-
PDF
- Split View
-
Views
-
Cite
Cite
Vanessa Grubbs, Eric Vittinghoff, George Taylor, Donna Kritz-Silverstein, Neil Powe, Kirsten Bibbins-Domingo, Areef Ishani, Steven R. Cummings, for the Osteoporotic Fractures in Men (MrOS) Study Research Group, The association of periodontal disease with kidney function decline: a longitudinal retrospective analysis of the MrOS dental study, Nephrology Dialysis Transplantation, Volume 31, Issue 3, March 2016, Pages 466–472, https://doi.org/10.1093/ndt/gfv312
Close - Share Icon Share
Abstract
Identifying modifiable risk factors for chronic kidney disease (CKD) is essential for reducing its burden. Periodontal disease is common, modifiable and has been implicated as a novel potential CKD risk factor, but evidence of its association with kidney function decline over time is limited.
In a longitudinal retrospective cohort of 761 elderly men with preserved kidney function [estimated glomerular filtration rate > 60 mL/min/1.73 m2 using a calibrated creatinine and cystatin C (eGFRcr-cys) equation] at baseline, we performed multivariable Poisson's regression to examine the association of severe periodontal disease with incident CKD, defined as incident eGFRcr-cys <60 mL/min/1.73 m2and rapid (>5% annualized) eGFRcr-cys decline. Severe periodontal disease was defined in two ways: (i) ≥5 mm proximal attachment loss in 30% of teeth examined (European Workshop in Periodontology Group C, European Workshop); and (ii) 2+ interproximal sites with attachment loss ≥6 mm and 1+ interproximal sites with probing depth ≥5 mm (Centers for Disease Control/American Academy of Periodontology, CDC/AAP).
At baseline, the mean age was 73.4 (SD 4.8) years, the median eGFRcr-cys was 82.4 mL/min/1.73 m2, and 35.5 and 25.4% of participants had severe periodontal disease by European Workshop and CDC/AAP criteria, respectively. After a mean follow-up of 4.9 years (SD 0.3), 56 (7.4%) participants had incident CKD. Severe periodontal disease was associated with a 2-fold greater rate of incident CKD [incidence rate ratio (IRR) 2.01 (1.21–3.44), P = 0.007] after adjusting for confounders compared with not severe periodontal disease by European Workshop criteria but did not reach statistical significance by CDC/AAP criteria [IRR 1.10 (0.63–1.91), P = 0.9].
Severe periodontal disease may be associated with incident clinically significant kidney function decline among a cohort of elderly men.
INTRODUCTION
Despite improvements in the management of traditional chronic kidney disease (CKD) risk factors in the general US population, the prevalence of moderate and advanced CKD continues to rise [1]. Identifying modifiable risk factors for CKD and its progression to end-stage renal disease (ESRD) is essential for reducing its significant burden.
Periodontal disease, which is both common and modifiable, has recently been implicated as a promising focus for impacting CKD and is thought to cause renal impairment via an inflammatory pathway. Several cross-sectional studies report that adults with significant periodontal disease were up to twice as likely to have CKD as were their counterparts without periodontal disease [2–5]. To date, two studies have found that periodontal disease is associated with kidney function decline over time—one among a small cohort study of Pima Indians with diabetes and another among a small cohort of elderly Japanese adults [6, 7]. However, an association between periodontal disease and kidney function decline among an elderly US population has not been explored. Since periodontal disease disproportionately affects those also disproportionately affected by CKD—namely poor, minority and elderly populations—further elucidation in this area is needed.
The purpose of this study was to evaluate the association of periodontal disease with kidney function decline over approximately a 5-year period among a cohort of elderly men. We hypothesized that individuals with severe periodontal disease would have greater kidney function decline than those without severe periodontal disease.
MATERIALS AND METHODS
Study design and population
We conducted a longitudinal analysis of participants in the Osteoporotic Fractures in Men (MrOS) study. MrOS is a prospective, observational, multicenter cohort study designed to evaluate the development and determinants of osteoporosis and fractures among older men (Figure 1). From March 2000 through April 2002, 5994 men aged 65 years and older were recruited from six clinical sites (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California) for Visit 1.
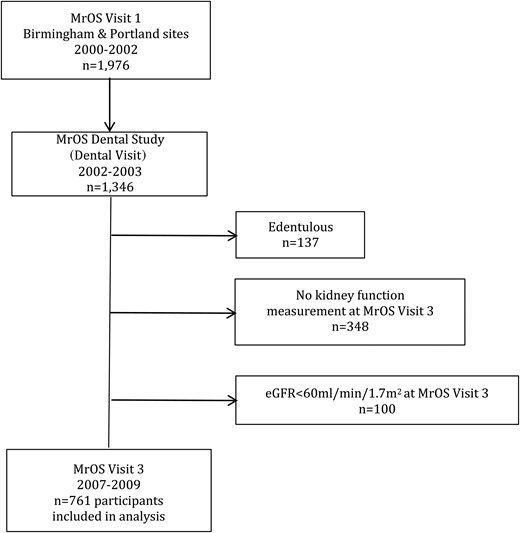
From September 2002 to May 2003, MrOS participants from the Birmingham and Portland clinical sites were invited to participate in the MrOS Dental Study (Dental Visit). Visit 3 was conducted between March 2007 and March 2009, ∼5 years after the Dental Visit. A detailed description of MrOS and MrOS Dental Study recruitment strategies and study design has been published elsewhere [8–10]. Each site obtained institutional review board approval for the examination protocol, and written informed consent was obtained at every examination. Cystatin C was measured as part of an ancillary study to MrOS on all available stored serum samples from participants attending the Dental Visit and Visit 3. The University of California, San Francisco, Institutional Review Board approved this ancillary study.
