (14.6) | 31 (11.0) | 13 (6.1) | 32 (11.2) | 19 (140.4) |
| Other | 4 (2.3) | 4 (3.3) | 4 (4.4) | 5 (2.4) | 7 (2.4) | 3 (1.6) |
| BMI (kg/m2) | 30.5 (6.5) | 30.2 (6.6) | 29.7 (6.3) | 30.2 (5.9) | 30.4 (5.9) | 30.5 (6.1) |
| Smoker, n (%) | 26 (14.9) | 27 (22.0) | 12 (13.2) | 39 (18.4) | 58 (20.3) | 35 (19.2) |
| Systolic BP (mmHg) | 157.8 (19.8) | 151.7 (19.8) | 151.3 (20.2) | 150.0 (18.6) | 153.8 (18.3) | 156.0 (20.6) |
| Diastolic BP (mmHg) | 82.6 (10.9) | 84.6 (11.5) | 84.0 (11.5) | 83.7 (10.4) | 84.5 (10.1) | 84.5 (11.8) |
| eGFR (mL/min/1.73 m2) | 40.9 (12.6) | 43.1 (14.1) | 44.1 (16.6) | 46.5 (16.0) | 43.8 (16.0) | 41.9 (14.6) |
| Haemoglobin (g/dL) | 12.9 (1.8) | 12.8 (1.7) | 12.9 (1.8) | 12.8 (1.8) | 12.8 (1.9) | 12.7 (1.9) |
| HbA1c (%) | 8.2 (1.6) | 8.7 (1.7) | 8.4 (1.7) | 8.5 (1.8) | 8.3 (1.7) | 8.2 (1.5) |
| Total cholesterol (mg/dL) | 217 (52) | 231 (58) | 222 (54) | 222 (49) | 229 (65) | 221 (55) |
| ACR (mg/g)c | 1713 (1683) | 1854 (1701) | 1558 (1417) | 1185 (1014) | 2153 (1780) | 1852 (1501) |
| Sodium excretion (mmol/24 h)d | 199 (107) | 167 (78) | 179 (84) | 184 (90) | 181 (86) | 172 (82) |
| Follow-up: absolute change from Month 3 to Month 12 | ||||||
| Systolic BP (mmHg)† | −0.1 (11.4) | 0.8 (12.1) | 3.7 (12.2) | −0.4 (10.3) | −1.2 (12.0) | −5.0 (13.5) |
| Diastolic BP (mmHg)† | −0.2 (6.3) | −0.2 (6.6) | 1.4 (5.9) | −0.8 (5.9) | −1.1 (6.6) | −2.6 (6.7) |
| Potassium† | 0.03 (0.45) | 0.07 (0.39) | −0.03 (0.43) | 0.05 (0.39) | 0.03 (0.42) | 0.03 (0.41) |
| . | Initial responders (N = 388) . | Initial non-responders (N = 682) . | ||||
|---|---|---|---|---|---|---|
| Enhanced response . | Sustained response . | Escape response . | Enhanced non-response . | Sustained non-responder . | Switch to response . | |
| Cases, n (%)a | 174 (44.8) | 123 (31.7) | 91 (23.5) | 212 (31.1) | 287 (42.1) | 183 (26.8) |
| Age (years) | 60.6 (7.6) | 59.0 (8.1) | 58.6 (7.3) | 58.8 (7.7) | 59.1 (7.1) | 60.6 (7.6) |
| Gender, n male (%)b | 108 (62.1) | 78 (63.4) | 59 (64.8) | 150 (70.8) | 195 (67.9) | 129 (70.49) |
| Race, n (%)b | ||||||
| White | 101 (58.1) | 70 (56.9) | 101 (46.2) | 120 (56.6) | 168 (64.8) | 103 (56.3) |
| Black | 22 (12.6) | 13 (10.6) | 22 (19.8) | 44 (20.8) | 35 (12.2) | 37 (20.2) |
| Hispanic | 16 (9.2) | 18 (14.6) | 16 (18.7) | 30 (14.2) | 27 (9.4) | 21 (11.5) |
| Asian | 31 (17.8) | 18 (14.6) | 31 (11.0) | 13 (6.1) | 32 (11.2) | 19 (140.4) |
| Other | 4 (2.3) | 4 (3.3) | 4 (4.4) | 5 (2.4) | 7 (2.4) | 3 (1.6) |
