-
PDF
- Split View
-
Views
-
Cite
Cite
Ying Zhou, Haruichi Asahara, Eric A. Gaucher, Shaorong Chong, Reconstitution of translation from Thermus thermophilus reveals a minimal set of components sufficient for protein synthesis at high temperatures and functional conservation of modern and ancient translation components, Nucleic Acids Research, Volume 40, Issue 16, 1 September 2012, Pages 7932–7945, https://doi.org/10.1093/nar/gks568
Close - Share Icon Share
Abstract
Thermus thermophilus is a thermophilic model organism distantly related to the mesophilic model organism E. coli. We reconstituted protein translation of Thermus thermophilus in vitro from purified ribosomes, transfer ribonucleic acids (tRNAs) and 33 recombinant proteins. This reconstituted system was fully functional, capable of translating natural messenger RNA (mRNA) into active full-length proteins at temperatures up to 65°C and with yields up to 60 μg/ml. Surprisingly, the synthesis of active proteins also occurred at 37°C, a temperature well below the minimal growth temperature for T. thermophilus. A polyamine was required, with tetraamine being most effective, for translation at both high and low temperatures. Using such a defined in vitro system, we demonstrated a minimal set of components that are sufficient for synthesizing active proteins at high temperatures, the functional compatibility of key translation components between T. thermophilus and E. coli, and the functional conservation of a number of resurrected ancient elongation factors. This work sets the stage for future experiments that apply abundant structural information to biochemical characterization of protein translation and folding in T. thermophilus. Because it contains significantly reduced nucleases and proteases, this reconstituted thermostable cell-free protein synthesis system may enable in vitro engineering of proteins with improved thermostability.
INTRODUCTION
Protein translation in bacteria comprises four main steps: initiation, elongation, termination and recycling. Initiation involves three initiation factors, IF1, IF2 and IF3. The elongation cycle is catalyzed by elongation factors, EF-Tu, EF-Ts and EF-G. The termination step is facilitated by a release factor, RF1 or RF2, which recognizes the stop codon on messenger ribonucleic acid (mRNA) and catalyzes the release of the nascent polypeptide chain. In the last step, a ribosome recycling factor (RRF) together with EF-G, prepares the ribosome for the next round of translation. In Escherichiacoli, another release factor, RF3, functions to facilitate the release of RF1 or RF2 from the ribosome after the polypeptide release (1,2).
Based on information from the completed genome sequence of Thermus thermophilus (T. thermophilus) (3), the translation factors (TFs) and aminoacyl-transfer (tRNA) RNA synthetases are generally homologous to those in E. coli (Supplementary Table S1). However, unlike E. coli, T. thermophilus does not have RF3. In addition to 20 aminoacyl-tRNA synthetases (aaRS), T. thermophilus has a second genetically distinct aspartyl-tRNA synthetase (AspRS2) and an indirect tRNA asparaginylation pathway similar to those in some gram-positive bacteria and archaebacteria (4).
Our understanding of bacterial translation has been greatly advanced by the structural information of ribosomes from T. thermophilus and E. coli [see (1) and references therein]. Though the ribosome from T. thermophilus features one of the first and best characterized crystal structures (5–7), genetic and biochemical information of protein translation in T. thermophilus has been far less abundant than that of E. coli. Despite of the fact that most of T. thermophilus TFs and aaRS have been characterized individually or in the context of individual steps of protein translation (references in Supplementary Table S1) (8–10), it is still not known whether these T. thermophilus components, together with T. thermophilus ribosomes and tRNAs, are sufficient for translation of a natural mRNA. In particular, the question arises whether additional factors are necessary in order for protein translation to occur at high temperatures, e.g. at the optimal growth temperature (∼73°C) of T. thermophilus. Of the ∼2000 protein coding genes of T. thermophilus, at least 7% are functionally unknown (3). Protein synthesis at high temperatures has been demonstrated in vitro in the cell extract of T. thermophilus and has been shown to require certain polyamines produced by T. thermophilus (11). However, using the cell extract of T. thermophilus, it is not possible to determine with certainty whether TFs and aaRS are the sole protein factors responsible for protein synthesis at high temperatures, and whether polyamines alone or other accessory components are also required.
