-
PDF
- Split View
-
Views
-
Cite
Cite
Ferruccio Galletti, Pasquale Strazzullo, The blood pressure–salt sensitivity paradigm: pathophysiologically sound yet of no practical value, Nephrology Dialysis Transplantation, Volume 31, Issue 9, September 2016, Pages 1386–1391, https://doi.org/10.1093/ndt/gfw295
Close - Share Icon Share
Abstract
Sodium plays an important pathophysiological role in blood pressure (BP) values and in the development of hypertension, and epidemiological studies such as the Intersalt Study have shown that the increase in BP occurring with age is determined by salt intake. Recently, a meta-analysis of 13 prospective studies has also shown the close relationship between excess sodium intake and higher risk of stroke and total cardiovascular events. However, the BP response to changing salt intake displayed a marked variability, as first suggested by Kawasaki et al. (The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med 1978; 64: 193–198) and later by Weinberger et al. (Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension 1986; 8: II127–II134), who recognized the heterogeneity of the BP response to salt and developed the concept of salt sensitivity. We have a large body of evidence in favour of a major role of metabolic and neuro-hormonal factors in determining BP salt sensitivity in association with the effect of genetic variation. There is evidence that salt sensitivity influences the development of organ damage, even independently—at least in part—of BP levels and the occurrence of hypertension. In addition, several observational studies indicate that salt sensitivity is clearly associated with a higher rate of cardiovascular events and mortality, independently of BP levels and hypertension. A cluster of factors with well-known atherogenic potential such as hyperinsulinaemia, dyslipidaemia and microalbuminuria—all known to be prevalent in salt-sensitive hypertension—might at least partially explain the increased cardiovascular risk observed in salt sensitive individuals. The gold standard for the evaluation of BP salt sensitivity is the BP response to a moderate reduction of salt intake for several weeks; nevertheless, these protocols often suffer of poor patient compliance to dietary instructions. To overcome this problem, short-term tests have been proposed that evaluate either large differences in salt intake for a few days or the response to intravenous administration of saline solution and short-acting diuretics. Recently, the use of ambulatory BP measurement has been proposed for the clinical assessment of BP salt sensitivity. Noteworthy, BP salt sensitivity, in whomever or however assessed, behaves as a continuous variable but salt sensitivity is used as a categorical parameter, with salt-sensitive individuals being defined as those with a difference in BP between low- and high-sodium intake >10%, and salt-resistant subjects those in whom BP does not increase or shows an increase <5% under sodium loading. The general conclusion that can and should be drawn from the above considerations is that the paradigm of salt sensitivity, despite its important pathophysiological meaning, is not helpful, so far, to the practising physician in clinical practice nor is it relevant or useful to the design and implementation of a population-based strategy of salt intake reduction; however, further studies are warranted for an accurate assessment of the salt-sensitivity phenotype in clinical practice. In the absence of a population strategy for salt intake reduction, the aim should be the generation of a ‘low sodium environment’ allowing for a dietary salt intake tailored on true human requirements and not on deleterious lifestyle habits.
INTRODUCTION
The sodium content of the habitual diet in Italy—as in most other countries worldwide—largely exceeds WHO recommendations [1] and Italian recommended dietary reference intakes [2]. This occurs in adults [3], children [4] and even in hypertensive patients [5], despite the consolidated evidence of the noxious effects of this widespread dietary habit on cardiovascular health. The Intersalt study provided unequivocal evidence of the direct association between average sodium intake of different populations around the world and the respective average blood pressure (BP) increase with age [6–9]. More recently, a systematic review and meta-analysis of prospective observational studies has shown the relationship between excess sodium intake and risk of stroke and total cardiovascular events [10]. The efficacy of moderate salt intake reduction in lowering BP in both hypertensive and normotensive adults and children has also been demonstrated by two meta-analyses of numerous randomized control trials [11, 12]. In addition to epidemiological and clinical studies, the milestone research conducted by Denton and coworkers in chimpanzees, an animal species sharing over 98% of its genotype with humans, has clearly shown that BP gradually increases over several months in these animals as they are exposed to a diet enriched with sodium chloride in the amount commonly consumed by human beings, to then recover to normal values once they are switched back to their regular low-sodium diet [13].
