-
PDF
- Split View
-
Views
-
Cite
Cite
Paul J. Roderick, Robin F. Jeffrey, Ho M. Yuen, Keith M. Godfrey, Jane West, John Wright, Smaller kidney size at birth in South Asians: findings from the Born in Bradford birth cohort study, Nephrology Dialysis Transplantation, Volume 31, Issue 3, March 2016, Pages 455–465, https://doi.org/10.1093/ndt/gfv274
Close - Share Icon Share
Abstract
Rates of advanced chronic kidney disease and renal replacement therapy are higher in South Asian than in white British populations. Low birth weight is also more frequent in South Asian populations and has been associated with increased risks of kidney disease, perhaps due to a reduced nephron endowment.
Using ultrasound scans at 34 weeks of gestation, we measured fetal kidney dimensions (transverse and anteroposterior diameters, length and circumference) and derived volume in a random sample of 872 white British and 715 South Asian participants in the Born in Bradford cohort study. Kidney measurements were compared between ethnic groups.
Birth weight for gestational age at 40 weeks was 200 g less in South Asian babies compared with white British babies. The mean kidney volume for gestational age was 16% lower in South Asian than in white British babies [8.79 versus 10.45 cm3, difference 1.66 cm3 (95% confidence interval 1.40–1.93, P < 0.001)]. The difference was robust after adjustment for maternal age, socio-economic factors, marital status, body mass index, smoking and alcohol use in pregnancy, parity, baby's gender and birth weight for gestational age [adjusted difference 1.38 cm3 (0.97–1.84), P < 0.001]. There were smaller reductions in other fetal measures.
South Asian babies have smaller kidneys compared with white British babies, even after adjusting for potential confounders including birth weight. This finding may contribute to increased risks of adult kidney disease in South Asian populations.
INTRODUCTION
The incidence of end-stage kidney failure (ESKF) requiring dialysis is higher and occurs earlier in adults of South Asian origin compared with those of white European origin across all diagnostic categories [1–3]. The reasons for this are not fully understood. There are limited data on the prevalence of chronic kidney disease (CKD) [4, 5].
Markers of kidney damage such as microalbuminuria are more common in South Asians, secondary to both diabetic and non-diabetic causes [6–8]. It is unclear whether CKD progresses faster to ESKF in South Asians [9–11].
Intrauterine growth restriction (IUGR) and low birth weight (LBW) are more common in South Asians [12–15] and are associated with a number of adult chronic diseases including type 2 diabetes and cardiovascular disease [16–19]. A systematic review has shown the association between LBW and kidney disease [20] at various stages of severity from microalbuminuria and reduced estimated glomerular filtration rate (eGFR) to ESKF on renal replacement therapy (RRT), although there are no specific data for this association among South Asians [21–23].
One hypothesis to explain the increased adult kidney risk secondary to LBW is that growth restriction, especially in the third trimester, leads to a prenatal reduction in the number of nephrons [24, 25] and consequently smaller kidney size at birth [26–28]. As nephron number is fixed by birth [24], Brenner and Chertow [29] hypothesized that such postnatal oligonephronia may lead to compensatory hypertrophy, glomerular hyperfiltration and hypertension with increase in glomerular size, followed by glomerular damage and sclerosis, further reduction in nephron number and increased susceptibility to damage from other renal insults and development of progressive kidney damage [29, 30].
The excess incidence of advanced CKD in South Asians has major implications for the burden of kidney disease in an ageing South Asian population. CKD can not only progress to ESKF requiring costly RRT, but can also be an independent factor for cardiovascular disease [31]. Understanding causation is, therefore, key to the prevention of CKD in such populations.
Bradford is the sixth largest metropolitan area in the UK, with the eighth most deprived health community; it has high ethnic diversity and half of the 5500 babies born each year are from South Asia. This study compared fetal intrauterine kidney development in South Asian and white British pregnant mothers in a substudy of the ‘Born in Bradford’ birth cohort [32].
MATERIALS AND METHODS
Born in Bradford (BiB) is a longitudinal multi-ethnic birth cohort study that aims to examine the impact of environmental, psychological and genetic factors on maternal and child health and well-being [32].
Women were recruited while attending for their glucose tolerance test (OGTT), a routine procedure offered to all pregnant women registered at the Bradford Royal Infirmary at 26–28 weeks of gestation. For those consenting, a baseline questionnaire was completed via an interview with a study administrator. It was transliterated into Urdu and Mirpuri using a standardized process, so that words and phrases corresponded with the original English version. The full BiB cohort recruited 12 453 women involving 13 776 pregnancies between 2007 and 2010. The cohort was broadly representative of the characteristics of the city's maternal population. Ethical approval for data collection was granted by the Bradford Research Ethics Committee (ref. 07/H1302/112).
Renal substudy recruitment
This study involved a nested cohort within the full BiB cohort. From early 2008, those women who were attending for the OGTT at 26–28 weeks of gestation, who had completed the baseline questionnaire and consented for the main BiB cohort study, were invited to undertake a further fetal ultrasound scan (USS) at 34 weeks for standard anthropometrics and fetal renal dimensions. Recruitment was sequential and offered to all women from the BiB cohort, except those with twins or known fetal abnormalities. A maximum of 20 women were enrolled each week (with reserves) to fill USS lists 8 weeks thereafter. The window for the USS was 33 weeks minus 4 days to 34 weeks plus 4 days based on a USS dating at 8–14 weeks.
From May 2008 to December 2010, a total of 3805 women were approached at 26–28 weeks, of whom 3028 were willing to take part; 2026 were scheduled a USS appointment; 167 failed to attend for USS and 26 were excluded at the time of scanning (Figure 1). Our focus of interest was mothers with fetal renal ultrasound measurements and of white British or South Asian ethnic origin with full-term live births. We therefore excluded women from other ethnic groups, those who went on to have premature births (<37 weeks of gestation), still births, those with congenital anomalies, twins or solitary kidneys and those who delivered where birth outcome data were unavailable.
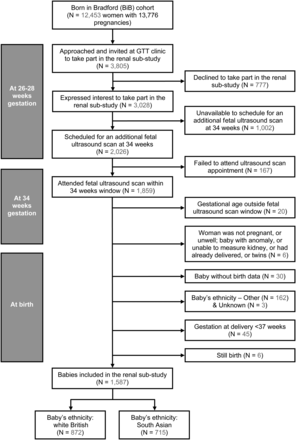
The present study focuses on 1587 pregnancies, comprising 872 white British and 715 South Asian women. The South Asian group included women of Pakistani, Indian and Bangladeshi origin. The ethnic mix was similar to the whole birth cohort.
Renal outcome and fetal anthropometric measures
The scans were performed by four experienced sonographers. The Philips Envisor ultrasound machine was used with a 3.5–5.0 curvilinear transducer. Standard fetal anthropometric measures included head and abdominal circumference (CF). Renal dimensions comprised left and right length (LH), anteroposterior (AP) diameter, transverse (TS) diameter and CF. Renal volume was derived from the volume of an ellipsoid using the formula: length × width × depth × 0.523 [33]. Estimated fetal weight was derived from the formula of Hadlock [34].
In a random subsample of mothers (n = 587) who took part in this study, each of the four fetal kidney measures was repeated three times by one of the four sonographers after training. The intraclass correlation (ICC) was estimated for each sonographer (the number of mothers per sonographer was 33, 115, 189 and 250, respectively). The ICCs were all over 0.96, showing good intra-observer reliability. In a smaller subsample (n = 136), inter-observer reliability was assessed by comparing two sonographers at a time (six pairs in total) who measured all dimensions three times in the same pregnant woman. Using the Bland–Altman method [35], all mean differences were close to zero, with no evidence of systematic bias between any pair of sonographers (bias from most comparisons ≤0.05 cm for every renal dimension measured, compared with mean dimensions LH 3.84 cm, AP 2.12 cm, TS 2.18 cm and CF 7.18 cm). Limits of agreement were, on average, within the range of approximately 7–15% from each side of the mean dimensions, and this reflected the small sample sizes.
Birth outcomes
Birth weight in participants was extracted from medical records and was recorded immediately after birth. Head, abdominal and mid-upper arm CF were not routinely measured, but were added to the routine neonatal examination, which was generally performed within 24 h of birth by a paediatrician. We have shown previously good reliability for such measurements [36]. We did not record birth length.
Covariates
Ethnicity was self-reported by mothers at interview, with participants given response options based on the UK Office of National Statistics guidance [37]. We were interested in characteristics that are known to be influenced by ethnicity and that might be on a causal pathway explaining kidney size differences, as well as potential confounders such as parity [38]. In addition, we were aware that some characteristics might mask differences, such as smoking during pregnancy. A priori, we considered maternal early pregnancy body mass index (BMI), height, age, parity, smoking, alcohol consumption, socio-economic position (maternal education), housing tenure, employment, living with a partner, place of birth, consanguinity, mental health using the General Health Questionnaire (GHQ-12) score [39], fasting and post-load glucose, fasting insulin, infant gender and gestational age to be characteristics that might explain or mask ethnic differences in outcomes. Data on self-reported number of cigarettes smoked were missing in 50% of the white British and 92% of the South Asian women, whereas the percentages missing on alcohol units consumed were 37 and 97%, respectively. We therefore created dichotomized variables of smoked or drank alcohol during pregnancy.
Maternal weight (SECA digital scales) and height (Leicester Height Measure) were measured in light clothing and unshod. Clinical details were obtained from the Eclipse electronic maternity information system. These included 12-week scan for dating, antenatal history (including parity), date of last menstrual period, past medical history (diabetes, hypertension and CKD), pregnancy medical history (gestational diabetes and pre-eclampsia), baby's birth weight and gestation at delivery and head, arm and abdominal CF. Gestational age was determined from the booking USS at 12 weeks by standard methods [40]. Prematurity was defined as gestational age below 37 weeks and LBW as <2.5 kg. Data on pre-pregnancy diabetes and hypertension, gestational diabetes, gestational hypertension and pre-eclampsia were missing in over 70% of the cases in both ethnic groups in routine medical records and were therefore not included in our models.
Fasting and post-load glucose and fasting insulin were obtained from the OGTT samples, which were assayed immediately after sampling at the Biochemistry Department of Bradford Royal Infirmary using the glucose oxidase method on Siemen's Advia 2400 chemistry auto-analysers and Siemen's Advia Centaur assay.
Information for all other covariables was obtained from medical records or from the mother's questionnaire.
Statistical analysis
Baseline and pregnancy-related factors and birth outcomes are presented for the two ethnic groups using basic descriptive statistics.