| BMI (kg/m2) | 30.5 (6.5) | 30.2 (6.6) | 29.7 (6.3) | 30.2 (5.9) | 30.4 (5.9) | 30.5 (6.1) |
| Smoker, n (%) | 26 (14.9) | 27 (22.0) | 12 (13.2) | 39 (18.4) | 58 (20.3) | 35 (19.2) |
| Systolic BP (mmHg) | 157.8 (19.8) | 151.7 (19.8) | 151.3 (20.2) | 150.0 (18.6) | 153.8 (18.3) | 156.0 (20.6) |
| Diastolic BP (mmHg) | 82.6 (10.9) | 84.6 (11.5) | 84.0 (11.5) | 83.7 (10.4) | 84.5 (10.1) | 84.5 (11.8) |
| eGFR (mL/min/1.73 m2) | 40.9 (12.6) | 43.1 (14.1) | 44.1 (16.6) | 46.5 (16.0) | 43.8 (16.0) | 41.9 (14.6) |
| Haemoglobin (g/dL) | 12.9 (1.8) | 12.8 (1.7) | 12.9 (1.8) | 12.8 (1.8) | 12.8 (1.9) | 12.7 (1.9) |
| HbA1c (%) | 8.2 (1.6) | 8.7 (1.7) | 8.4 (1.7) | 8.5 (1.8) | 8.3 (1.7) | 8.2 (1.5) |
| Total cholesterol (mg/dL) | 217 (52) | 231 (58) | 222 (54) | 222 (49) | 229 (65) | 221 (55) |
| ACR (mg/g)c | 1713 (1683) | 1854 (1701) | 1558 (1417) | 1185 (1014) | 2153 (1780) | 1852 (1501) |
| Sodium excretion (mmol/24 h)d | 199 (107) | 167 (78) | 179 (84) | 184 (90) | 181 (86) | 172 (82) |
| Follow-up: absolute change from Month 3 to Month 12 | ||||||
| Systolic BP (mmHg)† | −0.1 (11.4) | 0.8 (12.1) | 3.7 (12.2) | −0.4 (10.3) | −1.2 (12.0) | −5.0 (13.5) |
| Diastolic BP (mmHg)† | −0.2 (6.3) | −0.2 (6.6) | 1.4 (5.9) | −0.8 (5.9) | −1.1 (6.6) | −2.6 (6.7) |
| Potassium† | 0.03 (0.45) | 0.07 (0.39) | −0.03 (0.43) | 0.05 (0.39) | 0.03 (0.42) | 0.03 (0.41) |
aPercent of the initial response strata.
bPercent of the total angiotensin receptor blocker population.
cACR, albumin:creatinine ratio reported as geometric mean.
dData on 24-h sodium excretion were available in 389 (36%) subjects. Characteristics of these subjects did not differ from the overall cohort.
†P for trend >0.5.
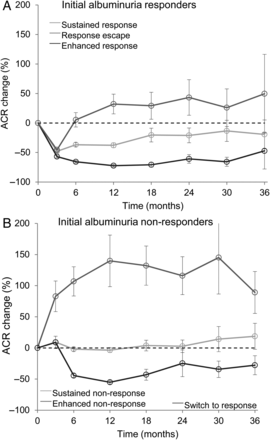
Mean albuminuria levels (95% confidence interval) over time in (A) responder and (B) non-responder patients.
During follow-up, 287 (26.7%) patients experienced a composite renal event. The Kaplan–Meier curves for renal outcomes for the three different albuminuria response categories in responders and non-responders are shown in Figure 2. Patients with an enhanced response compared with the reference group of a sustained response showed a lower risk for renal events. In contrast, patients with a non-sustained response showed a significantly higher renal risk compared with the reference group. A similar pattern was observed in the non-responder subgroup. These effects persisted after adjustment for other risk markers or Month 12 changes in systolic blood pressure or serum potassium (Table 2).