THE CONCEPT OF BP SALT SENSITIVITY
When Denton's experimental protocol was reproduced a few years later, the authors noticed that the relationship between salt intake and BP in this animal model was actually affected by a number of factors such as sex, age and concomitant occurrence of overweight and insulin resistance. Thus, the BP response to changing salt intake was not quantitatively similar in all individuals but showed a remarkable variability [13], much the same as observed in human studies [14–16].
Kawasaki et al. [17] and, later on, Weinberger et al. [18] systematically recognized the heterogeneity of the BP response to changing salt intake and formally developed the concept of BP salt sensitivity. Other, more recent, studies have highlighted the genetic and metabolic bases of this variable response leading to the unravelling of the central role of the kidney in a pathophysiological process that involves a number of neuro-endocrine effectors.
METABOLIC AND NEURO-ENDOCRINE FACTORS INVOLVED IN BP SALT SENSITIVITY
Among the neuro-endocrine factors involved in the salt sensitivity of BP are the renin-angiotensin-aldosterone system, the sympathetic nervous system, the system of natriuretic peptides, insulin, leptin and various endothelial effectors with endocrine and/or paracrine activity. Most of these affect the regulation of tubular sodium and water reabsorption and, thus, volume homeostasis [19]. Both alterations in the activity of these neuro-endocrine effectors and primary deviations in the response of the kidney to the effectors due to genetic variation determine the extent of the BP response to a similar change in salt intake [20]. Therefore, BP salt sensitivity is a partly inherited and partly acquired individual feature.
Paradigmatic examples of the influence of genetic factors are given by the monogenic forms of hypertension, in which loss-of-function or gain-of-function mutations in a single gene encoding a molecule involved in sodium transport through the tubular epithelium cause profound alterations in fluid volume homeostasis and lead to an early increase in BP, which is sensitive to salt restriction or to the administration of diuretics [21].
In addition to these very rare mutations, several genetic polymorphisms have been associated with higher rates of hypertension such as those affecting the α-adducin molecule [22–24], the glucagon receptor [25], the serum and glucocorticoid-regulated kinase, SGK1 [26], the G-protein beta-3 subunit [27], the renal isoenzyme of 11beta-hydroxysteroid dehydrogenase [28–30] and several others. In some reports, the clustering of two or more specific allelic variants in a given subject was associated with salt sensitivity, as in the study by Siani and colleagues, who observed that a group of participants in the Olivetti Heart Study featuring triple homozygosity for the angiotensinogen M235T, the angiotensin II AT1 receptor A1166C and the CYP11B2 C-344T allelic variants, in the presence of a D/D ACE genotype, displayed a considerable increase in the rate of proximal tubular reabsorption of sodium and a particularly high rate of hypertension [31]. On the other hand, a striking example of acquired salt sensitivity is the one associated with obesity, as elegantly shown by Rocchini et al., who demonstrated that high salt intake caused a substantial increase in BP in obese but not as much in lean adolescents, and that this greater salt sensitivity of BP was reversed following a successful weight-lowering diet [32]. Strazzullo et al. later found that both abdominal adiposity [33] and the metabolic syndrome [34] are associated with an enhanced rate of proximal tubular sodium reabsorption, which is at least partially explained by the effects of hyperinsulinaemia [35] and hyperleptinaemia [36], two common conditions in this type of subjects, both inducing sympathetic stimulation [37] and, in turn, activation of the renin-angiotensin system [38]. The relationship between salt sensitivity and insulin resistance was evaluated in a study adopting the hyperinsulinaemic euglycemic clamp, the gold standard method to measure insulin sensitivity [15]. This study showed that, within a population of essential hypertensive patients with normal glucose tolerance, insulin-stimulated whole-body glucose uptake was inversely associated with salt sensitivity of BP and that this association was independent of age and body mass index. Another mechanism whereby insulin may affect salt sensitivity is through its modulation of endothelial relaxation. ‘Salt-sensitive’ hypertensive and normotensive individuals presented significantly lower vascular distensibility compared with ‘salt-sensitive’ subjects [39].