Each scan and birth measures were compared by the ethnic group adjusted for gestational age. Group means and standard deviations, gestational age-adjusted mean difference and standardized difference (reference to the standard deviation of the white British group), 95% confidence interval and P-values were calculated. The three South Asian groups (Pakistani, Indian and Bangladeshi) were combined as there was no statistically significant difference between any kidney dimension when examined individually (data not shown). Not all babies had both kidney measurements, the number varying slightly by dimension. For volume, there were 645 white British and 542 South Asian pregnancies with values for both kidneys. The remainder had only the left or the right (white British, 114 left and 110 right; South Asians, 87 left and 83 right). We took the average of the two when both were present and the value of either right or left when only one was present; although there was a very small difference in the kidney size (right greater than left), there was no difference in the distribution of location by ethnic group. Renal volume was adjusted for estimated fetal weight at scan and birth weight.
Univariate and multivariable linear regression was used to explore ethnic differences in renal outcome measures adjusted for gestation and birth weight. We present data on renal volume as our primary outcome because it will be most closely associated with nephron number [41]. We also modelled head CF at birth and scan, abdominal CF and mid-upper arm CF.
We had three multivariable models to take account of confounding and explanatory factors:
socio-demographic: maternal age, maternal education, marital status, housing tenure and employment status (all from questionnaires);
maternal/pregnancy: BMI at booking, height, parity, smoking and alcohol use at recruitment at 27–29 weeks, fasting glucose, post-load glucose, fasting insulin and GHQ total score;
infant: gender and birth weight for gestational age.
We standardized the absolute differences in each measure from our fully adjusted models to the standard deviation of the measure in the white British group and expressed this as a proportion. There were small percentages of missing data on several maternal/pregnancy factors, which cumulatively led to reduced numbers in models which included these factors. We undertook sensitivity analysis by modelling complete cases only.
We modelled maternal height and BMI at booking separately as they were highly correlated. We modelled consanguinity and place of births in South Asian mothers only, univariate and fully adjusted.
All analyses were performed using Stata version 12.
Sample size
Based on the distribution of kidney size found on ultrasound by Konje [28], a sample size of 746 per ethnic group was required to detect a 1 mm difference in CF (SD 5.0 mm) with 90% power at the 1% significance level or of 439 per group to detect a 0.6 mm difference in length (SD 2.3 mm).
RESULTS
Baseline characteristics
South Asian mothers were predominantly of Pakistani origin, with an even mix of those born in the UK or in the Indian subcontinent (Table 1). South Asian mothers were slightly older, shorter, of higher parity, more likely to be married, consanguineous, less likely to work, more likely to own their home and take supplements and much less likely to smoke or drink alcohol during pregnancy. They had higher fasting insulin, and of those with data recorded, there were higher percentages of gestational diabetes in South Asian mothers (23.0% versus 10.5% in white British), whereas levels of pre-pregnancy hypertension, gestational hypertension and pre-eclampsia were all higher though non-significant in white British mothers. There were no recorded cases of pre-pregnancy diabetes or CKD.
| Variables . | White British (N = 872) . | South Asian (N = 715) . | P-valuea . |
|---|---|---|---|
| Mother’s details | |||
| Mother's ethnic group | |||
| White British | 872 (100) | 0 (0) | <0.001 |
| Pakistani | 0 (0) | 607 (84.9) | |
| Indian | 0 (0) | 76 (10.6) | |
| Bangladeshi | 0 (0) | 32 (4.5) | |
| Mother's country of birth | |||
| UK and Ireland | 863 (99.0) | 353 (49.4) | <0.001 |
| Indian subcontinent | 0 (0) | 351 (49.1) | |
| Other | 9 (1.0) | 11 (1.5) | |
| Mother related to baby's father other than by marriage | |||
| Yes | 2 (0.2) | 378 (52.9) | <0.001 |
| No | 870 (99.8) | 337 (47.1) | |
| Age at questionnaire completion (year), mean (SD) (N = 871/715) | 27.45 (5.84) | 28.57 (4.91) | <0.001 |
| Derived equivalized mother education | |||
| None (<5 GCSE or equivalent) | 142 (16.3) | 136 (19.1) | 0.019 |
| School (≥5 GCSE or equivalent) | 287 (32.9) | 221 (31.0) | |
| Further and higher (a level or equivalent or higher) | 374 (42.9) | 323 (45.4) | |
| Others (other, overseas, unknown) | 69 (7.9) | 32 (4.5) | |
| Marital status | |||
| Married (first marriage or re-married) | 308 (35.3) | 703 (98.5) | <0.001 |
| Single (never married, divorced or separated) | 564 (64.7) | 11 (1.5) | |
| Housing tenure | |||
| Buying/own | 501 (57.5) | 500 (70.1) | <0.001 |
| Renting or other related | 370 (42.5) | 213 (29.9) | |
| Mother's employment status | |||
| Currently employed | 599 (68.8) | 253 (35.4) | <0.001 |
| Previously employed | 209 (24.0) | 206 (28.8) | |
| Never employed | 63 (7.2) | 256 (35.8) | |
| BMI at booking (kg/m2), mean (SD) (N = 804/671) | 27.39 (6.20) | 25.71 (5.11) | <0.001 |
| Height at booking (cm), mean (SD) (N = 860/705) | 163.95 (5.90) | 159.61 (5.76) | <0.001 |
| Parity | |||
| 0 | 411 (47.7) | 237 (33.7) | <0.001 |
| 1 | 299 (34.7) | 186 (26.5) | |
| 2 | 102 (11.9) | 153 (21.8) | |
| 3+ | 49 (5.7) | 127 (18.1) | |
| Mother smoked at any time during pregnancy | |||
| Yes | 263 (32.4) | 24 (3.4) | <0.001 |
| No | 549 (67.6) | 685 (96.6) | |
| Mother drank alcohol at any time during pregnancy | |||
| Yes | 251 (29.0) | 9 (1.3) | <0.001 |
| No | 614 (71.0) | 706 (98.7) | |
| General Health Questionnaire total score, median (LQ, UQ) (N = 868/701) | 21 (16, 28) | 23 (16, 31) | <0.001b |
| Fasting glucose (mmol/L), mean (SD) (N = 850/694) | 4.42 (0.42) | 4.63 (0.67) | <0.001 |
| Post-load glucose (mmol/L), mean (SD) (N = 850/694) | 5.43 (1.19) | 5.80 (1.66) | <0.001 |
| Fasting insulin (pmol/L), mean (SD) (N = 844/658) | 81.93 (47.92) | 99.94 (56.32) | <0.001 |
| Vitamin or iron supplement taken in the last 4 weeks | |||
| Yes | 280 (32.1) | 383 (53.6) | <0.001 |
| No | 592 (67.9) | 332 (46.4) | |
| Pre-pregnancy hypertension | |||
| Yes | 8 (3.4) | 3 (1.6) | 0.359c |
| No | 226 (96.6) | 187 (98.4) | |
| Gestational diabetes | |||
| Yes | 27 (10.5) | 46 (23.0) | <0.001 |
| No | 230 (89.5) | 154 (77.0) | |
| Gestational hypertension | |||
| Yes | 65 (25.2) | 39 (19.4) | 0.141 |
| No | 193 (74.8) | 162 (80.6) | |
| Pre-eclampsia | |||
| Yes | 30 (11.7) | 18 (9.0) | 0.339 |
| No | 226 (88.3) | 183 (91.0) | |
| Variables . | White British (N = 872) . | South Asian (N = 715) . | P-valuea . |
|---|---|---|---|
| Mother’s details | |||
| Mother's ethnic group | |||
| White British | 872 (100) | 0 (0) | <0.001 |
| Pakistani | 0 (0) | 607 (84.9) | |
| Indian | 0 (0) | 76 (10.6) | |
| Bangladeshi | 0 (0) | 32 (4.5) | |
| Mother's country of birth | |||
| UK and Ireland | 863 (99.0) | 353 (49.4) | <0.001 |
| Indian subcontinent | 0 (0) | 351 (49.1) | |
| Other | 9 (1.0) | 11 (1.5) | |
| Mother related to baby's father other than by marriage | |||
| Yes | 2 (0.2) | 378 (52.9) | <0.001 |
| No | 870 (99.8) | 337 (47.1) | |
| Age at questionnaire completion (year), mean (SD) (N = 871/715) | 27.45 (5.84) | 28.57 (4.91) | <0.001 |
| Derived equivalized mother education | |||
| None (<5 GCSE or equivalent) | 142 (16.3) | 136 (19.1) | 0.019 |
| School (≥5 GCSE or equivalent) | 287 (32.9) | 221 (31.0) | |
| Further and higher (a level or equivalent or higher) | 374 (42.9) | 323 (45.4) | |
| Others (other, overseas, unknown) | 69 (7.9) | 32 (4.5) | |
| Marital status | |||
| Married (first marriage or re-married) | 308 (35.3) | 703 (98.5) | <0.001 |
| Single (never married, divorced or separated) | 564 (64.7) | 11 (1.5) | |
| Housing tenure | |||
| Buying/own | 501 (57.5) | 500 (70.1) | <0.001 |
| Renting or other related | 370 (42.5) | 213 (29.9) | |
| Mother's employment status | |||
| Currently employed | 599 (68.8) | 253 (35.4) | <0.001 |
| Previously employed | 209 (24.0) | 206 (28.8) | |
| Never employed | 63 (7.2) | 256 (35.8) | |
| BMI at booking (kg/m2), mean (SD) (N = 804/671) | 27.39 (6.20) | 25.71 (5.11) | <0.001 |
| Height at booking (cm), mean (SD) (N = 860/705) | 163.95 (5.90) | 159.61 (5.76) | <0.001 |
| Parity | |||
| 0 | 411 (47.7) | 237 (33.7) | <0.001 |
| 1 | 299 (34.7) | 186 (26.5) | |
| 2 | 102 (11.9) | 153 (21.8) | |
| 3+ | 49 (5.7) | 127 (18.1) | |
| Mother smoked at any time during pregnancy | |||
| Yes | 263 (32.4) | 24 (3.4) | <0.001 |
| No | 549 (67.6) | 685 (96.6) | |
| Mother drank alcohol at any time during pregnancy | |||
| Yes | 251 (29.0) | 9 (1.3) | <0.001 |
| No | 614 (71.0) | 706 (98.7) | |
| General Health Questionnaire total score, median (LQ, UQ) (N = 868/701) | 21 (16, 28) | 23 (16, 31) | <0.001b |
| Fasting glucose (mmol/L), mean (SD) (N = 850/694) | 4.42 (0.42) | 4.63 (0.67) | <0.001 |
| Post-load glucose (mmol/L), mean (SD) (N = 850/694) | 5.43 (1.19) | 5.80 (1.66) | <0.001 |
| Fasting insulin (pmol/L), mean (SD) (N = 844/658) | 81.93 (47.92) | 99.94 (56.32) | <0.001 |
| Vitamin or iron supplement taken in the last 4 weeks | |||
| Yes | 280 (32.1) | 383 (53.6) | <0.001 |
| No | 592 (67.9) | 332 (46.4) | |
| Pre-pregnancy hypertension | |||
| Yes | 8 (3.4) | 3 (1.6) | 0.359c |
| No | 226 (96.6) | 187 (98.4) | |
| Gestational diabetes | |||
| Yes | 27 (10.5) | 46 (23.0) | <0.001 |
| No | 230 (89.5) | 154 (77.0) | |
| Gestational hypertension | |||
| Yes | 65 (25.2) | 39 (19.4) | 0.141 |
| No | 193 (74.8) | 162 (80.6) | |
| Pre-eclampsia | |||
| Yes | 30 (11.7) | 18 (9.0) | 0.339 |
| No | 226 (88.3) | 183 (91.0) | |
Numbers (%) are presented, unless stated otherwise. For continuous variables, group sample size is presented, respectively.