Of the 1976 men enrolled in MrOS from the Birmingham and Portland sites, 1346 (68%) attended the Dental Visit. We excluded 137 men who were edentulous and 348 without kidney function measurement at Visit 3. Severe periodontal disease by the European Workshop in Periodontology Group C definition (see below) was more prevalent among participants missing Visit 3 kidney function data compared with the 861 participants with available kidney function data (43.4 versus 36.0%, P = 0.02), but was not different by the Centers for Disease Control/American Academy of Periodontology (CDC/AAP) definition (P = 0.5). Because our primary outcome included incident estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, we excluded an additional 100 participants with eGFR <60 mL/min/1.73 m2 at the Dental Visit. Severe periodontal disease was not different by either definition between these participants and the 761 participants with Dental Visit eGFR ≥60 mL/min/1.73 m2 included in the analysis (P > 0.4).
Predictor
Our primary predictor was periodontal disease. Six calibrated dentists and hygienists conducted random half-mouth examinations using a dental light, mirror, air and periodontal probe (UNC 15). Random half-mouth examinations were performed on the right or left depending upon participant's study ID number. The distance from cemento-enamel junction to the base of the pocket (clinical attachment loss) and the distance from the gingival margin to the base of the pocket (pocket probing depth) were measured at all six sites per tooth—disto-buccal, buccal, mesio-buccal, disto-lingual, lingual and mesio-lingual (excluding third molars). All measurements were made in millimeters and rounded to the nearest whole millimeter [10].
There is a lack of uniformity within the literature regarding the criteria used to define periodontal disease. We used two case definitions of periodontal disease provided and previously published by the MrOS Study Research Group for this analysis: the European Workshop in Periodontology Group C (European Workshop) and CDC/AAP [10]. With the European Workshop definition, severe periodontal disease is defined as the presence of a proximal clinical attachment loss of ≥5 mm in 30% of teeth examined [11]. The CDC/AAP defined moderate periodontal disease as two or more interproximal sites with clinical attachment loss ≥4 mm (not on the same tooth) or two or more interproximal sites with probing depth ≥5 mm (not on the same tooth). Severe periodontal disease was defined by two or more interproximal sites with clinical attachment loss ≥6 mm (not on the same tooth) and one or more interproximal sites with probing depth ≥5 mm [12]. Because data by European Workshop were available only as ‘not severe’ and ‘severe’, we collapsed CDC/AAP data similarly, where ‘not severe’ included participants with none, mild and moderate periodontal disease.
Main outcome
Our outcome of interest was incident CKD at follow-up (Visit 3), defined by incident eGFR <60 mL/min/1.73 m2accompanied by rapid eGFR decline (>5% annualized loss). Our definition of rapid decline is consistent with the Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group definition of CKD progression and has been applied to other studies [13–15]. Serum creatinine was measured on all available participants at study visits. Cystatin C was measured at part of an ancillary study to MrOS on all available stored serum samples from the Dental Visit and Visit 3. Kidney function (eGFR in mL/min/1.73 m2) was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for calibrated creatinine and cystatin C (eGFRcr-cys) as it has been shown to perform better than eGFRcr or eGFRcys alone even among community-dwelling elderly individuals [16, 17].
Covariates
All covariates were defined using Visit 1 data, except age and smoking status, which were defined from the Dental Visit data. Age, race (white or nonwhite), hypertension, education and smoking status were obtained by self-administered questionnaires [9]. Age was considered as a continuous variable. Hypertension was defined by self-report or average of two systolic blood pressures ≥140 mmHg. Diastolic blood pressure was not measured at Visit 1. We defined diabetes by self-report, fasting blood glucose ≥126 mg/dL, or using hypoglycemic medications or insulin. Glycosylated hemoglobin data were not available. Because income data were not available, we included educational attainment (defined as ‘more than high school’ or ‘high school equivalent or less’) as a proxy for socioeconomic status [18]. We defined smoking status as ‘current use’ or ‘no current use’.
Statistical analyses
We used multivariable Poisson's regression to examine the association of periodontal disease defined by the European Workshop and CDC/AAP with incident CKD over the study period. We adjusted for age, comorbidities (diabetes and hypertension) and health-related behavior (smoking), and sociodemographic characteristics (race and education) as potential confounders. We added covariates to the model sequentially to examine incremental effects of each confounder category on the adjusted association of periodontal disease with incident CKD. Finally, we examined the association of periodontal disease with incident CKD stratified by race and tested for interaction. Analyses were performed in Stata Version 13.0 (Stata Corp, College Station, TX).