In addition, Liu et al. reported that salt sensitivity was associated with reduced flow-mediated dilation in patients with higher BP salt sensitivity and that endothelial dysfunction apparently contributed to the long-term greater occurrence of target organ injury and to the higher mortality rate of these patients [40].
More recently, the in vitro studies by Oberleithner and coworkers showed that relatively small increases in plasma sodium concentration in the presence of aldosterone significantly impaired endothelial cell elasticity and nitric oxide production [41]. In this regard, it is worth noting that changes in the order of 2 to 3 mmol/L do occur in the absorptive and post-absorptive phase after a salty meal and are apparently associated with significant changes in BP [42].
To summarize, a large body of good quality evidence is in favour of an important role of metabolic and neuro-hormonal factors in the determination of BP salt sensitivity, in addition to the effect of a genetic variation (Figure 1).
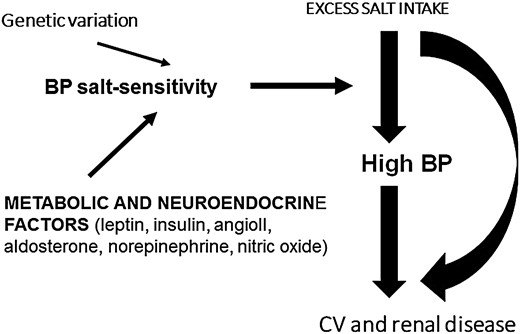
Metabolic, neuro-hormonal and genetic factors involved in the salt-sensitivity of BP. CV, cardiovascular.
SALT SENSITIVITY, A PREDICTOR OF CARDIOVASCULAR MORBIDITY AND MORTALITY
There is evidence from several observational studies that salt-sensitivity of BP is associated with a higher rate of cardiovascular events. Barba et al., studying a group of originally normotensive and clinically healthy men with different BP salt sensitivity, found that the incidence of hypertension over 15 years was significantly higher among individuals with a higher degree of salt-sensitivity at baseline compared with subjects with lower salt sensitivity [43]. The study did not have the potential to clarify the mechanisms explaining this association: nevertheless, a cluster of factors with well-known atherogenic potential such as hyperinsulinaemia, dyslipidaemia and microalbuminuria, known to be prevalent in salt-sensitive hypertension, might at least partially explain the increased cardiovascular risk observed in these individuals. In accordance with these findings, another study of untreated patients with moderate essential hypertension found that various features of hypertensive target organ damage including renal dysfunction, hypertensive retinopathy and left ventricular hypertrophy were more often associated with BP salt sensitivity [44]. The authors reported that these forms of organ damage clustered together with hyperinsulinaemia, hyperlipidaemia, microalbuminuria and evidence of endothelial dysfunction.
In a study by Morimoto et al., sodium sensitivity predicted a higher rate of cardiovascular events, even independently of BP and cigarette smoking [45]. Weinberger and co-workers showed that hypertensive patients and, to some extent, also normotensive individuals with higher BP salt sensitivity had a lower rate of survival in comparison with respectively hypertensive and normotensive subjects of similar age and lower BP salt sensitivity [18].
Finally, a genetic variance in NEDD4L, a sodium channel regulatory protein in the distal nephron, was associated with enhanced BP salt sensitivity, higher mean BP levels and a higher rate of cardiovascular disease and mortality [46].
SALT-SENSITIVITY ASSESSMENT IN RESEARCH AND IN CLINICAL PRACTICE
The assessment of BP salt sensitivity for research purposes has been the object of extensive investigation. There is general agreement that BP salt sensitivity, in whichever population is measured and with whatever test, behaves as a continuous variable. Depending on the characteristics of the population under study, with regard to e.g. age, body mass or hypertensive status, the population mean BP change upon a given change in sodium intake may be different; however, a widespread and continuous distribution of the individual BP responses will be observed in all cases. Despite this indisputable evidence, the existence of so-called ‘salt-sensitive’ and ‘salt-resistant’ individuals is very often purported on the basis of the identification of totally arbitrary thresholds. Indeed, the vast majority of individuals (more so if elderly and/or hypertensive) experience a dose-dependent drop in BP in response to a reduction of sodium intake, with only a small proportion of subjects showing no response or even some increase in BP [47–49].