Hazard ratio and percentage of events in initial responder and non-responder patients further stratified by the change in albuminuria from Month 3 to Month 12
| . | No. of patients . | No. of events (%) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| End-stage renal disease or doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 34 (27.6) | Ref (1.0) | |
| Enhanced response | 174 | 22 (12.6) | 0.42 (0.23–0.77) | 0.005 |
| Escaped response | 91 | 24 (26.4) | 2.03 (1.13–3.66) | 0.019 |
| Non-responders | ||||
| Sustained non-response | 287 | 84 (29.3) | Ref (1.0) | |
| Enhanced non-response | 212 | 77 (36.3) | 2.97 (2.05–4.30) | <0.0001 |
| Switch to response | 183 | 46 (25.1) | 0.64 (0.43–0.94) | 0.024 |
| End-stage renal disease | ||||
| Responders | ||||
| Sustained response | 123 | 21 (17.1) | Ref (1.0) | |
| Enhanced response | 174 | 15 (20.3) | 0.34 (0.15–0.77) | 0.009 |
| Escaped response | 91 | 13 (14.3) | 1.68 (0.78–3.63) | 0.185 |
| Non-responders | ||||
| Sustained non-response | 287 | 48 (16.7) | Ref (1.0) | |
| Enhanced non-response | 212 | 44 (20.8) | 3.96 (2.33–6.74) | <0.0001 |
| Switch to response | 183 | 25 (13.7) | 0.65 (0.38–1.11) | 0.118 |
| Doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 24 (19.5) | Ref (1.0) | |
| Enhanced response | 174 | 18 (10.4) | 0.35 (0.15–0.78) | 0.011 |
| Escaped response | 91 | 13 (14.3) | 2.70 (1.30–5.61) | 0.008 |
| Non-responders | ||||
| Sustained non-response | 287 | 72 (25.1) | Ref (1.0) | |
| Enhanced non-response | 212 | 67 (31.6) | 2.97 (2.00–4.41) | <0.0001 |
| Switch to response | 183 | 32 (17.5) | 0.50 (0.32–0.78) | 0.002 |
| . | No. of patients . | No. of events (%) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| End-stage renal disease or doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 34 (27.6) | Ref (1.0) | |
| Enhanced response | 174 | 22 (12.6) | 0.42 (0.23–0.77) | 0.005 |
| Escaped response | 91 | 24 (26.4) | 2.03 (1.13–3.66) | 0.019 |
| Non-responders | ||||
| Sustained non-response | 287 | 84 (29.3) | Ref (1.0) | |
| Enhanced non-response | 212 | 77 (36.3) | 2.97 (2.05–4.30) | <0.0001 |
| Switch to response | 183 | 46 (25.1) | 0.64 (0.43–0.94) | 0.024 |
| End-stage renal disease | ||||
| Responders | ||||
| Sustained response | 123 | 21 (17.1) | Ref (1.0) | |
| Enhanced response | 174 | 15 (20.3) | 0.34 (0.15–0.77) | 0.009 |
| Escaped response | 91 | 13 (14.3) | 1.68 (0.78–3.63) | 0.185 |
| Non-responders | ||||
| Sustained non-response | 287 | 48 (16.7) | Ref (1.0) | |
| Enhanced non-response | 212 | 44 (20.8) | 3.96 (2.33–6.74) | <0.0001 |
| Switch to response | 183 | 25 (13.7) | 0.65 (0.38–1.11) | 0.118 |
| Doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 24 (19.5) | Ref (1.0) | |
| Enhanced response | 174 | 18 (10.4) | 0.35 (0.15–0.78) | 0.011 |
| Escaped response | 91 | 13 (14.3) | 2.70 (1.30–5.61) | 0.008 |
| Non-responders | ||||
| Sustained non-response | 287 | 72 (25.1) | Ref (1.0) | |
| Enhanced non-response | 212 | 67 (31.6) | 2.97 (2.00–4.41) | <0.0001 |
| Switch to response | 183 | 32 (17.5) | 0.50 (0.32–0.78) | 0.002 |
Covariates: age, gender, race, baseline eGFR, albuminuria, systolic and diastolic blood pressure, haemoglobin, HbA1c and the change in systolic blood pressure and potassium from Month 3 to Month 12.