aχ2 test was performed unless stated otherwise.
bMann–Whitney U-test was performed.
cFisher's exact test was performed.
| Variables . | White British (N = 872) . | South Asian (N = 715) . | P-valuea . |
|---|---|---|---|
| Mother’s details | |||
| Mother's ethnic group | |||
| White British | 872 (100) | 0 (0) | <0.001 |
| Pakistani | 0 (0) | 607 (84.9) | |
| Indian | 0 (0) | 76 (10.6) | |
| Bangladeshi | 0 (0) | 32 (4.5) | |
| Mother's country of birth | |||
| UK and Ireland | 863 (99.0) | 353 (49.4) | <0.001 |
| Indian subcontinent | 0 (0) | 351 (49.1) | |
| Other | 9 (1.0) | 11 (1.5) | |
| Mother related to baby's father other than by marriage | |||
| Yes | 2 (0.2) | 378 (52.9) | <0.001 |
| No | 870 (99.8) | 337 (47.1) | |
| Age at questionnaire completion (year), mean (SD) (N = 871/715) | 27.45 (5.84) | 28.57 (4.91) | <0.001 |
| Derived equivalized mother education | |||
| None (<5 GCSE or equivalent) | 142 (16.3) | 136 (19.1) | 0.019 |
| School (≥5 GCSE or equivalent) | 287 (32.9) | 221 (31.0) | |
| Further and higher (a level or equivalent or higher) | 374 (42.9) | 323 (45.4) | |
| Others (other, overseas, unknown) | 69 (7.9) | 32 (4.5) | |
| Marital status | |||
| Married (first marriage or re-married) | 308 (35.3) | 703 (98.5) | <0.001 |
| Single (never married, divorced or separated) | 564 (64.7) | 11 (1.5) | |
| Housing tenure | |||
| Buying/own | 501 (57.5) | 500 (70.1) | <0.001 |
| Renting or other related | 370 (42.5) | 213 (29.9) | |
| Mother's employment status | |||
| Currently employed | 599 (68.8) | 253 (35.4) | <0.001 |
| Previously employed | 209 (24.0) | 206 (28.8) | |
| Never employed | 63 (7.2) | 256 (35.8) | |
| BMI at booking (kg/m2), mean (SD) (N = 804/671) | 27.39 (6.20) | 25.71 (5.11) | <0.001 |
| Height at booking (cm), mean (SD) (N = 860/705) | 163.95 (5.90) | 159.61 (5.76) | <0.001 |
| Parity | |||
| 0 | 411 (47.7) | 237 (33.7) | <0.001 |
| 1 | 299 (34.7) | 186 (26.5) | |
| 2 | 102 (11.9) | 153 (21.8) | |
| 3+ | 49 (5.7) | 127 (18.1) | |
| Mother smoked at any time during pregnancy | |||
| Yes | 263 (32.4) | 24 (3.4) | <0.001 |
| No | 549 (67.6) | 685 (96.6) | |
| Mother drank alcohol at any time during pregnancy | |||
| Yes | 251 (29.0) | 9 (1.3) | <0.001 |
| No | 614 (71.0) | 706 (98.7) | |
| General Health Questionnaire total score, median (LQ, UQ) (N = 868/701) | 21 (16, 28) | 23 (16, 31) | <0.001b |
| Fasting glucose (mmol/L), mean (SD) (N = 850/694) | 4.42 (0.42) | 4.63 (0.67) | <0.001 |
| Post-load glucose (mmol/L), mean (SD) (N = 850/694) | 5.43 (1.19) | 5.80 (1.66) | <0.001 |
| Fasting insulin (pmol/L), mean (SD) (N = 844/658) | 81.93 (47.92) | 99.94 (56.32) | <0.001 |
| Vitamin or iron supplement taken in the last 4 weeks | |||
| Yes | 280 (32.1) | 383 (53.6) | <0.001 |
| No | 592 (67.9) | 332 (46.4) | |
| Pre-pregnancy hypertension | |||
| Yes | 8 (3.4) | 3 (1.6) | 0.359c |
| No | 226 (96.6) | 187 (98.4) | |
| Gestational diabetes | |||
| Yes | 27 (10.5) | 46 (23.0) | <0.001 |
| No | 230 (89.5) | 154 (77.0) | |
| Gestational hypertension | |||
| Yes | 65 (25.2) | 39 (19.4) | 0.141 |
| No | 193 (74.8) | 162 (80.6) | |
| Pre-eclampsia | |||
| Yes | 30 (11.7) | 18 (9.0) | 0.339 |
| No | 226 (88.3) | 183 (91.0) | |
| Variables . | White British (N = 872) . | South Asian (N = 715) . | P-valuea . |
|---|---|---|---|
| Mother’s details | |||
| Mother's ethnic group | |||
| White British | 872 (100) | 0 (0) | <0.001 |
| Pakistani | 0 (0) | 607 (84.9) | |
| Indian | 0 (0) | 76 (10.6) | |
| Bangladeshi | 0 (0) | 32 (4.5) | |
| Mother's country of birth | |||
| UK and Ireland | 863 (99.0) | 353 (49.4) | <0.001 |
| Indian subcontinent | 0 (0) | 351 (49.1) | |
| Other | 9 (1.0) | 11 (1.5) | |
| Mother related to baby's father other than by marriage | |||
| Yes | 2 (0.2) | 378 (52.9) | <0.001 |
| No | 870 (99.8) | 337 (47.1) | |
| Age at questionnaire completion (year), mean (SD) (N = 871/715) | 27.45 (5.84) | 28.57 (4.91) | <0.001 |
| Derived equivalized mother education | |||
| None (<5 GCSE or equivalent) | 142 (16.3) | 136 (19.1) | 0.019 |
| School (≥5 GCSE or equivalent) | 287 (32.9) | 221 (31.0) | |
| Further and higher (a level or equivalent or higher) | 374 (42.9) | 323 (45.4) | |
| Others (other, overseas, unknown) | 69 (7.9) | 32 (4.5) | |
| Marital status | |||
| Married (first marriage or re-married) | 308 (35.3) | 703 (98.5) | <0.001 |
| Single (never married, divorced or separated) | 564 (64.7) | 11 (1.5) | |
| Housing tenure | |||
| Buying/own | 501 (57.5) | 500 (70.1) | <0.001 |
| Renting or other related | 370 (42.5) | 213 (29.9) | |
| Mother's employment status | |||
| Currently employed | 599 (68.8) | 253 (35.4) | <0.001 |
| Previously employed | 209 (24.0) | 206 (28.8) | |
| Never employed | 63 (7.2) | 256 (35.8) | |
| BMI at booking (kg/m2), mean (SD) (N = 804/671) | 27.39 (6.20) | 25.71 (5.11) | <0.001 |
| Height at booking (cm), mean (SD) (N = 860/705) | 163.95 (5.90) | 159.61 (5.76) | <0.001 |
| Parity | |||
| 0 | 411 (47.7) | 237 (33.7) | <0.001 |
| 1 | 299 (34.7) | 186 (26.5) | |
| 2 | 102 (11.9) | 153 (21.8) | |
| 3+ | 49 (5.7) | 127 (18.1) | |
| Mother smoked at any time during pregnancy | |||
| Yes | 263 (32.4) | 24 (3.4) | <0.001 |
| No | 549 (67.6) | 685 (96.6) | |
| Mother drank alcohol at any time during pregnancy | |||
| Yes | 251 (29.0) | 9 (1.3) | <0.001 |
| No | 614 (71.0) | 706 (98.7) | |
| General Health Questionnaire total score, median (LQ, UQ) (N = 868/701) | 21 (16, 28) | 23 (16, 31) | <0.001b |
| Fasting glucose (mmol/L), mean (SD) (N = 850/694) | 4.42 (0.42) | 4.63 (0.67) | <0.001 |
| Post-load glucose (mmol/L), mean (SD) (N = 850/694) | 5.43 (1.19) | 5.80 (1.66) | <0.001 |
| Fasting insulin (pmol/L), mean (SD) (N = 844/658) | 81.93 (47.92) | 99.94 (56.32) | <0.001 |
| Vitamin or iron supplement taken in the last 4 weeks | |||
| Yes | 280 (32.1) | 383 (53.6) | <0.001 |
| No | 592 (67.9) | 332 (46.4) | |
| Pre-pregnancy hypertension | |||
| Yes | 8 (3.4) | 3 (1.6) | 0.359c |
| No | 226 (96.6) | 187 (98.4) | |
| Gestational diabetes | |||
| Yes | 27 (10.5) | 46 (23.0) | <0.001 |
| No | 230 (89.5) | 154 (77.0) | |
| Gestational hypertension | |||
| Yes | 65 (25.2) | 39 (19.4) | 0.141 |
| No | 193 (74.8) | 162 (80.6) | |
| Pre-eclampsia | |||
| Yes | 30 (11.7) | 18 (9.0) | 0.339 |
| No | 226 (88.3) | 183 (91.0) | |
Numbers (%) are presented, unless stated otherwise. For continuous variables, group sample size is presented, respectively.
aχ2 test was performed unless stated otherwise.
bMann–Whitney U-test was performed.
cFisher's exact test was performed.