Here we report the reconstitution of protein translation of T. thermophilus using a constructive approach similar to that previously demonstrated for E. coli (12). The reconstituted T. thermophilus translation system comprised purified ribosomes, total tRNAs and 33 recombinant proteins of T. thermophilus. The recombinant proteins, each of which was individually overproduced and purified from E. coli, included 9 TFs, 20 canonical aaRS and 4 energy regeneration enzymes (ER) (see the list in Supplementary Table S1). Energy regeneration [regeneration of guanosine triphosphate (GTP) and adenosine triphosphate (ATP)] is necessary for in vitro protein synthesis in both reconstituted and cell extract-based E. coli cell-free systems (12,13). We have previously established a reconstituted E. coli translation system following the reported protocols (12,14). The availability of a reconstituted E. coli system has facilitated the reconstitution and characterization of the translation of T. thermophilus. With a successfully reconstituted T. thermophilus translation system, we not only defined a minimal set of components sufficient for protein translation at high temperatures, but also began to probe the evolutionary conservation of the translational machinery.
MATERIALS AND METHODS
Reagents
Unless specified otherwise, the chemicals were purchased from Sigma-Aldrich (St. Louis, MO), the reagents were from New England Biolabs (NEB) (Ipswich, MA) and the primers were ordered from Integrated DNA Technologies (Coralville, IA).
Cloning of target proteins of thermophilic origins to generate mRNA templates for in vitro translation at high temperatures
Thermusthermophilus alkaline phosphatase (TAP) and T. thermophilus amylomaltase (AMase) were cloned from T. thermophilus HB8 genomic deoxyribonucleic acid (DNA) using the following polymerase chain reaction (PCR) primers: TAP5NdeI (ATTATTCAT ATG AAG CGA AGG GAC ATC CTG AAA GGT G), TAP3NotI (ATATATGCGGCCGC TTA GGC CCA GAC GTC CTC GGG), AMase5NdeI (ATTATTCAT ATG GAG CTT CCC CGC GCT TT) and AMase3NotI (ATATATGCGGCCGC T TAG AGC CGT TCC GTG GCC T). The PCR products were cut with NdeI and NotI-HF and cloned into the pCOATexp vector, a derivative of pTYB1 vector (NEB). The genes for BstYI and PspGI (restriction enzymes from Bacillus stearothermophilus and Pyroccocus species, respectively) and Vent DNAP (a DNA polymerase from hyperthermophilc Thermocuccus litoralis) were obtained from the plasmids pET21a-BstYI, pET21a-PspGI and pAII17-Ventoptexo-, respectively, which were constructed at NEB.
Linear PCR templates were used for in vitro transcription reactions to generate mRNA for each target protein. The PCR reactions were performed using Phusion Hot Start High-Fidelity DNA polymerase. All PCR templates included 64–104 bp upstream of the T7 promoter and downstream of the T7 terminator. The primers used for the PCR reactions are listed as follows: BstYI (pETatfw: 5′- GGG GAT CGG AAT TAA TTC CCG GGG ATC TCG A -3′, pETatrv: 5′- GCT GGC AAG TGT AGC GGT CAC GCT GC -3′), PspGI (pET21fw: 5′- CCC ATC GGT GAT GTC GGC GAT ATA GGC -3′, pET21rv: 5′-TGT AGC GGT CAC GCT GCG CGT AA -3′), Vent DNAP (Vent-fw: GCC ATA AAC TGC CAG GAA TTG GGG ATC GGA, Vent-rv: CCG GGC CTC TTG CGG GAT ATC CGG ATA TA); TAP and AMase used the same set of primers (pCOAT-fw: ACT GCC AGG AAT TGG GGA TCG, pCOAT-rv: GTG CCA CCT GAC GTA GTT ATC CG).
The in vitro transcription reactions (20 μl) contained 100 ng of PCR template for each target protein and were conducted at 37°C for 4 h using the HiScribe T7 In vitro Transcription Kit (NEB). The resulting mRNA templates were purified using the MEGAclear kit (Ambion, Inc, Austin, TX).