RESULTS
The average age in the study population at baseline was 73.4 (SD 4.8) years. The mean number of teeth examined on the randomized side was 11.5 (SD 0.5, range 1–14), and 53.0% of participants had gingival bleeding around one or more teeth. The mean prevalence of clinical attachment loss ≥4 mm and ≥6 mm was 16.5% (SD 13.5, range 0–73) and 2.7% (SD 4.8, range 0–43), respectively. Mean clinical attachment loss was 3.0 mm (SD 0.8, range 0.7–8.9). The mean prevalence of pocket probing depth ≥5 mm was 3.2% (SD 5.2, range 0–38). Mean pocket probing depth was 2.5 mm (SD 0.5, range 1.3–5.5). Severe periodontal disease was present among 35.5 and 25.4% of participants by European Workshop and CDC/AAP definitions, respectively. Participant baseline characteristics by periodontal disease status by each definition are shown in Table 1. Baseline (Dental Visit) eGFRcr-cys was similar across periodontal disease groups as was the prevalence of hypertension. Participants with severe periodontal disease by European Workshop were older, had fewer teeth and had a higher prevalence of low educational attainment and diabetes than those without; these factors were similar across periodontal disease status by CDC/AAP. Participants with severe periodontal disease by either definition were more likely to be nonwhite and have current tobacco use than those without severe periodontal disease.
MrOS dental cohort baseline (Dental Visit) characteristicsa by periodontal disease statusb
| . | Entire cohort, N = 761 . | Periodontal disease by European workshop definition . | Periodontal disease by CDC/AAP definition . | ||||
|---|---|---|---|---|---|---|---|
| Not severe, n = 491 (64.5%) . | Severe, n = 270 (35.5%) . | P-valuec . | Not severe, n = 568 (74.6%) . | Severe, n = 193 (25.4%) . | P-valuec . | ||
| Mean age (SD), in years | 73.4 (4.8) | 73.1 (4.7) | 74.0 (4.9) | 0.01 | 73.3 (4.8) | 73.7 (4.7) | 0.3 |
| Nonwhite, n (% column) | 72 (9.5) | 35 (7.1) | 37 (13.7) | 0.003 | 45 (7.9) | 27 (14.0) | 0.01 |
| High school education or less, n (% column) | 114 (15.0) | 62 (12.6) | 52 (19.3) | 0.01 | 81 (14.3) | 33 (17.1) | 0.3 |
| Diabetes (self-report, fasting glucose ≥126 mg/dL, or on diabetes medication), n (% column) | 80 (10.5) | 43 (8.8) | 37 (13.7) | 0.03 | 54 (9.5) | 26 (13.5) | 0.1 |
| Hypertension (self-report or average of two systolic blood pressures ≥140 mmHg), n (% column) | 267 (35.1) | 170 (34.6) | 97 (35.9) | 0.7 | 197 (34.7) | 70 (36.3) | 0.7 |
| Current tobacco use, n (% column) | 29 (3.8) | 9 (1.8) | 20 (7.4) | <0.001 | 12 (2.1) | 17 (8.8) | <0.001 |
| eGFR [eGFRcr-cys mL/min/1.73 m2, median (interquartile range)] | 82.4 (73.3, 90.9) | 82.8 (73.4, 91.3) | 81.3 (72.8, 89.9) | 0.2 | 82.5 (73.3, 90.8) | 82.4 (73.1, 91.5) | 0.4 |
| Number of teeth examined (randomized side), mean (SD) | 11.5 (0.5) | 12.0 (2.6) | 10.5 (3.2) | <0.001 | 11.5 (3.0) | 11.4 (2.7) | 0.8 |
| Any bleeding on probing, n (%) | 403 (53.0) | 247 (50.3) | 156 (57.8) | 0.05 | 272 (47.9) | 131 (67.9) | <0.001 |
| Clinical attachment loss in millimeters (mm), mean (SD) | 3.0 (0.8) | 2.6 (0.5) | 3.7 (0.9) | <0.001 | 2.7 (0.5) | 3.8 (0.9) | <0.001 |
| Mean prevalence of examined sites with clinical attachment loss | |||||||