Hazard ratio and percentage of events in initial responder and non-responder patients further stratified by the change in albuminuria from Month 3 to Month 12
| . | No. of patients . | No. of events (%) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| End-stage renal disease or doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 34 (27.6) | Ref (1.0) | |
| Enhanced response | 174 | 22 (12.6) | 0.42 (0.23–0.77) | 0.005 |
| Escaped response | 91 | 24 (26.4) | 2.03 (1.13–3.66) | 0.019 |
| Non-responders | ||||
| Sustained non-response | 287 | 84 (29.3) | Ref (1.0) | |
| Enhanced non-response | 212 | 77 (36.3) | 2.97 (2.05–4.30) | <0.0001 |
| Switch to response | 183 | 46 (25.1) | 0.64 (0.43–0.94) | 0.024 |
| End-stage renal disease | ||||
| Responders | ||||
| Sustained response | 123 | 21 (17.1) | Ref (1.0) | |
| Enhanced response | 174 | 15 (20.3) | 0.34 (0.15–0.77) | 0.009 |
| Escaped response | 91 | 13 (14.3) | 1.68 (0.78–3.63) | 0.185 |
| Non-responders | ||||
| Sustained non-response | 287 | 48 (16.7) | Ref (1.0) | |
| Enhanced non-response | 212 | 44 (20.8) | 3.96 (2.33–6.74) | <0.0001 |
| Switch to response | 183 | 25 (13.7) | 0.65 (0.38–1.11) | 0.118 |
| Doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 24 (19.5) | Ref (1.0) | |
| Enhanced response | 174 | 18 (10.4) | 0.35 (0.15–0.78) | 0.011 |
| Escaped response | 91 | 13 (14.3) | 2.70 (1.30–5.61) | 0.008 |
| Non-responders | ||||
| Sustained non-response | 287 | 72 (25.1) | Ref (1.0) | |
| Enhanced non-response | 212 | 67 (31.6) | 2.97 (2.00–4.41) | <0.0001 |
| Switch to response | 183 | 32 (17.5) | 0.50 (0.32–0.78) | 0.002 |
| . | No. of patients . | No. of events (%) . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| End-stage renal disease or doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 34 (27.6) | Ref (1.0) | |
| Enhanced response | 174 | 22 (12.6) | 0.42 (0.23–0.77) | 0.005 |
| Escaped response | 91 | 24 (26.4) | 2.03 (1.13–3.66) | 0.019 |
| Non-responders | ||||
| Sustained non-response | 287 | 84 (29.3) | Ref (1.0) | |
| Enhanced non-response | 212 | 77 (36.3) | 2.97 (2.05–4.30) | <0.0001 |
| Switch to response | 183 | 46 (25.1) | 0.64 (0.43–0.94) | 0.024 |
| End-stage renal disease | ||||
| Responders | ||||
| Sustained response | 123 | 21 (17.1) | Ref (1.0) | |
| Enhanced response | 174 | 15 (20.3) | 0.34 (0.15–0.77) | 0.009 |
| Escaped response | 91 | 13 (14.3) | 1.68 (0.78–3.63) | 0.185 |
| Non-responders | ||||
| Sustained non-response | 287 | 48 (16.7) | Ref (1.0) | |
| Enhanced non-response | 212 | 44 (20.8) | 3.96 (2.33–6.74) | <0.0001 |
| Switch to response | 183 | 25 (13.7) | 0.65 (0.38–1.11) | 0.118 |
| Doubling of serum creatinine | ||||
| Responders | ||||
| Sustained response | 123 | 24 (19.5) | Ref (1.0) | |
| Enhanced response | 174 | 18 (10.4) | 0.35 (0.15–0.78) | 0.011 |
| Escaped response | 91 | 13 (14.3) | 2.70 (1.30–5.61) | 0.008 |
| Non-responders | ||||
| Sustained non-response | 287 | 72 (25.1) | Ref (1.0) | |
| Enhanced non-response | 212 | 67 (31.6) | 2.97 (2.00–4.41) | <0.0001 |
| Switch to response | 183 | 32 (17.5) | 0.50 (0.32–0.78) | 0.002 |
Covariates: age, gender, race, baseline eGFR, albuminuria, systolic and diastolic blood pressure, haemoglobin, HbA1c and the change in systolic blood pressure and potassium from Month 3 to Month 12.