Birth outcomes
Duration of gestation at birth was slightly shorter in South Asians (Table 2). Birth weight was greater in white British babies, with and without adjustment for gestation duration, and the percentage of LBW (<2.5 kg) was higher in South Asian babies (3.6 versus 1.5%). The other anthropometric measures such as head CF (scan and birth), mid-upper arm CF and abdominal CF were also higher in white British babies. Standardized differences varied between 0.3 and 0.6 SDs.
| Birth outcome . | Ethnicity . | Number with data . | Mean (SD) . | Difference (95% CI) . | P-value . | Standardized difference (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|
| Gender malea, N (%) | WB | 872 | 417 (47.8%) | 3.9% (−1.0%, 8.9%) | 0.120 | — | — |
| SA | 715 | 370 (51.7%) | |||||
| Gestational age at scan (week)b | WB | 872 | 34.13 (0.36) | −0.003 (−0.04, 0.03) | 0.852 | −0.01 (−0.11, −0.09) | 0.852 |
| SA | 714 | 34.13 (0.38) | |||||
| Gestation at delivery (week)b | WB | 872 | 39.97 (1.19) | −0.21 (−0.33, −0.10) | <0.001 | −0.18 (−0.27, −0.08) | <0.001 |
| SA | 715 | 39.76 (1.13) | |||||
| Birth weight for gestational age at 40 weeks (kg)2 | WB | 872 | 3451.84 (449.52) | −200.17 (−243.18, −157.16) | <0.001 | −0.45 (−0.54, −0.35) | <0.001 |
| SA | 715 | 3251.67 (415.72) | |||||
| Low birth weight (<2500 g)a, N (%) | WB | 872 | 13 (1.5%) | 2.1% (0.6%, 3.7%) | 0.006 | — | — |
| SA | 715 | 26 (3.6%) | |||||
| Head circumference at scan (cm)c | WB | 866 | 31.66 (0.93) | −0.38 (−0.47, −0.30) | <0.001 | −0.41 (−0.51, −0.32) | <0.001 |
| SA | 708 | 31.28 (0.84) | |||||
| Head circumference at birth (cm)d | WB | 842 | 34.76 (1.37) | −0.41 (−0.54, −0.29) | <0.001 | −0.30 (−0.39, −0.21) | <0.001 |
| SA | 698 | 34.26 (1.26) | |||||
| Mid-upper arm circumference at birth (cm)d | WB | 830 | 10.96 (1.03) | −0.32 (−0.42, −0.22) | <0.001 | −0.31 (−0.40, −0.21) | <0.001 |
| SA | 684 | 10.59 (1.01) | |||||
| Abdominal circumference at birth (cm)d | WB | 832 | 32.04 (2.33) | −1.35 (−1.60, −1.11) | <0.001 | −0.58 (−0.69, −0.48) | <0.001 |
| SA | 680 | 30.57 (2.62) |
| Birth outcome . | Ethnicity . | Number with data . | Mean (SD) . | Difference (95% CI) . | P-value . | Standardized difference (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|
| Gender malea, N (%) | WB | 872 | 417 (47.8%) | 3.9% (−1.0%, 8.9%) | 0.120 | — | — |
| SA | 715 | 370 (51.7%) | |||||
| Gestational age at scan (week)b | WB | 872 | 34.13 (0.36) | −0.003 (−0.04, 0.03) | 0.852 | −0.01 (−0.11, −0.09) | 0.852 |
| SA | 714 | 34.13 (0.38) | |||||
| Gestation at delivery (week)b | WB | 872 | 39.97 (1.19) | −0.21 (−0.33, −0.10) | <0.001 | −0.18 (−0.27, −0.08) | <0.001 |
| SA | 715 | 39.76 (1.13) | |||||
| Birth weight for gestational age at 40 weeks (kg)2 | WB | 872 | 3451.84 (449.52) | −200.17 (−243.18, −157.16) | <0.001 | −0.45 (−0.54, −0.35) | <0.001 |
| SA | 715 | 3251.67 (415.72) | |||||
| Low birth weight (<2500 g)a, N (%) | WB | 872 | 13 (1.5%) | 2.1% (0.6%, 3.7%) | 0.006 | — | — |
| SA | 715 | 26 (3.6%) | |||||
| Head circumference at scan (cm)c | WB | 866 | 31.66 (0.93) | −0.38 (−0.47, −0.30) | <0.001 | −0.41 (−0.51, −0.32) | <0.001 |
| SA | 708 | 31.28 (0.84) | |||||
| Head circumference at birth (cm)d | WB | 842 | 34.76 (1.37) | −0.41 (−0.54, −0.29) | <0.001 | −0.30 (−0.39, −0.21) | <0.001 |
| SA | 698 | 34.26 (1.26) | |||||
| Mid-upper arm circumference at birth (cm)d | WB | 830 | 10.96 (1.03) | −0.32 (−0.42, −0.22) | <0.001 | −0.31 (−0.40, −0.21) | <0.001 |
| SA | 684 | 10.59 (1.01) | |||||
| Abdominal circumference at birth (cm)d | WB | 832 | 32.04 (2.33) | −1.35 (−1.60, −1.11) | <0.001 | −0.58 (−0.69, −0.48) | <0.001 |
| SA | 680 | 30.57 (2.62) |
Mean (SD) is presented unless stated otherwise. WB, white British; SA, South Asian.
aDifference in proportions was unadjusted, with WB as the reference category.
bDifference in means was unadjusted, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
cDifference in means was adjusted for gestational age at scan, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
dDifference in means was adjusted for gestation at delivery, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
| Birth outcome . | Ethnicity . | Number with data . | Mean (SD) . | Difference (95% CI) . | P-value . | Standardized difference (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|
| Gender malea, N (%) | WB | 872 | 417 (47.8%) | 3.9% (−1.0%, 8.9%) | 0.120 | — | — |
| SA | 715 | 370 (51.7%) | |||||
| Gestational age at scan (week)b | WB | 872 | 34.13 (0.36) | −0.003 (−0.04, 0.03) | 0.852 | −0.01 (−0.11, −0.09) | 0.852 |
| SA | 714 | 34.13 (0.38) | |||||
| Gestation at delivery (week)b | WB | 872 | 39.97 (1.19) | −0.21 (−0.33, −0.10) | <0.001 | −0.18 (−0.27, −0.08) | <0.001 |
| SA | 715 | 39.76 (1.13) | |||||
| Birth weight for gestational age at 40 weeks (kg)2 | WB | 872 | 3451.84 (449.52) | −200.17 (−243.18, −157.16) | <0.001 | −0.45 (−0.54, −0.35) | <0.001 |
| SA | 715 | 3251.67 (415.72) | |||||
| Low birth weight (<2500 g)a, N (%) | WB | 872 | 13 (1.5%) | 2.1% (0.6%, 3.7%) | 0.006 | — | — |
| SA | 715 | 26 (3.6%) | |||||
| Head circumference at scan (cm)c | WB | 866 | 31.66 (0.93) | −0.38 (−0.47, −0.30) | <0.001 | −0.41 (−0.51, −0.32) | <0.001 |
| SA | 708 | 31.28 (0.84) | |||||
| Head circumference at birth (cm)d | WB | 842 | 34.76 (1.37) | −0.41 (−0.54, −0.29) | <0.001 | −0.30 (−0.39, −0.21) | <0.001 |
| SA | 698 | 34.26 (1.26) | |||||
| Mid-upper arm circumference at birth (cm)d | WB | 830 | 10.96 (1.03) | −0.32 (−0.42, −0.22) | <0.001 | −0.31 (−0.40, −0.21) | <0.001 |
| SA | 684 | 10.59 (1.01) | |||||
| Abdominal circumference at birth (cm)d | WB | 832 | 32.04 (2.33) | −1.35 (−1.60, −1.11) | <0.001 | −0.58 (−0.69, −0.48) | <0.001 |
| SA | 680 | 30.57 (2.62) |
| Birth outcome . | Ethnicity . | Number with data . | Mean (SD) . | Difference (95% CI) . | P-value . | Standardized difference (95% CI) . | P-value . |
|---|---|---|---|---|---|---|---|
| Gender malea, N (%) | WB | 872 | 417 (47.8%) | 3.9% (−1.0%, 8.9%) | 0.120 | — | — |
| SA | 715 | 370 (51.7%) | |||||
| Gestational age at scan (week)b | WB | 872 | 34.13 (0.36) | −0.003 (−0.04, 0.03) | 0.852 | −0.01 (−0.11, −0.09) | 0.852 |
| SA | 714 | 34.13 (0.38) | |||||
| Gestation at delivery (week)b | WB | 872 | 39.97 (1.19) | −0.21 (−0.33, −0.10) | <0.001 | −0.18 (−0.27, −0.08) | <0.001 |
| SA | 715 | 39.76 (1.13) | |||||
| Birth weight for gestational age at 40 weeks (kg)2 | WB | 872 | 3451.84 (449.52) | −200.17 (−243.18, −157.16) | <0.001 | −0.45 (−0.54, −0.35) | <0.001 |
| SA | 715 | 3251.67 (415.72) | |||||
| Low birth weight (<2500 g)a, N (%) | WB | 872 | 13 (1.5%) | 2.1% (0.6%, 3.7%) | 0.006 | — | — |
| SA | 715 | 26 (3.6%) | |||||
| Head circumference at scan (cm)c | WB | 866 | 31.66 (0.93) | −0.38 (−0.47, −0.30) | <0.001 | −0.41 (−0.51, −0.32) | <0.001 |
| SA | 708 | 31.28 (0.84) | |||||
| Head circumference at birth (cm)d | WB | 842 | 34.76 (1.37) | −0.41 (−0.54, −0.29) | <0.001 | −0.30 (−0.39, −0.21) | <0.001 |
| SA | 698 | 34.26 (1.26) | |||||
| Mid-upper arm circumference at birth (cm)d | WB | 830 | 10.96 (1.03) | −0.32 (−0.42, −0.22) | <0.001 | −0.31 (−0.40, −0.21) | <0.001 |
| SA | 684 | 10.59 (1.01) | |||||
| Abdominal circumference at birth (cm)d | WB | 832 | 32.04 (2.33) | −1.35 (−1.60, −1.11) | <0.001 | −0.58 (−0.69, −0.48) | <0.001 |
| SA | 680 | 30.57 (2.62) |
Mean (SD) is presented unless stated otherwise. WB, white British; SA, South Asian.
aDifference in proportions was unadjusted, with WB as the reference category.
bDifference in means was unadjusted, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
cDifference in means was adjusted for gestational age at scan, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
dDifference in means was adjusted for gestation at delivery, with WB as the reference category; standardized difference was standardized to WB, i.e. the difference between the SA mean and the WB mean in terms of standard deviation of the WB, expressed as a proportion.