| ≥4 mm, % column (SD) | 16.5 (13.5) | 10.6 (8.6) | 27.3 (14.2) | <0.001 | 11.6 (9.3) | 30.9 (13.8) | <0.001 |
| ≥6 mm, % column (SD) | 2.7 (4.8) | 0.8 (1.4) | 6.3 (6.5) | <0.001 | 1.0 (2.0) | 7.9 (6.7) | <0.001 |
| Pocket probing depth in mm, mean (SD) | 2.5 (0.5) | 2.4 (0.3) | 2.8 (0.6) | <0.001 | 2.4 (0.3) | 3.0 (0.6) | <0.001 |
| Mean prevalence of examined sites with pocket probing depth ≥5 mm, % column (SD) | 3.2 (5.2) | 1.7 (2.6) | 6.2 (7.3) | <0.001 | 1.6 (2.6) | 8.1 (7.6) | <0.001 |
| . | Entire cohort, N = 761 . | Periodontal disease by European workshop definition . | Periodontal disease by CDC/AAP definition . | ||||
|---|---|---|---|---|---|---|---|
| Not severe, n = 491 (64.5%) . | Severe, n = 270 (35.5%) . | P-valuec . | Not severe, n = 568 (74.6%) . | Severe, n = 193 (25.4%) . | P-valuec . | ||
| Mean age (SD), in years | 73.4 (4.8) | 73.1 (4.7) | 74.0 (4.9) | 0.01 | 73.3 (4.8) | 73.7 (4.7) | 0.3 |
| Nonwhite, n (% column) | 72 (9.5) | 35 (7.1) | 37 (13.7) | 0.003 | 45 (7.9) | 27 (14.0) | 0.01 |
| High school education or less, n (% column) | 114 (15.0) | 62 (12.6) | 52 (19.3) | 0.01 | 81 (14.3) | 33 (17.1) | 0.3 |
| Diabetes (self-report, fasting glucose ≥126 mg/dL, or on diabetes medication), n (% column) | 80 (10.5) | 43 (8.8) | 37 (13.7) | 0.03 | 54 (9.5) | 26 (13.5) | 0.1 |
| Hypertension (self-report or average of two systolic blood pressures ≥140 mmHg), n (% column) | 267 (35.1) | 170 (34.6) | 97 (35.9) | 0.7 | 197 (34.7) | 70 (36.3) | 0.7 |
| Current tobacco use, n (% column) | 29 (3.8) | 9 (1.8) | 20 (7.4) | <0.001 | 12 (2.1) | 17 (8.8) | <0.001 |
| eGFR [eGFRcr-cys mL/min/1.73 m2, median (interquartile range)] | 82.4 (73.3, 90.9) | 82.8 (73.4, 91.3) | 81.3 (72.8, 89.9) | 0.2 | 82.5 (73.3, 90.8) | 82.4 (73.1, 91.5) | 0.4 |
| Number of teeth examined (randomized side), mean (SD) | 11.5 (0.5) | 12.0 (2.6) | 10.5 (3.2) | <0.001 | 11.5 (3.0) | 11.4 (2.7) | 0.8 |
| Any bleeding on probing, n (%) | 403 (53.0) | 247 (50.3) | 156 (57.8) | 0.05 | 272 (47.9) | 131 (67.9) | <0.001 |
| Clinical attachment loss in millimeters (mm), mean (SD) | 3.0 (0.8) | 2.6 (0.5) | 3.7 (0.9) | <0.001 | 2.7 (0.5) | 3.8 (0.9) | <0.001 |
| Mean prevalence of examined sites with clinical attachment loss | |||||||
| ≥4 mm, % column (SD) | 16.5 (13.5) | 10.6 (8.6) | 27.3 (14.2) | <0.001 | 11.6 (9.3) | 30.9 (13.8) | <0.001 |
| ≥6 mm, % column (SD) | 2.7 (4.8) | 0.8 (1.4) | 6.3 (6.5) | <0.001 | 1.0 (2.0) | 7.9 (6.7) | <0.001 |
| Pocket probing depth in mm, mean (SD) | 2.5 (0.5) | 2.4 (0.3) | 2.8 (0.6) | <0.001 | 2.4 (0.3) | 3.0 (0.6) | <0.001 |
| Mean prevalence of examined sites with pocket probing depth ≥5 mm, % column (SD) | 3.2 (5.2) | 1.7 (2.6) | 6.2 (7.3) | <0.001 | 1.6 (2.6) | 8.1 (7.6) | <0.001 |
aPeriodontal disease status, eGFRcr-cys and tobacco use were defined using baseline (Dental Visit) data. All other characteristics were defined using Study Visit 1 data.
bUsing European Workshop in Periodontology Group C (European Workshop) or CDC/AAP criteria.
cP-value is Kruskal–Wallis test (eGFRcr-cys), t-test (age, number of teeth, mean pocket probing depth and clinical attachment loss) or chi-square test (all other) of association between periodontal disease status and characteristic.