Synthesis of super-thermostable green fluorescent protein and testing the thermostability of purified stGFP protein
Super-thermostable green fluorescent protein (stGFP) was engineered from a superfolder GFP (sGFP) (15) by changing Leu64 and Thr65 in sGFP into Phe and Ser, respectively. As a result, stGFP has the same residues in these positions as the wild-type GFP and also the same excitation and emission wavelengths (ex. 398 nm, em. 508 nm). In our study, stGFP synthesized in the translation reactions exhibited more stable fluorescence signals than sGFP when measured at high temperatures in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The gene for sGFP was synthesized (Integrated DNA Technologies, Coralville, IA, USA) and cloned into pUC105T7 vector (14). The L64F and T65S mutations were made using QuickChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) with following primers: stGFP+: ACC CTG GTG ACC ACT TTC TCC TAC GGC GTC CAG TGC and stGFP: GCA CTG GAC GCC GTA GGA GAA AGT GGT CAC CAG GGT. The resulting stGFP gene was cut with NdeI and XhoI from pUC105T7 and cloned into pCOATexp for expression of the stGFP protein with an N-terminal His-tag. The recombinant stGFP was expressed from pCOATexp stGFP in E. coli and purified following the same protocol as described for other His-tagged proteins (see Supplemental Data).
To test the thermostability of purified stGFP, the stGFP solution (1 μM stGFP in 50 mM Hepes-KOH, pH 8, 100 mM K-Glutamate, 10 mM Mg(OAc)2) was incubated at temperatures ranging from 50 to 80°C for 4 h. Aliquots were taken at different time points, and after cooling on ice for 2 min, the fluorescence (ex. 398 nm, em. 508 nm) was measured in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) (Supplementary Figure S2).
To generate stGFP mRNA for real-time monitoring of in vitro protein synthesis (Figure 1), the in vitro transcription reaction was conducted using the linear PCR template amplified from the plasmid pCOATexp stGFP, following the same protocol as described in the previous section.
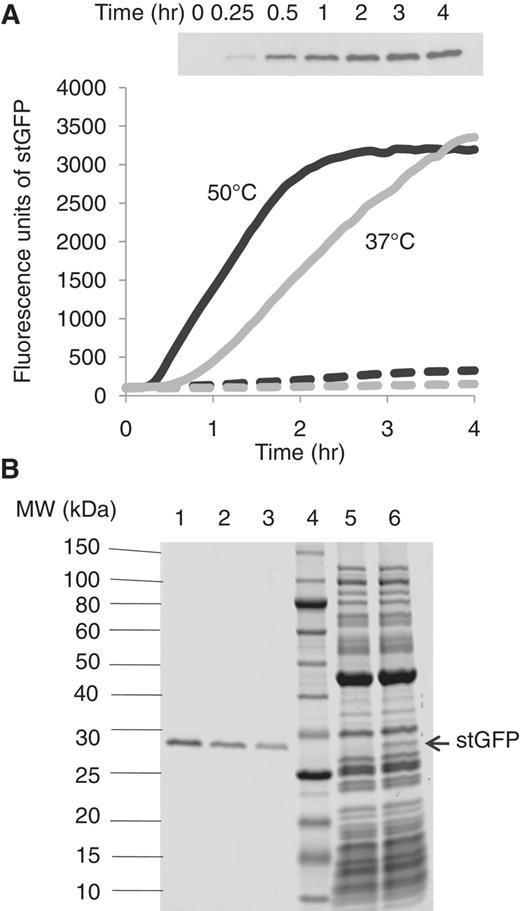
(A) Real-time monitoring of the fluorescence of stGFP synthesized in the reconstituted T. thermophilus system at 37°C (grey solid line: with stGFP mRNA and grey dashed line: without mRNA) and 50°C (black solid line: with stGFP mRNA and black dashed line: without mRNA). Inset: western blot analysis of aliquots taken from the 50°C reaction containing stGFP mRNA at different time points using an antibody specific to stGFP. (B) SDS–PAGE analysis of aliquots of the translation reactions at 4 h in the presence (lane 6) or absence (lane 5) of stGFP mRNA. Different amounts of purified recombinant stGFP are loaded on the same gel in lane 1 (200 μg/ml), lane 2 (100 μg/ml) and lane 3 (50 μg/ml). The MW standards (run in lane 4) are indicated on the left.