Renal anthropometry
After adjustment for gestational age at assessment, all South Asian fetal renal dimensions were significantly smaller (Table 3). The proportional reduction was greater for TS diameter, AP diameter and CF than LH, indicating a tendency to a ‘sausage’-like shape. All the dimensions were significantly (P < 0.001) correlated with birth weight (data not shown).
| Renal measurement at scan . | Ethnicity . | Number with data . | Mean (SD) . | Gestational age-adjusted mean difference (95% CI)a . | P-value . | Standardized difference (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|---|
| Transverse diameter (cm) | WB | 870 | 2.26 (0.27) | −0.13 (−0.15, −0.10) | <0.001 | −0.48 (−0.57, −0.38) | <0.001 |
| SA | 712 | 2.13 (0.24) | |||||
| Anteroposterior diameter (cm) | WB | 870 | 2.21 (0.26) | −0.15 (−0.17, −0.12) | <0.001 | −0.56 (−0.66, −0.46) | <0.001 |
| SA | 712 | 2.07 (0.26) | |||||
| Length (cm) | WB | 870 | 3.91 (0.38) | −0.18 (−0.22, −0.14) | <0.001 | −0.47 (−0.57, −0.37) | <0.001 |
| SA | 712 | 3.73 (0.37) | |||||
| Circumference (cm) | WB | 870 | 7.49 (0.79) | −0.46 (−0.54, −0.39) | <0.001 | −0.59 (−0.68, −0.49) | <0.001 |
| SA | 712 | 7.03 (0.74) | |||||
| Volume (cm3) | WB | 870 | 10.45 (2.85) | −1.66 (−1.93, −1.40) | <0.001 | −0.58 (−0.68, −0.49) | <0.001 |
| SA | 712 | 8.79 (2.48) | |||||
| Estimated fetal weight at scan (kg) | WB | 846 | 2.30 (0.25) | −0.12 (−0.14, −0.09) | <0.001 | −0.46 (−0.55, −0.37) | <0.001 |
| SA | 707 | 2.18 (0.22) | |||||
| Volume/estimated fetal weight (cm3/kg) | WB | 864 | 4.57 (1.18) | −0.54 (−0.65, −0.43) | <0.001 | −0.46 (−0.55, −0.36) | <0.001 |
| SA | 705 | 4.03 (1.05) | |||||
| Volume/birth weight (cm3/kg) | WB | 870 | 3.06 (0.83) | −0.30 (−0.38, −0.22) | <0.001 | −0.36 (−0.46, −0.27) | <0.001 |
| SA | 712 | 2.76 (0.76) |
| Renal measurement at scan . | Ethnicity . | Number with data . | Mean (SD) . | Gestational age-adjusted mean difference (95% CI)a . | P-value . | Standardized difference (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|---|
| Transverse diameter (cm) | WB | 870 | 2.26 (0.27) | −0.13 (−0.15, −0.10) | <0.001 | −0.48 (−0.57, −0.38) | <0.001 |
| SA | 712 | 2.13 (0.24) | |||||
| Anteroposterior diameter (cm) | WB | 870 | 2.21 (0.26) | −0.15 (−0.17, −0.12) | <0.001 | −0.56 (−0.66, −0.46) | <0.001 |
| SA | 712 | 2.07 (0.26) | |||||
| Length (cm) | WB | 870 | 3.91 (0.38) | −0.18 (−0.22, −0.14) | <0.001 | −0.47 (−0.57, −0.37) | <0.001 |
| SA | 712 | 3.73 (0.37) | |||||
| Circumference (cm) | WB | 870 | 7.49 (0.79) | −0.46 (−0.54, −0.39) | <0.001 | −0.59 (−0.68, −0.49) | <0.001 |
| SA | 712 | 7.03 (0.74) | |||||
| Volume (cm3) | WB | 870 | 10.45 (2.85) | −1.66 (−1.93, −1.40) | <0.001 | −0.58 (−0.68, −0.49) | <0.001 |
| SA | 712 | 8.79 (2.48) | |||||
| Estimated fetal weight at scan (kg) | WB | 846 | 2.30 (0.25) | −0.12 (−0.14, −0.09) | <0.001 | −0.46 (−0.55, −0.37) | <0.001 |
| SA | 707 | 2.18 (0.22) | |||||
| Volume/estimated fetal weight (cm3/kg) | WB | 864 | 4.57 (1.18) | −0.54 (−0.65, −0.43) | <0.001 | −0.46 (−0.55, −0.36) | <0.001 |
| SA | 705 | 4.03 (1.05) | |||||
| Volume/birth weight (cm3/kg) | WB | 870 | 3.06 (0.83) | −0.30 (−0.38, −0.22) | <0.001 | −0.36 (−0.46, −0.27) | <0.001 |
| SA | 712 | 2.76 (0.76) |
WB, white British; SA, South Asian.
aReference category: WB.
bThe adjusted mean difference was standardized to WB, i.e. the difference between the SA mean and the WB mean was divided by the standard deviation of WB and expressed as a proportion.
| Renal measurement at scan . | Ethnicity . | Number with data . | Mean (SD) . | Gestational age-adjusted mean difference (95% CI)a . | P-value . | Standardized difference (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|---|
| Transverse diameter (cm) | WB | 870 | 2.26 (0.27) | −0.13 (−0.15, −0.10) | <0.001 | −0.48 (−0.57, −0.38) | <0.001 |
| SA | 712 | 2.13 (0.24) | |||||
| Anteroposterior diameter (cm) | WB | 870 | 2.21 (0.26) | −0.15 (−0.17, −0.12) | <0.001 | −0.56 (−0.66, −0.46) | <0.001 |
| SA | 712 | 2.07 (0.26) | |||||
| Length (cm) | WB | 870 | 3.91 (0.38) | −0.18 (−0.22, −0.14) | <0.001 | −0.47 (−0.57, −0.37) | <0.001 |
| SA | 712 | 3.73 (0.37) | |||||
| Circumference (cm) | WB | 870 | 7.49 (0.79) | −0.46 (−0.54, −0.39) | <0.001 | −0.59 (−0.68, −0.49) | <0.001 |
| SA | 712 | 7.03 (0.74) | |||||
| Volume (cm3) | WB | 870 | 10.45 (2.85) | −1.66 (−1.93, −1.40) | <0.001 | −0.58 (−0.68, −0.49) | <0.001 |
| SA | 712 | 8.79 (2.48) | |||||
| Estimated fetal weight at scan (kg) | WB | 846 | 2.30 (0.25) | −0.12 (−0.14, −0.09) | <0.001 | −0.46 (−0.55, −0.37) | <0.001 |
| SA | 707 | 2.18 (0.22) | |||||
| Volume/estimated fetal weight (cm3/kg) | WB | 864 | 4.57 (1.18) | −0.54 (−0.65, −0.43) | <0.001 | −0.46 (−0.55, −0.36) | <0.001 |
| SA | 705 | 4.03 (1.05) | |||||
| Volume/birth weight (cm3/kg) | WB | 870 | 3.06 (0.83) | −0.30 (−0.38, −0.22) | <0.001 | −0.36 (−0.46, −0.27) | <0.001 |
| SA | 712 | 2.76 (0.76) |
| Renal measurement at scan . | Ethnicity . | Number with data . | Mean (SD) . | Gestational age-adjusted mean difference (95% CI)a . | P-value . | Standardized difference (95% CI)b . | P-value . |
|---|---|---|---|---|---|---|---|
| Transverse diameter (cm) | WB | 870 | 2.26 (0.27) | −0.13 (−0.15, −0.10) | <0.001 | −0.48 (−0.57, −0.38) | <0.001 |
| SA | 712 | 2.13 (0.24) | |||||
| Anteroposterior diameter (cm) | WB | 870 | 2.21 (0.26) | −0.15 (−0.17, −0.12) | <0.001 | −0.56 (−0.66, −0.46) | <0.001 |
| SA | 712 | 2.07 (0.26) | |||||
| Length (cm) | WB | 870 | 3.91 (0.38) | −0.18 (−0.22, −0.14) | <0.001 | −0.47 (−0.57, −0.37) | <0.001 |
| SA | 712 | 3.73 (0.37) | |||||
| Circumference (cm) | WB | 870 | 7.49 (0.79) | −0.46 (−0.54, −0.39) | <0.001 | −0.59 (−0.68, −0.49) | <0.001 |
| SA | 712 | 7.03 (0.74) | |||||
| Volume (cm3) | WB | 870 | 10.45 (2.85) | −1.66 (−1.93, −1.40) | <0.001 | −0.58 (−0.68, −0.49) | <0.001 |
| SA | 712 | 8.79 (2.48) | |||||
| Estimated fetal weight at scan (kg) | WB | 846 | 2.30 (0.25) | −0.12 (−0.14, −0.09) | <0.001 | −0.46 (−0.55, −0.37) | <0.001 |
| SA | 707 | 2.18 (0.22) | |||||
| Volume/estimated fetal weight (cm3/kg) | WB | 864 | 4.57 (1.18) | −0.54 (−0.65, −0.43) | <0.001 | −0.46 (−0.55, −0.36) | <0.001 |
| SA | 705 | 4.03 (1.05) | |||||
| Volume/birth weight (cm3/kg) | WB | 870 | 3.06 (0.83) | −0.30 (−0.38, −0.22) | <0.001 | −0.36 (−0.46, −0.27) | <0.001 |
| SA | 712 | 2.76 (0.76) |
WB, white British; SA, South Asian.
aReference category: WB.
bThe adjusted mean difference was standardized to WB, i.e. the difference between the SA mean and the WB mean was divided by the standard deviation of WB and expressed as a proportion.
The adjusted mean difference for volume reduced with adjustment for birth weight, but volume remained higher and statistically significant in white British women, with a standardized difference of 0.36. There was a similar finding adjusting for estimated fetal weight. The differences were similar when comparing left or right kidney only or when restricting to only those with both measures (data not shown).
Regression models of renal volume
The univariable- and multivariable-adjusted models of association between ethnicity and renal volume are shown in Table 4. In the univariate analysis, South Asian and non-smoking mothers had significantly reduced kidney volume; ethnicity confounded the relation with maternal smoking and it was no longer apparent in multivariable models. The ethnicity difference persisted in multivariable models; adjusting for baby's weight and gender attenuated the difference, but the kidney volume was nonetheless 1.38 cm3 (95% CI −1.84 to −0.93) smaller in the fully adjusted model. Independent factors associated with larger kidney volumes were older maternal age, male gender and higher birth weight. Sensitivity analysis using 1352 complete cases only in all models (Supplementary data, Table S1) and substituting BMI (as a continuous variable) for height gave similar results.