MrOS dental cohort baseline (Dental Visit) characteristicsa by periodontal disease statusb
| . | Entire cohort, N = 761 . | Periodontal disease by European workshop definition . | Periodontal disease by CDC/AAP definition . | ||||
|---|---|---|---|---|---|---|---|
| Not severe, n = 491 (64.5%) . | Severe, n = 270 (35.5%) . | P-valuec . | Not severe, n = 568 (74.6%) . | Severe, n = 193 (25.4%) . | P-valuec . | ||
| Mean age (SD), in years | 73.4 (4.8) | 73.1 (4.7) | 74.0 (4.9) | 0.01 | 73.3 (4.8) | 73.7 (4.7) | 0.3 |
| Nonwhite, n (% column) | 72 (9.5) | 35 (7.1) | 37 (13.7) | 0.003 | 45 (7.9) | 27 (14.0) | 0.01 |
| High school education or less, n (% column) | 114 (15.0) | 62 (12.6) | 52 (19.3) | 0.01 | 81 (14.3) | 33 (17.1) | 0.3 |
| Diabetes (self-report, fasting glucose ≥126 mg/dL, or on diabetes medication), n (% column) | 80 (10.5) | 43 (8.8) | 37 (13.7) | 0.03 | 54 (9.5) | 26 (13.5) | 0.1 |
| Hypertension (self-report or average of two systolic blood pressures ≥140 mmHg), n (% column) | 267 (35.1) | 170 (34.6) | 97 (35.9) | 0.7 | 197 (34.7) | 70 (36.3) | 0.7 |
| Current tobacco use, n (% column) | 29 (3.8) | 9 (1.8) | 20 (7.4) | <0.001 | 12 (2.1) | 17 (8.8) | <0.001 |
| eGFR [eGFRcr-cys mL/min/1.73 m2, median (interquartile range)] | 82.4 (73.3, 90.9) | 82.8 (73.4, 91.3) | 81.3 (72.8, 89.9) | 0.2 | 82.5 (73.3, 90.8) | 82.4 (73.1, 91.5) | 0.4 |
| Number of teeth examined (randomized side), mean (SD) | 11.5 (0.5) | 12.0 (2.6) | 10.5 (3.2) | <0.001 | 11.5 (3.0) | 11.4 (2.7) | 0.8 |
| Any bleeding on probing, n (%) | 403 (53.0) | 247 (50.3) | 156 (57.8) | 0.05 | 272 (47.9) | 131 (67.9) | <0.001 |
| Clinical attachment loss in millimeters (mm), mean (SD) | 3.0 (0.8) | 2.6 (0.5) | 3.7 (0.9) | <0.001 | 2.7 (0.5) | 3.8 (0.9) | <0.001 |
| Mean prevalence of examined sites with clinical attachment loss | |||||||
| ≥4 mm, % column (SD) | 16.5 (13.5) | 10.6 (8.6) | 27.3 (14.2) | <0.001 | 11.6 (9.3) | 30.9 (13.8) | <0.001 |
| ≥6 mm, % column (SD) | 2.7 (4.8) | 0.8 (1.4) | 6.3 (6.5) | <0.001 | 1.0 (2.0) | 7.9 (6.7) | <0.001 |
| Pocket probing depth in mm, mean (SD) | 2.5 (0.5) | 2.4 (0.3) | 2.8 (0.6) | <0.001 | 2.4 (0.3) | 3.0 (0.6) | <0.001 |
| Mean prevalence of examined sites with pocket probing depth ≥5 mm, % column (SD) | 3.2 (5.2) | 1.7 (2.6) | 6.2 (7.3) | <0.001 | 1.6 (2.6) | 8.1 (7.6) | <0.001 |
| . | Entire cohort, N = 761 . | Periodontal disease by European workshop definition . | Periodontal disease by CDC/AAP definition . | ||||
|---|---|---|---|---|---|---|---|
| Not severe, n = 491 (64.5%) . | Severe, n = 270 (35.5%) . | P-valuec . | Not severe, n = 568 (74.6%) . | Severe, n = 193 (25.4%) . | P-valuec . | ||
| Mean age (SD), in years | 73.4 (4.8) | 73.1 (4.7) | 74.0 (4.9) | 0.01 | 73.3 (4.8) | 73.7 (4.7) | 0.3 |
| Nonwhite, n (% column) | 72 (9.5) | 35 (7.1) | 37 (13.7) | 0.003 | 45 (7.9) | 27 (14.0) | 0.01 |
| High school education or less, n (% column) | 114 (15.0) | 62 (12.6) | 52 (19.3) | 0.01 | 81 (14.3) | 33 (17.1) | 0.3 |
| Diabetes (self-report, fasting glucose ≥126 mg/dL, or on diabetes medication), n (% column) | 80 (10.5) | 43 (8.8) | 37 (13.7) | 0.03 | 54 (9.5) | 26 (13.5) | 0.1 |
| Hypertension (self-report or average of two systolic blood pressures ≥140 mmHg), n (% column) | 267 (35.1) | 170 (34.6) | 97 (35.9) | 0.7 | 197 (34.7) | 70 (36.3) | 0.7 |
| Current tobacco use, n (% column) | 29 (3.8) | 9 (1.8) | 20 (7.4) | <0.001 | 12 (2.1) | 17 (8.8) | <0.001 |
| eGFR [eGFRcr-cys mL/min/1.73 m2, median (interquartile range)] | 82.4 (73.3, 90.9) | 82.8 (73.4, 91.3) | 81.3 (72.8, 89.9) | 0.2 | 82.5 (73.3, 90.8) | 82.4 (73.1, 91.5) | 0.4 |
| Number of teeth examined (randomized side), mean (SD) | 11.5 (0.5) | 12.0 (2.6) | 10.5 (3.2) | <0.001 | 11.5 (3.0) | 11.4 (2.7) | 0.8 |
| Any bleeding on probing, n (%) | 403 (53.0) | 247 (50.3) | 156 (57.8) | 0.05 | 272 (47.9) | 131 (67.9) | <0.001 |
| Clinical attachment loss in millimeters (mm), mean (SD) | 3.0 (0.8) | 2.6 (0.5) | 3.7 (0.9) | <0.001 | 2.7 (0.5) | 3.8 (0.9) | <0.001 |
| Mean prevalence of examined sites with clinical attachment loss | |||||||
| ≥4 mm, % column (SD) | 16.5 (13.5) | 10.6 (8.6) | 27.3 (14.2) | <0.001 | 11.6 (9.3) | 30.9 (13.8) | <0.001 |
| ≥6 mm, % column (SD) | 2.7 (4.8) | 0.8 (1.4) | 6.3 (6.5) | <0.001 | 1.0 (2.0) | 7.9 (6.7) | <0.001 |
| Pocket probing depth in mm, mean (SD) | 2.5 (0.5) | 2.4 (0.3) | 2.8 (0.6) | <0.001 | 2.4 (0.3) | 3.0 (0.6) | <0.001 |
| Mean prevalence of examined sites with pocket probing depth ≥5 mm, % column (SD) | 3.2 (5.2) | 1.7 (2.6) | 6.2 (7.3) | <0.001 | 1.6 (2.6) | 8.1 (7.6) | <0.001 |
aPeriodontal disease status, eGFRcr-cys and tobacco use were defined using baseline (Dental Visit) data. All other characteristics were defined using Study Visit 1 data.