In vitro reconstitution of protein translation of T. thermophilus and demonstration of protein synthesis at high temperatures
The purification processes and assays of 33 recombinant proteins, ribosome and total tRNAs of T. thermophilus (listed in Supplementary Table S1) are described in the Supplementary ‘Materials and Methods’ section.
The translation of T. thermophilus was reconstituted in vitro by mixing the Tt factor solution, containing the purified TFs, aaRS, ER and ribosome (see Supplementary Table S1 for the final concentration of each component), with the Tt translation buffer, containing the final concentrations of 50 mM HEPES–KOH, pH 8, 100 mM K-Glutamate, 10 mM Mg(OAc)2, 2 mM spermine, 7.2 mM dithiothreitol (DTT), 2 mM ATP, 2 mM GTP, 0.02 mM N10-formyl-tetrahydrofolate, 15 mM phospho(enol)pyruvic acid, 0.15 mM each of 19 amino acids, 0.1 mM cold methionine, 0.66 μM [35S] Met (1175 Ci/mmol; PerkinElmer, Waltham, MA); 2 mg/ml of purified T. thermophilus tRNA and 1 U/μl of of RNase inhibitor (NEB, Ipswich, MA, USA). The in vitro translation reactions (typically 25 μl for each reaction) were initiated by adding purified mRNA templates (4–5 μg for each reaction) and incubated at 60°C for 3 h. Aliquots of the final reaction mixtures were taken and run on a10–20% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) gel (Life Technologies, Carlsbad, CA). The gels were dried and exposed to a phosphor imager overnight (Figure 2A). For the synthesis of Vent DNAP mRNA at different temperatures, the reactions were incubated at temperatures ranging from 37 to 75°C for 3 h (Figure 2B). In the case of BstYI mRNA, the reactions were conducted at both 60°C and 37°C and in the presence or absence of the ER in the Tt factor solution (see Supplementary Figure S1 and the legend). As a comparison, BstYI was also synthesized under the same condition in the in vitro reaction, except that the T. thermophilus ribosome in the Tt factor solution was replaced by the S30 extract (8 μg/μl) (Supplementary Figure S1, lane 2). As a control, BstYI was synthesized in a reconstituted E. coli system (12,14) using the same concentrations of amino acids and [35S] Met as described earlier.
![(A) Detection of the synthesis of target proteins (indicated at the bottom of lane 2–7) with [35S] methionine labeling in the reconstituted T. thermophilus system. As a control, mRNA is not added (lane 1, mRNA). BstYI (23 kDa) and PspGI (32 kDa): restriction enzymes from Bacillus stearothermophilus and Pyroccocus species, respectively; TAP (57 kDa): an alkaline phosphatase from T. thermophilus; AMase: (59 kDa): an amylomaltase from T. thermophilus; Vent DNAP (90 kDa): a DNA polymerase from hyperthermophilc Thermocuccus litoralis; stGFP (29 kDa): an engineered thermostable GFP variant from this study. The MW standards (MW, kDa) are indicated on the left. (B) Synthesis of Vent DNAP with [35S] methionine labeling at different temperatures in the reconstituted T. thermophilus system. (C) Comparison of the stability of mRNA at 60°C in in vitro translation reactions of the reconstituted T. thermophilus system (left panel) or T. thermophilus cell extract (right panel). The stability of 32P-labeled BstYI mRNA at various time points (indicated on top) is analyzed on a SDS-PAGE gel, followed by autoradiography. Purified 32P-labeled BstYI mRNA without incubation is shown as the control (ctrl). The RNA standards in base pairs (bp) are indicated on the left.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/nar/40/16/10.1093_nar_gks568/4/m_gks568f2.jpeg?Expires=1750419871&Signature=lAchdVoiCsp9mZzp0xLyHqvDpkjlUiGqThbMR2gP-RnFDbdnI3eV~j99-W0q1VjFN2AdtTR4oM4P1h3NBmYZo58YXoBWVHswdFQU9qhTeQy6iFI-IoJCvkaMynwccU58yO48Y1KjgNXT3C1LwLkn04Aog5SnlakkeVWRWqTG7u25wsCM2FFIcSqet3vV~GvTMO~w4K0oVWfbw9HuvCtS8bzgjYGrq4y0xjYmm0bPQ1NSDyV7-s6wyyTDxwo6qVIrJnbBN4UZxGf10YAC6U0a8spT4tbpuM72SQDFhkekRgdX~ItWQiBIRXtz9fumqiEqM6sL4M8Y42KT1wQ1RdjEgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