Differences in renal volume at scan (cm3) by ethnic group: univariable and multivariable
| Model variable . | N . | Univariate modela (max. N = 1582) . | Socio-demographic modela (N = 1573) . | Maternal/pregnancy modela (N = 1358) . | Infant modela (N = 1582) . | Full modela (N = 1352) . |
|---|---|---|---|---|---|---|
| Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | ||
| Ethnicityb | ||||||
| White British | 870 | 0 | 0 | 0 | 0 | 0 |
| South Asian | 712 | −1.66 (−1.93, −1.40) (P < 0.001) | −1.88 (−2.27, −1.50) (P < 0.001) | −1.83 (−2.19, −1.47) (P < 0.001) | −1.33 (−1.59, −1.07) (P < 0.001) | −1.38 (−1.84, −0.93) (P < 0.001) |
| Age at questionnaire completion (year) | 1581 | 0.029 (0.003, 0.054) (P = 0.026) | 0.05 (0.02, 0.07) (P = 0.001) | 0.04 (0.01, 0.08) (P = 0.018) | ||
| Derived equivalized mother educationb | ||||||
| None (<5 GCSE or equivalent) | 276 | 0 | 0 | 0 | ||
| School (≥5 GCSE or equivalent) | 506 | 0.23 (−0.18, 0.64) (P = 0.278) | 0.18 (−0.23, 0.59) (P = 0.392) | 0.10 (−0.32, 0.53) (P = 0.633) | ||
| Further and higher (a level or equivalent or higher) | 696 | 0.05 (−0.34, 0.44) (P = 0.807) | −0.04 (−0.45, 0.38) (P = 0.856) | −0.09 (−0.53, 0.36) (P = 0.709) | ||
| Others (other, overseas, unknown) | 101 | 0.50 (−0.14, 1.14) (P = 0.125) | 0.14 (−0.49, 0.77) (P = 0.654) | −0.03 (−0.69, 0.63) (P = 0.923) | ||
| Marital statusb | ||||||
| Married | 1007 | 0 | 0 | 0 | ||
| Single | 574 | 0.93 (0.64, 1.21) (P < 0.001) | −0.28 (−0.68, −0.13) (P = 0.181) | −0.06 (−0.50, 0.37) (P = 0.783) | ||
| Housing tenureb | ||||||
| Buying/own | 999 | 0 | 0 | 0 | ||
| Renting or other related | 580 | 0.25 (−0.04, 0.54) (P = 0.091) | 0.21 (−0.10, 0.52) (P = 0.186) | 0.30 (−0.02, 0.62) (P = 0.065) | ||
| Employment statusb | ||||||
| Currently employed | 851 | 0 | 0 | 0 | ||
| Previously employed | 413 | −0.17 (−0.50, 0.16) (P = 0.306) | 0.20 (−0.14, 0.55) (P = 0.247) | 0.23 (−0.15, 0.61) (P = 0.232) | ||
| Never employed | 317 | −0.90 (1.26, −0.54) (P < 0.001) | 0.03 (−0.39, 0.45) (P = 0.889) | 0.08 (−0.37, 0.53) (P = 0.724) | ||
| BMI at booking (kg/m2)c | 1470 | 0.06 (0.04, 0.09) (P < 0.001) | ||||
| Height at booking (cm) | 1560 | 0.07 (0.04, 0.09) (P < 0.001) | 0.03 (0.002, 0.05) (P = 0.031) | 0.01 (−0.02, 0.03) (P = 0.554) | ||
| Parityb | ||||||
| 0 | 647 | 0 | 0 | 0 | ||
| 1 | 483 | −0.14 (−0.47, 0.20) (P = 0.418) | 0.07 (−0.27, 0.42) (P = 0.676) | −0.29 (−0.64, 0.07) (P = 0.113) | ||
| 2 | 255 | −0.25 (−0.66, 0.16) (P = 0.228) | 0.27 (−0.16, 0.70) (P = 0.221) | −0.25 (−0.71, 0.21) (P = 0.290) | ||
| 3+ | 174 | −0.01 (−0.48, 0.46) (P = 0.973) | 0.60 (0.10, 1.09) (P = 0.018) | −0.14 (−0.72, 0.45) (P = 0.650) | ||
| Smoked at any time during pregnancyb | ||||||
| No | 1230 | 0 | 0 | 0 | ||
| Yes | 286 | 0.58 (0.22, 0.95) (P = 0.002) | −0.19 (−0.59, 0.21) (P = 0.348) | 0.12 (−0.30, 0.54) (P = 0.567) | ||
| Drank alcohol at any time during pregnancyb | ||||||
| No | 1315 | 0 | 0 | 0 | ||
| Yes | 260 | 0.83 (0.46, 1.21) (P < 0.001) | 0.07 (−0.35, 0.49) (P = 0.741) | 0.08 (−0.32, 0.49) (P = 0.682) | ||
| Glucose tolerance test—fasting glucose (mmol/L) | 1539 | −0.08 (−0.34, 0.19) (P = 0.575) | −0.04 (−0.40, 0.32) (P = 0.841) | −0.18 (−0.52, 0.17) (P = 0.324) | ||
| Glucose tolerance test—post-glucose (mmol/L) | 1539 | −0.004 (−0.10, 0.10) (P = 0.944) | 0.06 (−0.06, 0.18) (P = 0.336) | 0.0003 (−0.12, 0.12) (P = 0.996) | ||
| Insulin level (pmol/L) | 1497 | 0.0001 (−0.0026, 0.0028) (P = 0.941) | 0.0034 (0.0002, 0.0066) (P = 0.038) | 0.0017 (−0.0014, 0.0048) (P = 0.286) | ||
| GHQ total score | 1582 | −0.011 (−0.024, 0.002) (P = 0.090) | ||||
| Baby's genderb | ||||||
| Male | 783 | 0 | 0 | 0 | ||
| Female | 799 | −0.73 (−1.01, −0.46) (P < 0.001) | −0.55 (−0.81, −0.30) (P < 0.001) | −0.52 (−0.80, −0.24) (P < 0.001) | ||
| Birth weight for gestational age (kg) | 1582 | 2.18 (1.89, 2.48) (P < 0.001) | 1.76 (1.47, 2.05) (P< 0.001) | 1.70 (1.36, 2.05) (P < 0.001) | ||
| Model variable . | N . | Univariate modela (max. N = 1582) . | Socio-demographic modela (N = 1573) . | Maternal/pregnancy modela (N = 1358) . | Infant modela (N = 1582) . | Full modela (N = 1352) . |
|---|---|---|---|---|---|---|
| Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | ||
| Ethnicityb | ||||||
| White British | 870 | 0 | 0 | 0 | 0 | 0 |
| South Asian | 712 | −1.66 (−1.93, −1.40) (P < 0.001) | −1.88 (−2.27, −1.50) (P < 0.001) | −1.83 (−2.19, −1.47) (P < 0.001) | −1.33 (−1.59, −1.07) (P < 0.001) | −1.38 (−1.84, −0.93) (P < 0.001) |
| Age at questionnaire completion (year) | 1581 | 0.029 (0.003, 0.054) (P = 0.026) | 0.05 (0.02, 0.07) (P = 0.001) | 0.04 (0.01, 0.08) (P = 0.018) | ||
| Derived equivalized mother educationb | ||||||
| None (<5 GCSE or equivalent) | 276 | 0 | 0 | 0 | ||
| School (≥5 GCSE or equivalent) | 506 | 0.23 (−0.18, 0.64) (P = 0.278) | 0.18 (−0.23, 0.59) (P = 0.392) | 0.10 (−0.32, 0.53) (P = 0.633) | ||
| Further and higher (a level or equivalent or higher) | 696 | 0.05 (−0.34, 0.44) (P = 0.807) | −0.04 (−0.45, 0.38) (P = 0.856) | −0.09 (−0.53, 0.36) (P = 0.709) | ||
| Others (other, overseas, unknown) | 101 | 0.50 (−0.14, 1.14) (P = 0.125) | 0.14 (−0.49, 0.77) (P = 0.654) | −0.03 (−0.69, 0.63) (P = 0.923) | ||
| Marital statusb | ||||||
| Married | 1007 | 0 | 0 | 0 | ||
| Single | 574 | 0.93 (0.64, 1.21) (P < 0.001) | −0.28 (−0.68, −0.13) (P = 0.181) | −0.06 (−0.50, 0.37) (P = 0.783) | ||
| Housing tenureb | ||||||
| Buying/own | 999 | 0 | 0 | 0 | ||
| Renting or other related | 580 | 0.25 (−0.04, 0.54) (P = 0.091) | 0.21 (−0.10, 0.52) (P = 0.186) | 0.30 (−0.02, 0.62) (P = 0.065) | ||
| Employment statusb | ||||||
| Currently employed | 851 | 0 | 0 | 0 | ||
| Previously employed | 413 | −0.17 (−0.50, 0.16) (P = 0.306) | 0.20 (−0.14, 0.55) (P = 0.247) | 0.23 (−0.15, 0.61) (P = 0.232) | ||
| Never employed | 317 | −0.90 (1.26, −0.54) (P < 0.001) | 0.03 (−0.39, 0.45) (P = 0.889) | 0.08 (−0.37, 0.53) (P = 0.724) | ||
| BMI at booking (kg/m2)c | 1470 | 0.06 (0.04, 0.09) (P < 0.001) | ||||
| Height at booking (cm) | 1560 | 0.07 (0.04, 0.09) (P < 0.001) | 0.03 (0.002, 0.05) (P = 0.031) | 0.01 (−0.02, 0.03) (P = 0.554) | ||
| Parityb | ||||||
| 0 | 647 | 0 | 0 | 0 | ||
| 1 | 483 | −0.14 (−0.47, 0.20) (P = 0.418) | 0.07 (−0.27, 0.42) (P = 0.676) | −0.29 (−0.64, 0.07) (P = 0.113) | ||
| 2 | 255 | −0.25 (−0.66, 0.16) (P = 0.228) | 0.27 (−0.16, 0.70) (P = 0.221) | −0.25 (−0.71, 0.21) (P = 0.290) | ||
| 3+ | 174 | −0.01 (−0.48, 0.46) (P = 0.973) | 0.60 (0.10, 1.09) (P = 0.018) | −0.14 (−0.72, 0.45) (P = 0.650) | ||
| Smoked at any time during pregnancyb | ||||||
| No | 1230 | 0 | 0 | 0 | ||
| Yes | 286 | 0.58 (0.22, 0.95) (P = 0.002) | −0.19 (−0.59, 0.21) (P = 0.348) | 0.12 (−0.30, 0.54) (P = 0.567) | ||
| Drank alcohol at any time during pregnancyb | ||||||
| No | 1315 | 0 | 0 | 0 | ||
| Yes | 260 | 0.83 (0.46, 1.21) (P < 0.001) | 0.07 (−0.35, 0.49) (P = 0.741) | 0.08 (−0.32, 0.49) (P = 0.682) | ||
| Glucose tolerance test—fasting glucose (mmol/L) | 1539 | −0.08 (−0.34, 0.19) (P = 0.575) | −0.04 (−0.40, 0.32) (P = 0.841) | −0.18 (−0.52, 0.17) (P = 0.324) | ||
| Glucose tolerance test—post-glucose (mmol/L) | 1539 | −0.004 (−0.10, 0.10) (P = 0.944) | 0.06 (−0.06, 0.18) (P = 0.336) | 0.0003 (−0.12, 0.12) (P = 0.996) | ||
| Insulin level (pmol/L) | 1497 | 0.0001 (−0.0026, 0.0028) (P = 0.941) | 0.0034 (0.0002, 0.0066) (P = 0.038) | 0.0017 (−0.0014, 0.0048) (P = 0.286) | ||
| GHQ total score | 1582 | −0.011 (−0.024, 0.002) (P = 0.090) | ||||
| Baby's genderb | ||||||
| Male | 783 | 0 | 0 | 0 | ||
| Female | 799 | −0.73 (−1.01, −0.46) (P < 0.001) | −0.55 (−0.81, −0.30) (P < 0.001) | −0.52 (−0.80, −0.24) (P < 0.001) | ||
| Birth weight for gestational age (kg) | 1582 | 2.18 (1.89, 2.48) (P < 0.001) | 1.76 (1.47, 2.05) (P< 0.001) | 1.70 (1.36, 2.05) (P < 0.001) | ||
aDependent variable: renal volume at scan (cm3).
bThe first category of each categorical variable is the reference category.
cSubstituting BMI for height gave similar results.