The gold standard for the evaluation of BP salt sensitivity is the BP response to moderate reduction of salt intake maintained over a period of several weeks. Protocols of long-term moderate sodium reduction, however, are limited by the often unsatisfactory patients' compliance to dietary instructions and by the need to ascertain this compliance repeatedly over time. To overcome this problem, the investigation of the effect of long-term moderate salt reduction is often replaced by short-term tests applying large differences in salt intake for a few days or, in some cases, by tests of the response to intravenous administration of saline solution and to short-acting diuretics [17, 47, 48]. Unfortunately, there is little or no evidence that the responses to these manoeuvres are truly predictive of the long-term response to salt intake reduction in real life conditions. In fact, drastic and abrupt short-term sodium restriction produces BP responses strongly dependent on the neuro-endocrine adjustments taking place in the immediacy of the sodium regimen change, with particular regard to the sympathetic and the renin-angiotensin-aldosterone system. There is clear evidence that these adjustments are much larger than the neuro-endocrine changes occurring with moderate salt reduction in controlled trials of at least 4-week duration, thus producing spurious results [49].
A widely used protocol for the assessment of BP salt-sensitivity in clinical investigation has been for quite a long time the test of Grim and Weinberger [47], which evaluates the BP response to volume expansion and contraction manoeuvres applied in sequence; this protocol has undergone several reproducibility and comparability tests versus the BP response to dietary salt restriction [14, 50–53]. Although a statistical association was detected between the different tests, the marked intra-individual variation in the response to the two tests made mis-classification in the salt sensitivity or salt resistance group very likely [51]. Furthermore, a careful analysis of the BP changes occurring during the two phases of the Grim–Weinberger test allowed the identification among hypertensive patients of two populations, one having a definite BP increase during the intravenous sodium chloride load administered on the first day of the test, and a second one experiencing a substantial BP fall after diuretic administration on the second day. These two responses were poorly interrelated and, indeed, it was found that the slope of the regression line of the BP changes over time during the saline infusion was the best correlate of the BP response to a reduced sodium diet observed in the same patients [53].
Recently, Castiglioni et al. proposed ambulatory BP measurement (ABPM) for the clinical assessment of BP salt sensitivity. They reasoned that, within a population on a high-sodium diet, the more salt-sensitive individuals could have an altered circadian BP pattern as a consequence of sodium and water retention during daytime and would integrate neuro-hormonal responses to the increase in fluid volume amenable to measurement by ABPM. They observed in 46 never-treated hypertensive patients that the average heart rate and systolic BP nocturnal fall was significantly related to a salt-sensitivity index derived by the systolic BP change upon switching from a 200 mmol to a 30 mmol sodium diet (each maintained for 1 week). Unfortunately, the size of the correlation coefficients was definitely too small to support the possibility of a practical application and, once again, their salt-sensitivity index used as a reference standard was based on the response to short-term drastic sodium intake restriction that has little in common with the BP response to long-term moderate salt reduction [54].
The Dietary Approaches to Stop Hypertension (DASH) study, which involved people with high-normal BP or stage 1 hypertension, showed very clearly that in real life conditions moderate salt reduction is associated in most individuals with a gradual decrease in BP that tends to plateau in no less than 4 weeks [55]. Thus, a meaningful assessment of BP salt sensitivity in a given individual would require that the subject adhere to the prescribed moderate salt reduction for such a period of time. On the other hand, the same DASH experience highlighted the widespread distribution of the spontaneous changes in BP occurring in any given subject even during constant sodium intake: this spontaneous intra-individual variability of BP makes it very difficult to discriminate on an individual basis whether a given change in BP observed in response to salt intake reduction was caused by salt intake reduction per se or was simply due to spontaneous intra-subject BP variation [56].
This problem could be perhaps overcome by multiple BP measurements over several days or by the use of ABPM.