(A) Detection of the synthesis of target proteins (indicated at the bottom of lane 2–7) with [35S] methionine labeling in the reconstituted T. thermophilus system. As a control, mRNA is not added (lane 1, mRNA). BstYI (23 kDa) and PspGI (32 kDa): restriction enzymes from Bacillus stearothermophilus and Pyroccocus species, respectively; TAP (57 kDa): an alkaline phosphatase from T. thermophilus; AMase: (59 kDa): an amylomaltase from T. thermophilus; Vent DNAP (90 kDa): a DNA polymerase from hyperthermophilc Thermocuccus litoralis; stGFP (29 kDa): an engineered thermostable GFP variant from this study. The MW standards (MW, kDa) are indicated on the left. (B) Synthesis of Vent DNAP with [35S] methionine labeling at different temperatures in the reconstituted T. thermophilus system. (C) Comparison of the stability of mRNA at 60°C in in vitro translation reactions of the reconstituted T. thermophilus system (left panel) or T. thermophilus cell extract (right panel). The stability of 32P-labeled BstYI mRNA at various time points (indicated on top) is analyzed on a SDS-PAGE gel, followed by autoradiography. Purified 32P-labeled BstYI mRNA without incubation is shown as the control (ctrl). The RNA standards in base pairs (bp) are indicated on the left.
For real-time monitoring and SDS-PAGE analysis of the stGFP synthesis (Figure 1), higher amino acid concentrations were used in the Tt translation buffer. In this case, the Tt translation buffer contained the final concentrations of 50 mM Hepes-KOH, pH 8, 100 mM K-Glutamate, 10 mM Mg(OAc)2, 2 mM spermine, 7.2 mM DTT, 2 mM ATP, 2 mM GTP, 0.02 mM N10-formyl-tetrahydrofolate, 1 mM each of 18 amino acids, 0.3 mM tyrosine, 0.6 mM cysteine, 25 mM phospho(enol)pyruvic acid, 2 mg/ml of T. thermophilus tRNA and 1U/μl of RNase. The in vitro translation reaction (25 μl) was initiated by adding purified stGFP mRNA templates (4 μg) and then loaded in a 384-well microplate (Corning, Lowell, MA). The fluorescence (ex. 398 nm, em. 508 nm) was measured kinetically (every 5 min) for up to 8 h in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA) in which the chamber temperature was maintained either at 37 or 50°C.
For SDS-PAGE and western analyses, aliquots were taken from the in vitro translation reaction (see Figure 1 and the legend) and run on a 10–20% Tris–glycine SDS-PAGE gels (Life Technologies, Carlsbad, CA, USA). After blotting, the gel was analyzed with primary anti-GFP antibody(Cell Signaling Technology, Beverly, MA, USA), and the detection was carried out using HRP-linked anti-rabbit IgG and LumiGLO chemiluminescent substrate (Cell Signaling Technology, Beverly, MA, USA).
Testing mRNA stability during in vitro translation
The 32P-labeled BstYI mRNA was prepared in an in vitro transcription reaction (20 μl) containing 10 μCi of [α-32P]UTP (GE healthcare, Piscataway, NJ, USA). The resulting 32P-labeled BstYI mRNA was purified and added (2 μl) to a typical in vitro translation reaction (25 μl) as described earlier. In the case of the T. thermophilus cell extract, the T. thermophilus ribosome in the Tt factor solution was replaced by the S30 extract of T. thermophilus (8 μg/μl). The reaction mixtures were incubated at 60°C for 2 h. Aliquots were taken at different time points and treated with 20 μg of protease K (NEB) for 10 min at 37°C. The samples were loaded onto 6% Tris/Borate/EDTA-urea gel (Life Technologies, Carlsbad, CA, USA), and the gel was dried and exposed to a phosphor imager overnight (Figure 2C).