Differences in renal volume at scan (cm3) by ethnic group: univariable and multivariable
| Model variable . | N . | Univariate modela (max. N = 1582) . | Socio-demographic modela (N = 1573) . | Maternal/pregnancy modela (N = 1358) . | Infant modela (N = 1582) . | Full modela (N = 1352) . |
|---|---|---|---|---|---|---|
| Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | ||
| Ethnicityb | ||||||
| White British | 870 | 0 | 0 | 0 | 0 | 0 |
| South Asian | 712 | −1.66 (−1.93, −1.40) (P < 0.001) | −1.88 (−2.27, −1.50) (P < 0.001) | −1.83 (−2.19, −1.47) (P < 0.001) | −1.33 (−1.59, −1.07) (P < 0.001) | −1.38 (−1.84, −0.93) (P < 0.001) |
| Age at questionnaire completion (year) | 1581 | 0.029 (0.003, 0.054) (P = 0.026) | 0.05 (0.02, 0.07) (P = 0.001) | 0.04 (0.01, 0.08) (P = 0.018) | ||
| Derived equivalized mother educationb | ||||||
| None (<5 GCSE or equivalent) | 276 | 0 | 0 | 0 | ||
| School (≥5 GCSE or equivalent) | 506 | 0.23 (−0.18, 0.64) (P = 0.278) | 0.18 (−0.23, 0.59) (P = 0.392) | 0.10 (−0.32, 0.53) (P = 0.633) | ||
| Further and higher (a level or equivalent or higher) | 696 | 0.05 (−0.34, 0.44) (P = 0.807) | −0.04 (−0.45, 0.38) (P = 0.856) | −0.09 (−0.53, 0.36) (P = 0.709) | ||
| Others (other, overseas, unknown) | 101 | 0.50 (−0.14, 1.14) (P = 0.125) | 0.14 (−0.49, 0.77) (P = 0.654) | −0.03 (−0.69, 0.63) (P = 0.923) | ||
| Marital statusb | ||||||
| Married | 1007 | 0 | 0 | 0 | ||
| Single | 574 | 0.93 (0.64, 1.21) (P < 0.001) | −0.28 (−0.68, −0.13) (P = 0.181) | −0.06 (−0.50, 0.37) (P = 0.783) | ||
| Housing tenureb | ||||||
| Buying/own | 999 | 0 | 0 | 0 | ||
| Renting or other related | 580 | 0.25 (−0.04, 0.54) (P = 0.091) | 0.21 (−0.10, 0.52) (P = 0.186) | 0.30 (−0.02, 0.62) (P = 0.065) | ||
| Employment statusb | ||||||
| Currently employed | 851 | 0 | 0 | 0 | ||
| Previously employed | 413 | −0.17 (−0.50, 0.16) (P = 0.306) | 0.20 (−0.14, 0.55) (P = 0.247) | 0.23 (−0.15, 0.61) (P = 0.232) | ||
| Never employed | 317 | −0.90 (1.26, −0.54) (P < 0.001) | 0.03 (−0.39, 0.45) (P = 0.889) | 0.08 (−0.37, 0.53) (P = 0.724) | ||
| BMI at booking (kg/m2)c | 1470 | 0.06 (0.04, 0.09) (P < 0.001) | ||||
| Height at booking (cm) | 1560 | 0.07 (0.04, 0.09) (P < 0.001) | 0.03 (0.002, 0.05) (P = 0.031) | 0.01 (−0.02, 0.03) (P = 0.554) | ||
| Parityb | ||||||
| 0 | 647 | 0 | 0 | 0 | ||
| 1 | 483 | −0.14 (−0.47, 0.20) (P = 0.418) | 0.07 (−0.27, 0.42) (P = 0.676) | −0.29 (−0.64, 0.07) (P = 0.113) | ||
| 2 | 255 | −0.25 (−0.66, 0.16) (P = 0.228) | 0.27 (−0.16, 0.70) (P = 0.221) | −0.25 (−0.71, 0.21) (P = 0.290) | ||
| 3+ | 174 | −0.01 (−0.48, 0.46) (P = 0.973) | 0.60 (0.10, 1.09) (P = 0.018) | −0.14 (−0.72, 0.45) (P = 0.650) | ||
| Smoked at any time during pregnancyb | ||||||
| No | 1230 | 0 | 0 | 0 | ||
| Yes | 286 | 0.58 (0.22, 0.95) (P = 0.002) | −0.19 (−0.59, 0.21) (P = 0.348) | 0.12 (−0.30, 0.54) (P = 0.567) | ||
| Drank alcohol at any time during pregnancyb | ||||||
| No | 1315 | 0 | 0 | 0 | ||
| Yes | 260 | 0.83 (0.46, 1.21) (P < 0.001) | 0.07 (−0.35, 0.49) (P = 0.741) | 0.08 (−0.32, 0.49) (P = 0.682) | ||
| Glucose tolerance test—fasting glucose (mmol/L) | 1539 | −0.08 (−0.34, 0.19) (P = 0.575) | −0.04 (−0.40, 0.32) (P = 0.841) | −0.18 (−0.52, 0.17) (P = 0.324) | ||
| Glucose tolerance test—post-glucose (mmol/L) | 1539 | −0.004 (−0.10, 0.10) (P = 0.944) | 0.06 (−0.06, 0.18) (P = 0.336) | 0.0003 (−0.12, 0.12) (P = 0.996) | ||
| Insulin level (pmol/L) | 1497 | 0.0001 (−0.0026, 0.0028) (P = 0.941) | 0.0034 (0.0002, 0.0066) (P = 0.038) | 0.0017 (−0.0014, 0.0048) (P = 0.286) | ||
| GHQ total score | 1582 | −0.011 (−0.024, 0.002) (P = 0.090) | ||||
| Baby's genderb | ||||||
| Male | 783 | 0 | 0 | 0 | ||
| Female | 799 | −0.73 (−1.01, −0.46) (P < 0.001) | −0.55 (−0.81, −0.30) (P < 0.001) | −0.52 (−0.80, −0.24) (P < 0.001) | ||
| Birth weight for gestational age (kg) | 1582 | 2.18 (1.89, 2.48) (P < 0.001) | 1.76 (1.47, 2.05) (P< 0.001) | 1.70 (1.36, 2.05) (P < 0.001) | ||
| Model variable . | N . | Univariate modela (max. N = 1582) . | Socio-demographic modela (N = 1573) . | Maternal/pregnancy modela (N = 1358) . | Infant modela (N = 1582) . | Full modela (N = 1352) . |
|---|---|---|---|---|---|---|
| Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | Beta estimate (95% CI) (P-value) . | ||
| Ethnicityb | ||||||
| White British | 870 | 0 | 0 | 0 | 0 | 0 |
| South Asian | 712 | −1.66 (−1.93, −1.40) (P < 0.001) | −1.88 (−2.27, −1.50) (P < 0.001) | −1.83 (−2.19, −1.47) (P < 0.001) | −1.33 (−1.59, −1.07) (P < 0.001) | −1.38 (−1.84, −0.93) (P < 0.001) |
| Age at questionnaire completion (year) | 1581 | 0.029 (0.003, 0.054) (P = 0.026) | 0.05 (0.02, 0.07) (P = 0.001) | 0.04 (0.01, 0.08) (P = 0.018) | ||
| Derived equivalized mother educationb | ||||||
| None (<5 GCSE or equivalent) | 276 | 0 | 0 | 0 | ||
| School (≥5 GCSE or equivalent) | 506 | 0.23 (−0.18, 0.64) (P = 0.278) | 0.18 (−0.23, 0.59) (P = 0.392) | 0.10 (−0.32, 0.53) (P = 0.633) | ||
| Further and higher (a level or equivalent or higher) | 696 | 0.05 (−0.34, 0.44) (P = 0.807) | −0.04 (−0.45, 0.38) (P = 0.856) | −0.09 (−0.53, 0.36) (P = 0.709) | ||
| Others (other, overseas, unknown) | 101 | 0.50 (−0.14, 1.14) (P = 0.125) | 0.14 (−0.49, 0.77) (P = 0.654) | −0.03 (−0.69, 0.63) (P = 0.923) | ||
| Marital statusb | ||||||
| Married | 1007 | 0 | 0 | 0 | ||
| Single | 574 | 0.93 (0.64, 1.21) (P < 0.001) | −0.28 (−0.68, −0.13) (P = 0.181) | −0.06 (−0.50, 0.37) (P = 0.783) | ||
| Housing tenureb | ||||||
| Buying/own | 999 | 0 | 0 | 0 | ||
| Renting or other related | 580 | 0.25 (−0.04, 0.54) (P = 0.091) | 0.21 (−0.10, 0.52) (P = 0.186) | 0.30 (−0.02, 0.62) (P = 0.065) | ||
| Employment statusb | ||||||
| Currently employed | 851 | 0 | 0 | 0 | ||
| Previously employed | 413 | −0.17 (−0.50, 0.16) (P = 0.306) | 0.20 (−0.14, 0.55) (P = 0.247) | 0.23 (−0.15, 0.61) (P = 0.232) | ||
| Never employed | 317 | −0.90 (1.26, −0.54) (P < 0.001) | 0.03 (−0.39, 0.45) (P = 0.889) | 0.08 (−0.37, 0.53) (P = 0.724) | ||
| BMI at booking (kg/m2)c | 1470 | 0.06 (0.04, 0.09) (P < 0.001) | ||||
| Height at booking (cm) | 1560 | 0.07 (0.04, 0.09) (P < 0.001) | 0.03 (0.002, 0.05) (P = 0.031) | 0.01 (−0.02, 0.03) (P = 0.554) | ||
| Parityb | ||||||
| 0 | 647 | 0 | 0 | 0 | ||
| 1 | 483 | −0.14 (−0.47, 0.20) (P = 0.418) | 0.07 (−0.27, 0.42) (P = 0.676) | −0.29 (−0.64, 0.07) (P = 0.113) | ||
| 2 | 255 | −0.25 (−0.66, 0.16) (P = 0.228) | 0.27 (−0.16, 0.70) (P = 0.221) | −0.25 (−0.71, 0.21) (P = 0.290) | ||
| 3+ | 174 | −0.01 (−0.48, 0.46) (P = 0.973) | 0.60 (0.10, 1.09) (P = 0.018) | −0.14 (−0.72, 0.45) (P = 0.650) | ||
| Smoked at any time during pregnancyb | ||||||
| No | 1230 | 0 | 0 | 0 | ||
| Yes | 286 | 0.58 (0.22, 0.95) (P = 0.002) | −0.19 (−0.59, 0.21) (P = 0.348) | 0.12 (−0.30, 0.54) (P = 0.567) | ||
| Drank alcohol at any time during pregnancyb | ||||||
| No | 1315 | 0 | 0 | 0 | ||
| Yes | 260 | 0.83 (0.46, 1.21) (P < 0.001) | 0.07 (−0.35, 0.49) (P = 0.741) | 0.08 (−0.32, 0.49) (P = 0.682) | ||
| Glucose tolerance test—fasting glucose (mmol/L) | 1539 | −0.08 (−0.34, 0.19) (P = 0.575) | −0.04 (−0.40, 0.32) (P = 0.841) | −0.18 (−0.52, 0.17) (P = 0.324) | ||
| Glucose tolerance test—post-glucose (mmol/L) | 1539 | −0.004 (−0.10, 0.10) (P = 0.944) | 0.06 (−0.06, 0.18) (P = 0.336) | 0.0003 (−0.12, 0.12) (P = 0.996) | ||
| Insulin level (pmol/L) | 1497 | 0.0001 (−0.0026, 0.0028) (P = 0.941) | 0.0034 (0.0002, 0.0066) (P = 0.038) | 0.0017 (−0.0014, 0.0048) (P = 0.286) | ||
| GHQ total score | 1582 | −0.011 (−0.024, 0.002) (P = 0.090) | ||||
| Baby's genderb | ||||||
| Male | 783 | 0 | 0 | 0 | ||
| Female | 799 | −0.73 (−1.01, −0.46) (P < 0.001) | −0.55 (−0.81, −0.30) (P < 0.001) | −0.52 (−0.80, −0.24) (P < 0.001) | ||
| Birth weight for gestational age (kg) | 1582 | 2.18 (1.89, 2.48) (P < 0.001) | 1.76 (1.47, 2.05) (P< 0.001) | 1.70 (1.36, 2.05) (P < 0.001) | ||
aDependent variable: renal volume at scan (cm3).