The problem of large intra-subject variation is similarly encountered when monitoring a patient's compliance to any given prescribed reduction of sodium intake using urinary sodium excretion: old and recent studies are consistent in showing that a large number of 24 h urine collections is actually necessary to get an accurate estimate of a patient's average daily sodium intake [57, 58], an intolerable burden for both the patient and the clinical laboratory. In addition, recent experimental work by Rakova and coworkers, carried out on healthy individuals kept on a fixed Na intake for a prolonged period, provided evidence of unpredicted variation in urinary sodium excretion, not associated with concomitant changes in body weight and water retention, but related to changes in aldosterone and cortisol production [59].
CONCLUSION: THE PARADIGM OF SALT SENSITIVITY IS NOT APPLICABLE TO PREVENTIVE AND THERAPEUTIC STRATEGIES OF SALT INTAKE REDUCTION
In summary, this short review on the merits and limitations of the concept of BP salt sensitivity leads to the following considerations:
The concept of BP salt sensitivity is useful to explain the pathogenesis of the BP increase with age experienced by different populations to an extent dependent on the average population salt intake.
It provides a mechanistic explanation for the development of hypertension both as related to genetic susceptibility (see the monogenic forms of hypertension and hypertension associated with certain allelic variants of genes affecting renal tubular sodium handling) and to acquired conditions such as advancing age, obesity, abdominal adiposity and diabetes, which are all associated with altered renal sodium handling.
It appears to be a predictor of the likelihood to develop hypertension over time and a predictor of higher cardiovascular morbidity and mortality.
On the other hand, BP salt sensitivity, in relation to its polygenic and multifactorial nature, is a continuous variable; therefore, any attempt to dichotomize a population in salt-sensitive and salt-resistant individuals is artefactual.
All the tests proposed over time for the quantitative assessment of BP salt sensitivity have proved inaccurate, poorly reproducible, often cumbersome and costly, and in general not applicable in real life conditions.
The large majority of individuals in any population do experience some BP decrease in response to gradual, long-term and moderate sodium intake reduction: this fall in BP, even when limited to a few mmHg, is expected to translate into a sizable reduction in cardiovascular risk.
In conclusion, further studies would be desirable for the accurate assessment of the salt-sensitivity phenotype in clinical practice. To date, the paradigm of salt sensitivity, despite its important pathophysiological meaning, cannot be used by the practising physician in day-to-day hypertensive patients' care nor is it relevant to the design and implementation of population-based strategies of salt intake reduction.
These strategies should be directed in the first place to the implementation of campaigns aimed at increasing the population awareness about the risks of excess salt intake and at guiding people to reduce salt intake at home and when consuming meals outside the home. It is hard to envisage any rationale for or any practical possibility to restrict these campaigns to supposedly ‘salt-sensitive’ individuals while leaving out the ‘others’, possibly within the same family.
Given the steady increase in the amount of meals consumed away from home, the participation of the restoration enterprises and the catering industry in any salt reduction programme is necessary.
In addition to calling the attention of consumers and those involved with meal preparation, a nation-wide salt reduction strategy requires the cooperation of the food industry as at least two-thirds of the dietary sodium content is given by salt hidden in processed foods bought as such at food shops and supermarkets. To reduce this burden, reformulation of transformed foods must be operated either through negotiation with industry or through regulation by law [60].
In essence, a population strategy for salt intake reduction should aim at generating a ‘reduced sodium environment’, allowing for a dietary salt intake tailored on the true human requirement (approximately 1 g of sodium equivalent to 2.5 g of salt per day) and not on deleterious lifestyle habits imposed on individuals since their childhood and based on unhealthy and no longer justifiable traditions, and as a tribute to the commercial interest of food industries to maintain these negative lifestyles [61]. This ‘reduced sodium environment’ must clearly be for all.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest. None of the data presented has been published in whole or in part.
REFERENCES
- dyslipidemias
- phenotype
- diuretics
- hypertension
- patient compliance
- heart disease risk factors
- hyponatremia
- dietary sodium chloride
- hypertension, essential
- blood pressure
- diet
- heterogeneity
- hypernatremia
- blood pressure determination
- epidemiologic studies
- habits
- hyperinsulinism
- intravenous infusion procedures
- life style
- sound
- genetics
- mortality
- sodium
- microalbuminuria
- sodium intake
- salt intake
- cardiovascular event
- stroke risk
- interval data
- gold standard
- intravenous route of drug administration
- saline solutions





Comments