Testing the effect of polyamines on protein translation in the reconstituted T. thermophilus system
The effect of polyamines was examined in the in vitro translation reactions described earlier for the real-time monitoring of the synthesis of stGFP, except that different polyamines (kindly provided by Dr Y. Terui) at the concentrations of 0–10 mM were used (Table 1). The reactions (25 μl) were conducted in a 384-well microplate (Corning, Lowell, MA, USA) at 50 (Table 1) or 37°C (Supplementary Figure S3), and the fluorescence (ex. 398 nm, em. 508 nm) was measured kinetically for up to 4–8 h in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The maximal fluorescence values for each polyamine at different concentrations were determined, and the corresponding stGFP yield (μg/ml) was calculated based on the correlation between the fluorescence value and the stGFP yield observed in Figure 1. From the aforementioned data, the optimal concentration (mM) for each polyamine to achieve the maximal stGFP yield (μg/ml) was obtained (Table 1).
The effect of polyamines on the synthesis of stGFP at 50°C in the reconstituted T. thermophilus system
 |
 |
The effect of polyamines on the synthesis of stGFP at 50°C in the reconstituted T. thermophilus system
 |
 |
Determining a minimal set of components sufficient for in vitro protein translation at high temperatures
The contribution of TFs and ER to the overall protein synthesis was examined in the in vitro translation reactions described earlier for the real-time monitoring of the synthesis of stGFP in the reconstituted T. thermophilus system (Figure 3). The reactions contained the Tt factor solutions in which one or several TFs or ER are removed. The reactions were conducted in a 384-well microplate (Corning, Lowell, MA, USA) at 50°C for 8 h in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The maximal fluorescence values were used to determine the relative protein synthesis yields.
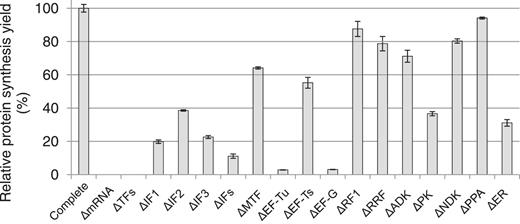
Contribution of TFs and ER to the overall protein synthesis in the reconstituted T. thermophilus system. The translation of stGFP mRNA is conducted at 50°C for 4 h in the reconstituted T. thermophilus system in which one or several TFs or ER are removed. The relative synthesis yields (%) are based on the fluorescence of the reactions compared with that of the complete reconstituted system (Complete, set as 100). ΔmRNA: missing stGFP mRNA; ΔTFs: missing all TFs; ΔIFs: missing all three initiation factors; ΔER: missing all ER; also see Supplementary Table S1 for the abbreviations. The data are based on at least two independent experiments.
Testing the functional compatibility of key translation components between T. thermophilus and E. coli
The experiments examining the compatibility of key translation components between T. thermophilus and E. coli were primarily conducted in in vitro translation reactions described earlier for the real-time monitoring of the synthesis of stGFP in the reconstituted T. thermophilus system (Figure 4A). The reactions contained the Tt factor solutions in which one or several T. thermophilus components were removed or replaced by equal amounts of their E. coli counterparts. The experiments examining the compatibility of Tu and Ts between T. thermophilus and E. coli were conducted in a reconstituted E. coli system (Figure 4B). The reconstituted E. coli system was constructed following the reported protocols (12,14), except that Ec Tu/Ts was either removed or replaced by equal amounts of Tt Tu/Ts.

Testing the functional compatibility of translation components between T. thermophilus and E. coli. (A) The exchange of key translation components (IFs, Tu/Ts, G, RRF and ribosome) between T. thermophilus (filled circles) and E. coli (open circles) in the reconstituted T. thermophilus system translating stGFP mRNA at 37°C. The relevant exchange experiments including the controls are boxed for clarity. (B) The exchange of EF-Tu and EF-Ts (Tu and Ts) between T. thermophilus (filled circles) and E. coli (open circles) in the reconstituted E. coli system translating stGFP mRNA at 37°C. The missing components are indicated by the minus symbol (−). The relative synthesis yields (%) are based on the fluorescence of the reactions compared with that of the original reconstituted systems (set as 100; lane 11 in A and lane 9 in B). All data are based on at least two independent experiments.