bThe first category of each categorical variable is the reference category.
cSubstituting BMI for height gave similar results.
Examining how the ethnicity difference in kidney volumes compared with ethnicity-associated differences in fetal and neonatal anthropometry in fully adjusted models, we show that the proportional reductions in terms of the WB SD (from Table 3) for South Asian offspring were greatest for renal volume (1.38/2.85) at 0.49 (95% CI −0.65 to −0.33), compared with 0.21 (95% CI −0.36 to −0.06) for head CF at scan and 0.12 (95% CI −0.26 to 0.02), 0.39 (95% CI −0.56 to −0.22) and 0.11 (95% CI −0.26 to 0.05) for head, abdominal and mid-upper arm CFs at birth, respectively.
There were no significant differences in renal volume in the South Asian population either by consanguinity or by country of birth, in both univariate and adjusted models.
DISCUSSION
In this large prospective birth cohort study of a multi-ethnic community in the UK, we have shown that all measures of fetal anthropometry were reduced in fetuses of South Asian origin compared with white British fetuses. This reduction was greatest for kidney volume, and it persisted after adjustment for potential confounders and mediators including LBW. As there is thought to be a critical period for kidney development between 26 and 34 weeks of gestation, our results suggest a greater propensity to renal growth restriction during this period for South Asian fetuses. Subsequent reduction in kidney volume may reflect reduced nephron numbers, as there is an inverse correlation between glomerular number and volume [24].
A human fetus normally has a full complement of nephrons by 34–36 weeks of gestation, with 60% of the nephrons developing in the third trimester [42, 43]. Brenner and Chertow [29] proposed that growth restriction in this period would result in a lower complement of nephrons. Nephron number is then fixed at birth, but post-natally the kidney grows to match the body size by hypertrophy [42]. This oligonephric state with consequent postnatal glomerular hypertrophy and sclerosis may predispose to hypertension and renal dysfunction, particularly in the presence of other genetic and environmental influences and with ageing. The link between IUGR and nephron number has been demonstrated in animal models: restriction of energy or protein reduces nephron number and leads to adult hypertension [44–47]. Postmortem studies of human fetuses and neonates have demonstrated that IUGR is associated with reduced nephron number, with no post-natal catch-up [24, 25]. Fetal ultrasound has shown significant reductions in renal volume and in CF, AP and TS diameters between 22 and 41 weeks in growth-restricted fetuses [27, 28]. In preliminary analyses from the Southampton Women's Survey, smaller infants and those whose mothers were thinner tended to have ‘sausage-shaped’ kidneys that were relatively narrow for their length [48]. We found similar relations with kidney shape in the BiB cohort, and this shape may be associated with fewer layers of normal nephrons.
Although there have been no studies in South Asian populations linking LBW to future risk of CKD, there is growing evidence in other populations [20, 49, 50], shown for populations at high risk of CKD (Aborigines in Australia, Pima Indians and Blacks in the USA) and with different measures of CKD (albuminuria, reduced eGFR and RRT). The association is not seen in all studies, and this may be due to selection bias, poor measures of exposure (kidney development), inaccuracies of eGFR measurement, biased ascertainment of CKD outcome and underpowered studies. According to the Brenner hypothesis, lower nephron numbers might partly explain the LBW–CKD association. There is some evidence to support this. Glomerular hypertrophy as a marker of reduced nephron number is associated with albuminuria in Aborigines [51]. LBW is associated with increased risks of developing hypertension [19, 52], and adult postmortem studies have demonstrated reduced nephron numbers in patients with existing essential hypertension when compared with normotensive controls [53].
The mechanism(s) for the reduction of interethnic differences in kidney size is unclear. Although part of the difference can be explained by LBW in South Asian infants, the differences persisted after adjustment for birth weight. This might indicate a greater propensity in South Asians to growth faltering in the third trimester when kidney development is maximal and that kidney volume is more sensitive to such growth restriction than the overall birth weight. We found greater reductions in kidney size (and related abdominal CF) than in fetal and neonatal head CF. This is in keeping with fetal blood flow adaptations, which maintain blood flow to the brain at the expense of abdominal viscera when the materno-placental supply of essential nutrients is inadequate for fetal demands [54]. Abdominal CF (a surrogate for visceral size) has been shown to be smaller in South Asians than in white Europeans in line with their LBWs [55].
The most common reasons for IUGR in the third trimester are reduced utero-placental function and impaired fetal nutrition. We were able to rule out several factors operating on utero-placental function such as smoking, alcohol and psychological stress (GHQ). We found no difference in rates of pre-eclampsia and gestational hypertension in South Asian mothers, although there were some data missing from the routine records. Fasting insulin was higher and gestational diabetes was more common in South Asian women, but differences in renal volume remained after adjustment. Regular physical activity might increase placental blood flow, but we had no data on activity to explore this.
Nutritional factors that have been proposed as mechanisms for IUGR include macronutrients and micronutrients imbalances or deficiencies. We had no data on dietary intake. The fetus is dependent on maternal vitamin D, either from the actions of sunlight or food and supplements. In rat models, vitamin D deficiency leads to upregulation of renin, which persists into adulthood and leads to altered renal function in male rats and slight reduction in kidney weight [56]. Mothers of South Asian origin in the BiB cohort have been shown to have significantly lower vitamin D levels [57].
Other potential reasons might be genetic or transgenerational. Genome-wide association studies have identified seven loci associated with birth weight; five were associated with other phenotypes (type 2 diabetes, height and blood pressure), although the variance explained was small (0.76% in European populations) and there were few data reported in South Asian populations [58]. We found no difference in kidney volume or birth weight by consanguinity, which would tend to exclude the impact of rarer recessive conditions. Low maternal birth weight has a strong influence on fetal size at birth [59]; although we had no information on maternal birth weight, kidney volume was similar in the offspring of UK and South Asian born mothers, which does not support a transgenerational effect.
Strengths and limitations
The study strengths included its size, the multi-ethnic-deprived population, availability of baseline data on several potential mediators, the detailed ultrasonography with high reliability and adjustment for gestation and birth weight. However, data that relied on routine maternity information recording were incomplete, and we did not attempt to impute missing values. These included smoking during pregnancy and hypertensive and diabetic disorders of pregnancy. Any residual confounding from smoking would have widened the ethnic differences in renal dimensions. We had no data on maternal dietary intake or birth weight. The findings cannot be extrapolated to other ethnic minorities; although there was no evidence of significant differences between separate South Asian ethnic minorities, it is possible that there were small differences. We excluded babies born prematurely; therefore, we cannot comment on the effects of prematurity on renal anthropometry.
IMPLICATIONS
South Asian adults are at high risk of both premature severe CKD and several of its antecedent causes, particularly type 2 diabetes. A greater propensity to third trimester growth restriction in South Asians impacts on the developing kidney and leads to reduced kidney volume and it is assumed, nephron number. This may increase propensity to future CKD and interact with other CKD risks. The causes of IUGR in South Asian populations require further research to identify potentially modifiable factors. Longitudinal follow-up is required to investigate the relationship between kidney volume, post-natal environment and growth and markers of kidney disease and blood pressure in childhood and early adulthood.
CONCLUSION
South Asian infants have smaller kidneys compared with white British infants, even after adjusting for potential confounders including birth weight. This finding may partly explain the increased risk of adult CKD.
DATA SHARING STATEMENT
Scientists are encouraged and able to use BiB data. Data requests are made to the BiB executive using the form available from the study website http://www.borninbradford.nhs.uk (click on ‘Science and Research’ to access the form). Guidance for researchers and collaborators, the study protocol and the data collection schedule are all available via the website. All requests are carefully considered and accepted where possible.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
ACKNOWLEDGEMENTS
Born in Bradford is only possible because of the enthusiasm and commitment of the children and parents in born in Bradford. The authors are grateful to all participants, health professionals and researchers who have made Born in Bradford happen. This paper presents independent research funded by the Yorkshire Kidney Research Fund and the National Institute for Health Research (NIHR) under its Collaboration for Applied Health Research and Care (CLAHRC) for Yorkshire and Humber. Core support for Born in Bradford is also provided by the Wellcome Trust (WT101597MA). K.M.G. is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement no. 289346.
REFERENCES





Comments