bUsing European Workshop in Periodontology Group C (European Workshop) or CDC/AAP criteria.
cP-value is Kruskal–Wallis test (eGFRcr-cys), t-test (age, number of teeth, mean pocket probing depth and clinical attachment loss) or chi-square test (all other) of association between periodontal disease status and characteristic.
After a mean follow-up of 4.9 years (SD 0.3), 56 (7.4%) participants had incident CKD (Table 2). Follow-up time did not differ by periodontal disease status by either definition (P > 0.1). In the unadjusted model, severe periodontal disease by European Workshop was associated with a 2.4-fold greater rate of incident CKD [incidence rate ratio (IRR) 2.42 (1.45–4.02), P = 0.001]. This association was partially attenuated with sequential adjustment but persisted in the fully adjusted model [IRR 2.01 (1.21–3.44), P = 0.007]. Severe periodontal disease by European Workshop was associated with each component of incident CKD: incident eGFR <60 mL/min/1.73 m2 [adjusted IRR 1.54 (1.08–2.21), P = 0.02] and rapid eGFR decline [adjusted IRR 1.80 (1.13–2.86), P = 0.01].
Incidence rate ratio of eGFRcr-cysa <60 mL/min/1.73 m2and rapid eGFRcr-cys lossb at 5-year follow-up by periodontal disease statusc, N = 761
| Model . | Periodontal disease by European Workshop definition (n events/N subgroup) . | Periodontal disease by CDC/AAP definition (n events/N subgroup) . | ||||
|---|---|---|---|---|---|---|
| Not severe (24/491) . | Severe (32/270) . | Not severe (39/568) . | Severe (17/193) . | |||
| IRR (95% CI) | IRR (95% CI) | P-value | IRR (95% CI) | IRR (95% CI) | P-value | |
| Unadjusted | 1.0 (reference) | 2.42 (1.45–4.02) | 0.001 | 1.0 (reference) | 1.27 (0.74–2.19) | 0.4 |
| +Age | 1.0 (reference) | 2.26 (1.36–3.73) | 0.002 | 1.0 (reference) | 1.24 (0.72–2.14) | 0.4 |
| +Age, diabetes, hypertension, tobacco use | 1.0 (reference) | 2.16 (1.29–3.62) | 0.003 | 1.0 (reference) | 1.14 (0.65–2.00) | 0.8 |
| +Age, diabetes, hypertension, tobacco use, race, education | 1.0 (reference) | 2.04 (1.21–3.44) | 0.007 | 1.0 (reference) | 1.10 (0.63–1.91) | 0.9 |
| Model . | Periodontal disease by European Workshop definition (n events/N subgroup) . | Periodontal disease by CDC/AAP definition (n events/N subgroup) . | ||||
|---|---|---|---|---|---|---|
| Not severe (24/491) . | Severe (32/270) . | Not severe (39/568) . | Severe (17/193) . | |||
| IRR (95% CI) | IRR (95% CI) | P-value | IRR (95% CI) | IRR (95% CI) | P-value | |
| Unadjusted | 1.0 (reference) | 2.42 (1.45–4.02) | 0.001 | 1.0 (reference) | 1.27 (0.74–2.19) | 0.4 |
| +Age | 1.0 (reference) | 2.26 (1.36–3.73) | 0.002 | 1.0 (reference) | 1.24 (0.72–2.14) | 0.4 |
| +Age, diabetes, hypertension, tobacco use | 1.0 (reference) | 2.16 (1.29–3.62) | 0.003 | 1.0 (reference) | 1.14 (0.65–2.00) | 0.8 |
| +Age, diabetes, hypertension, tobacco use, race, education | 1.0 (reference) | 2.04 (1.21–3.44) | 0.007 | 1.0 (reference) | 1.10 (0.63–1.91) | 0.9 |
aeGFRcr-cys = CKD-EPI combined creatinine–cystatin C estimated glomerular filtration rate, mL/min/1.73 m2.
bRapid eGFR loss, >5 mL/min/1.73 m2 loss per year.
cUsing European Workshop in Periodontology Group C (European Workshop) or CDC/AAP criteria.