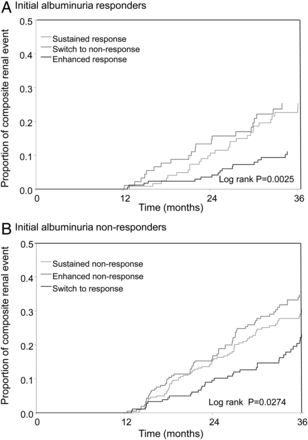
(A) Proportion of renal events in responder patients stratified by the change in albuminuria from Month 3 to Month 12. (B) Proportion of renal events in non-responder patients stratified by the change in albuminuria from Month 3 to Month 12.
Given the variations in albuminuria over time, we subsequently assessed whether the individual albuminuria exposure over time would be a better predictor of renal outcome than the initial Month 3 change in albuminuria. The discrimination of renal outcomes significantly increased when using the albuminuria exposure during the first 12 months versus the initial Month 3 change as indicated by the increase in the area under the receiver operating characteristics (ROC) curve from 0.68 (95% CI 0.64–0.72) to 0.78 (95% CI 0.75–0.82; P < 0.0001) (Figure 3). These findings were confirmed in a sensitivity analysis imputing missing albuminuria measurements (Supplementary Table S1). In another analysis, the total exposure to albuminuria over the first 12 months significantly increased the ROC curve for hospitalization for heart failure events compared with the initial Month 3 change (increase in the area under the ROC curve = 0.08; P = 0.027) but did not increase the ROC curve for all-cause mortality (Supplementary Table S1). Finally, analysis of individual variability in the placebo arm also showed considerable fluctuations in albuminuria over time (CV 32.6%; P = 0.083 versus ARB treatment). The total exposure to albuminuria from Month 3 to 12 in the placebo arm significantly increased the ROC curve for renal events compared with the initial change [increase in the ROC curve from 0.71 (95% CI 0.68–0.75) to 0.81 (95% CI 0.79–0.84); P < 0.0001].

Comparison of the predictive probability for renal outcomes between the initial drug-induced albuminuria reduction until Month 3 and the total exposure to albuminuria until Month 12. The predictive probability is assessed using logistic regression and expressed as the ROC curve and 95% CI. Subsequently the difference between the ROC curves was assessed.
DISCUSSION
This study showed considerable albuminuria variability during prolonged treatment with ARBs. Among initial responders to ARB treatment, the albuminuria levels remained stable during continued treatment in only one-third of the patients, whereas the other two-thirds of the population showed progressively worsening or improving albuminuria patterns despite continued treatment. We subsequently demonstrated that these individual variations in albuminuria during prolonged follow-up determine renal outcome and that the integrated albuminuria exposure over time during ARB treatment improves the renal risk prediction compared with the Month 3 albuminuria response. These results suggest that it may be more appropriate to use multiple albuminuria measurements over time during ARB treatment to monitor treatment effects and precisely quantify long-term renal risk.
The RENAAL trial and IDNT have shown that the initial reduction in albuminuria explains 40–50% of the treatment effect on ESRD. In the LIFE trial, the initial reduction in albuminuria explained 17% of the cardiovascular protective treatment effect of losartan. That the initial reduction in albuminuria does not completely explain the renal/cardiovascular protective treatment can be attributed to the fact that ARBs have short-term effects on multiple risk markers. Each of these additional short-term drug effects can influence the renal and cardioprotective treatment effect of ARBs, either positively or negatively, and thereby attenuate the predictive performance of the initial change in albuminuria [18]. In addition, the principle finding of this study is that albuminuria varies considerably over time in individual patients during ARB treatment. These variations in albuminuria may further attenuate the predictive performance of the initial change. Therefore, continuous monitoring of albuminuria during ARB treatment, in combination with other renal/cardiovascular risk markers, is recommended to accurately assess the degree of renoprotection.
Our results are in keeping with previous studies in glomerular diseases demonstrating that exposure to proteinuria over time is the strongest predictor of renal outcome. In a large Chinese observation study, it was shown that patients with immunoglobulin A (IgA) nephropathy with time-averaged urinary protein excretion of >1 g/day had a 46-fold increased risk compared with patients with a time-averaged urinary protein excretion of 0.5 g/day. In contrast, when the proteinuria level at entry into the study was considered, a proteinuria level >1.0 g/day conferred a 4.5-fold increased ESRD risk [19]. In addition, Reich et al. [20] analyzed a cohort of 542 IgA nephropathy patients and reported that time-averaged proteinuria was the most important independent determinant of renal function decline. In an observational study conducted in outpatient clinics in Alberta, Canada, Bello et al. found that omitting follow-up albuminuria measurements underestimates long-term renal risk as compared with using all available follow-up measures [21]. In our study we extended these findings to a large, internationally represented cohort of patients with type 2 diabetes and nephropathy treated with ARBs.