Incidence rate ratio of eGFRcr-cysa <60 mL/min/1.73 m2and rapid eGFRcr-cys lossb at 5-year follow-up by periodontal disease statusc, N = 761
| Model . | Periodontal disease by European Workshop definition (n events/N subgroup) . | Periodontal disease by CDC/AAP definition (n events/N subgroup) . | ||||
|---|---|---|---|---|---|---|
| Not severe (24/491) . | Severe (32/270) . | Not severe (39/568) . | Severe (17/193) . | |||
| IRR (95% CI) | IRR (95% CI) | P-value | IRR (95% CI) | IRR (95% CI) | P-value | |
| Unadjusted | 1.0 (reference) | 2.42 (1.45–4.02) | 0.001 | 1.0 (reference) | 1.27 (0.74–2.19) | 0.4 |
| +Age | 1.0 (reference) | 2.26 (1.36–3.73) | 0.002 | 1.0 (reference) | 1.24 (0.72–2.14) | 0.4 |
| +Age, diabetes, hypertension, tobacco use | 1.0 (reference) | 2.16 (1.29–3.62) | 0.003 | 1.0 (reference) | 1.14 (0.65–2.00) | 0.8 |
| +Age, diabetes, hypertension, tobacco use, race, education | 1.0 (reference) | 2.04 (1.21–3.44) | 0.007 | 1.0 (reference) | 1.10 (0.63–1.91) | 0.9 |
| Model . | Periodontal disease by European Workshop definition (n events/N subgroup) . | Periodontal disease by CDC/AAP definition (n events/N subgroup) . | ||||
|---|---|---|---|---|---|---|
| Not severe (24/491) . | Severe (32/270) . | Not severe (39/568) . | Severe (17/193) . | |||
| IRR (95% CI) | IRR (95% CI) | P-value | IRR (95% CI) | IRR (95% CI) | P-value | |
| Unadjusted | 1.0 (reference) | 2.42 (1.45–4.02) | 0.001 | 1.0 (reference) | 1.27 (0.74–2.19) | 0.4 |
| +Age | 1.0 (reference) | 2.26 (1.36–3.73) | 0.002 | 1.0 (reference) | 1.24 (0.72–2.14) | 0.4 |
| +Age, diabetes, hypertension, tobacco use | 1.0 (reference) | 2.16 (1.29–3.62) | 0.003 | 1.0 (reference) | 1.14 (0.65–2.00) | 0.8 |
| +Age, diabetes, hypertension, tobacco use, race, education | 1.0 (reference) | 2.04 (1.21–3.44) | 0.007 | 1.0 (reference) | 1.10 (0.63–1.91) | 0.9 |
aeGFRcr-cys = CKD-EPI combined creatinine–cystatin C estimated glomerular filtration rate, mL/min/1.73 m2.
bRapid eGFR loss, >5 mL/min/1.73 m2 loss per year.
cUsing European Workshop in Periodontology Group C (European Workshop) or CDC/AAP criteria.
There was no statistically significant association between severe periodontal disease by CDC/AAP and incident CKD [adjusted IRR 1.10 (0.63–1.91), P = 0.7]. Severe periodontal disease by CDC/AAP was not associated with incident eGFR <60 mL/min/1.73 m2 [adjusted IRR 0.89 (0.59–1.35), P = 0.6] or rapid eGFR decline 1.13 (0.69–1.85), P = 0.6].
Among whites (n = 689), severe periodontal disease by European Workshop was associated with a 1.8-fold greater rate of incident CKD after adjustment for confounders [IRR 1.84 (1.04–3.26), P = 0.03]. Among nonwhites (n = 72), the point estimate after adjustment for confounders was considerably higher but did not reach statistical significance [IRR 4.72 (0.69–32.20), P = 0.1]. There was no statistical evidence that the association between periodontal disease by either definition and kidney function decline varied by race (Pinteraction = 0.3).
DISCUSSION
In a cohort of elderly men with preserved renal function at baseline, we found that severe periodontal disease was associated with incident CKD relative to non-severe periodontal disease during a 5-year follow-up period. This finding is particularly meaningful given the robustness of our outcome requiring renal function deterioration below the clinically significant threshold of eGFR <60 mL/min/1.73 m2 in addition to rapid eGFR decline over the study period. While we found similar associations with incident eGFR <60 mL/min/1.73 m2 and rapid eGFR decline considered separately, the requirement that both end points be met removes the possibility that our findings are driven by participants with eGFR near 60 mL/min/1.73 m2 at baseline.
Prior investigation among a prospective cohort of 529 Pima Indian adults with diabetes and eGFR >60 mL/min/1.73 m2 at baseline found that severe periodontal disease, as defined by missing teeth and alveolar bone loss on panoramic radiograph, was associated with a 2-fold increased risk of incident macroalbuminuria and a 3.5-fold increased risk of incident ESRD over a follow-up of up to 22 years [7]. Iwasaki et al. [6] found that, among 317 Japanese elderly adults, severe periodontal inflammatory burden was associated with a 2-fold higher likelihood of worsening kidney function category (≥60, 30–59 and ≤29 mL/min/1.73 m2) over 2 years. Our study extends the current understanding of the association of periodontal disease with kidney function decline over time to a different population—both with and without diabetes and with preserved kidney function at baseline. A particular strength of our study is the use of a combined equation, which more accurately estimates renal function than creatinine only-based equations as employed by Iwasaki et al. Further, their outcome of worsening eGFR may be problematic in that declines of significantly different sizes would be classified similarly, such as those from 50 to 29 and from 31 to 29 mL/min/1.73 m2.
Our findings are particularly important because periodontal disease is disproportionately more common among racial and ethnic minorities [19–21]. The association between periodontal disease and kidney function decline may be greater among racial and ethnic minorities, potentially contributing to similar disparities in CKD prevalence and progression. We found no persuasive evidence that the association varied by race, but this may be in part attributable to the very small numbers of nonwhites in our study population. Similarly, the effect of periodontal disease on kidney function decline may be greater among a younger population, where a survivor bias is not a factor. Additional study in more diverse populations is needed to determine if associations are more pronounced than in this study of older, predominantly white men. If this is indeed the case, modifying periodontal disease could be a potential means for reducing the risk of CKD progression and disparities in CKD because periodontal disease is usually preventable and treatable with basic oral hygiene and routine dental care.
The mechanism by which periodontal disease is hypothesized to exert an effect on kidney dysfunction is via an inflammatory pathway. Though periodontal disease is a chronic, local bacterial infection of the oral cavity, periodontal pathogens can access systemic circulation and potentially induce kidney injury through an innate immune response through toll-like receptor 4 (TLR4) [22, 23]. TLR4 is one in a group of transmembrane proteins that play a key role in the innate immune response and is found throughout the kidney, where it can both recognize and bind bacterial lipopolysaccharide coating and launch an inflammatory cascade, leading to kidney dysfunction [24, 25]. This hypothesis is supported by a murine model of systemic lupus erythematosus in which transient exposure to bacterial cell wall components induced production of DNA autoantibodies, effector molecules of the innate immune response and massive albuminuria, but similar studies of periodontal pathogens in humans are needed [26].
Of note, IRRs with the CDC/AAP periodontal disease definition were imprecisely estimated but in the same direction as the European Workshop definition, with confidence intervals including substantial adverse effects, thus lending weight to the overall finding of an association between periodontal disease and kidney function decline. The difference in findings by periodontal disease definition may be attributable to differences in classifying severe disease. The sample prevalence of severe periodontal disease was 10 percentage points lower using the CDC/AAP definition, and 43% (n = 117) of cases classified as severe by the European Workshop definition were classified as not severe by the CDC/AAP definition. While there is no standard case definition for periodontal disease universally recognized in epidemiological studies, the CDC/AAP definition has been proposed as a standard definition of periodontal status for epidemiological studies. However, the CDC/AAP definition has been shown to underestimate periodontal disease when only a partial-mouth periodontal examination is performed, as is the case in the MrOS study [27]. The European Workshop definition may also underestimate the prevalence of periodontal disease in a partial-mouth examination, and yet it classified substantially more participants as having severe disease.
Our study has several limitations. First, because of the limited number of outcomes in our study population, we were restricted to a parsimonious model. Body mass index, for example, has been implicated as an independent risk factor for CKD progression [28]. Obesity was not included as a confounder in our analyses, but less than one-fifth of our study population had body mass index ≥ 30 kg/m2 and was not different by periodontal disease status by either definition (P = 0.4). As with all observational studies, the association of periodontal disease with kidney function decline may be subject to residual confounding. However, given that we have accounted for the most important known confounders for periodontal disease and CKD, only one or more powerful unmeasured confounders could explain the strength of the association we found with the European Workshop definition. A second limitation of our study is the lack of interval periodontal treatment data, but the treatment of periodontal disease would likely bias the findings toward the null. If we are correct in our hypothesis that severe periodontal disease leads to kidney function decline, then those with severe periodontal disease at baseline but later treated would have slower kidney function decline, while renal deterioration in those with interval worsening of periodontal disease would have been attributed to the milder periodontal disease at baseline. Finally, because our study population included very few nonwhite participants, our ability to test for differences in the strength of the association by race was limited. Our findings may not be generalizable to younger or more diverse cohorts or women. Further study is needed to determine if associations are similar across genders, racial/ethnic groups and age groups.
CONCLUSIONS
In conclusion, we found some evidence for an association of severe periodontal disease with incident clinically significant kidney function decline among a cohort of elderly men. Because periodontal disease is preventable and modifiable, further research is needed to determine if an association exists among more diverse populations and whether the treatment of periodontal disease will alter the trajectory of eGFR decline.
CONFLICT OF INTEREST STATEMENT
V.G. received investigator-initiated research funding from Valeant Pharmaceuticals.
ACKNOWLEDGEMENTS
We thank the participants and staff of the MrOS study. V.G. was supported by grant 1K23DK093710-01A1 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation. This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number TL1 TR000144. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The MrOS study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR) and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810 and UL1 TR000128. The National Institute for Dental and Craniofacial Research (NIDCR) provides funding for the MrOS Dental ancillary study ‘Oral and Skeletal Bone Loss in Older Men’ under the Grant Number R01 DE014386. Cystatin C measures from the Dental Visit and Visit 3 were funded by the Clinical and Translational Science Institute's (CTSI's) Strategic Opportunities Support (SOS) Program RAS Award ID# A117088.
REFERENCES





Comments