Our results seem to contrast with a previous study that concluded that multiple urine sampling does not improve the predictive power of albuminuria for cardiovascular events [22]. However, that study looked at the number of urine samples collected at a single visit (i.e. collected the days before the study visit) and did not take into account multiple urine sampling and albuminuria measurements over a prolonged period of time. In both the RENAAL trial and IDNT, single urine samples were collected at each study visit. We were therefore unable to determine the impact of additional urine collections at a single visit on the predictive performance in this population. In a previous study we also found that taking multiple urine samples the days before a single visit only marginally improved the precision of albuminuria measurements in a clinical trial setting. However, taking urine samples over a longer time period markedly improves the precision of albuminuria measurements and drug effect estimates in clinical trials in diabetic nephropathy [23].
The present study contradicts recent guideline recommendations from the American College of Physicians, which state that the evidence to date is insufficient to recommend monitoring of albuminuria in patients using ACEis or ARBs [24]. Our results unequivocally show large individual variations in albuminuria over time during long-term ARB treatment and show that incorporating the individual variations in albuminuria improves renal risk prediction; highlighting the importance of monitoring albuminuria over time.
The reason for the observed variability both in the initial albuminuria response to ARB treatment and in the subsequent albuminuria course remains unexplained to date. The available baseline characteristics could not explain the variability. Adherence to the treatment could play a role, although the subgroup of patients selected were adherent to medication. Nevertheless, efforts to accurately monitor drug adherence and improve drug adherence should be a high research priority. It seems that initiation or discontinuation of other blood pressure–lowering agents is unlikely to explain the variation, as few patients initiated or discontinued these drugs during the first 12 months of the trial. Finally, aldosterone escape could be another explanation and has been associated with albuminuria response escape [25, 26]. The value of aldosterone inhibition is highlighted by clinical trials of mineralocorticoid receptor antagonists in heart failure patients demonstrating that these agents prolong survival and prevent hospitalizations [27]. Unfortunately, neither plasma or urine drug levels, to verify drug adherence, nor aldosterone levels were available.
This study has limitations. First, this is a post hoc analysis with all its inherent limitations, and the results are only generalizable to the population who share the characteristics of the current cohort. We also excluded patients due to missing albuminuria values, which may have introduced a selection bias. However, a sensitivity analysis imputing missing albuminuria measurements did not change our findings. All patients received a fixed maximal antihypertensive dose of losartan or irbesartan. We were unable to determine if higher doses beyond the maximum antihypertensive dose would decrease the proportion of non-responders [28, 29]. Finally, the results are obtained in a standardized clinical trial setting that does not mimic general practice.
We conclude that following the initial response to ARB treatment there is a large within-patient variability in albuminuria response over time. This variability in response attenuates the predictive performance of a single 3-month albuminuria response. Hence, these results highlight the importance of continuously monitoring albuminuria over time in clinical practice and incorporating albuminuria levels during follow-up in risk algorithms to improve renal outcome prediction.
CONFLICT OF INTEREST STATEMENT
T.F.K. and F.A.H. have no disclosures. D.d.Z. has consultancy agreements with the following companies: AbbVie, Astellas, Bristol-Meyers Squibb, Hemocue, Johnson & Johnson, Merck Sharpe & Dohme, Novartis, Reata Pharmaceuticals and Vitae. All honoraria are paid to his institution. D.K.P. has consultancy agreements with Mesoblast, Nephrogenex and ZN Pharma. H.J.L.H. has consultancy agreements with the following companies: AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Reata Pharmaceuticals and ZN Pharma. All honoraria are paid to his institution. The results presented in this article have not been published previously in whole or part, except in abstract format.
ACKNOWLEDGEMENTS
We would like to acknowledge the supportive role of all RENAAL trial and IDNT patients, investigators and support staff.
REFERENCES





Comments