All reactions were conducted in a 384-well microplate (Corning, Lowell, MA, USA) at 37°C for 8 h in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The maximal fluorescence values were used to determine the relative protein synthesis yields.
Testing the functional conservation of resurrected ancient elongation factors in the reconstituted T. thermophilus system
The functions of purified ancient elongation factors were examined in the in vitro translation reactions described earlier for the real-time monitoring of the synthesis of stGFP in the reconstituted T. thermophilus system (Figure 5 and Supplementary Figure S5). The reactions contained the Tt factor solutions in which Tt Tu was replaced by the equal amount of each ancient elongation factor. The reactions were conducted in a 384-well microplate (Corning, Lowell, MA, USA) at 50°C for 8 h in Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA). The maximal fluorescence values were used to determine the relative protein synthesis yields.
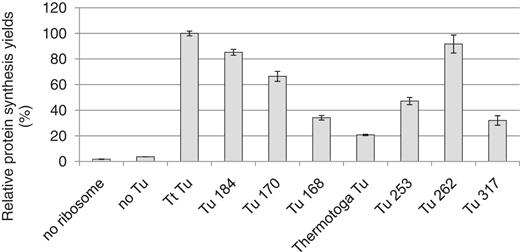
Testing the functional conservation of resurrected ancient elongation factors (indicated at the bottom) in the reconstituted T. thermophilus system in which Tt Tu is replaced by each of the ancient elongation factors (indicated at the bottom) (Supplementary Figure S5). The translation reactions containing stGFP mRNA are conducted at 50°C for 4 h. The relative synthesis yields (%) are based on the fluorescence of the reactions compared with that of the original reconstituted system (Tt Tu, set as 100). All data are based on at least two independent experiments.
RESULTS
In vitro reconstitution of protein translation of T. thermophilus and demonstration of protein synthesis at high temperatures
We attempted to reconstitute the translation of T. thermophilus by mixing purified ribosomes, total tRNAs, 33 recombinant proteins and small molecules (e.g. 20 amino acids, ATP and GTP, etc.) following similar protocols as those for the reconstituted E. coli system (12). The recombinant proteins were purified to near homogeneity as indicated by the SDS-PAGE analyses (Supplementary Figure S6). The activity of the ribosome was verified in the poly(Phe) assay (Supplementary Figure S8), and those of aaRSs were assessed either by their ability to replace their counterparts in the reconstituted E. coli system or in the tRNA aminoacylation assay (Supplementary Figure S9).
The final concentrations of almost all T. thermophilus components (Supplementary Table S1) were the same as those in the reconstituted E. coli system used in this study. The exceptions are as follows: RF1 and MetRS were used in the reconstituted T. thermophilus system at 2-fold higher concentrations than their counterparts in the reconstituted E. coli system. Nontagged recombinant T. thermophilus EF-Tu was used, as the His-tagged versions (N- or C-terminal) resulted in lower activities. In contrast, the recombinant EF-Tu from E. coli was highly active with the His-tag at the C-terminus (12). To achieve the energy regeneration at high temperatures, the thermostable pyruvate kinase (PK) from T. thermophilus and the small molecule phospho(enol)pyruvic acid were used in the reconstituted T. thermophilus system. In comparison, creatine kinase from rabbit muscle and creatine phosphate were used as the energy source for the reconstituted E. coli system (12).
Six thermostable proteins from a variety of sources were chosen as target proteins for in vitro translation. The mRNAs encoding these proteins, purified from in vitro transcription reactions, were added to the in vitro translation reactions containing the reconstituted T. thermophilus system and [35S] methionine for labeling newly synthesized polypeptides. After incubation at 60°C for 3 h, aliquots were taken and analyzed by SDS-PAGE, followed by autoradiography. As shown in Figure 2A, all but PspGI mRNA resulted in one predominant band corresponding to the molecular weight (MW) of the encoding protein. PspGI mRNA produced a small truncated protein (∼10 kDa) and a near full-length protein (∼30 kDa), suggesting that the ribosome stalled during translation of PspGI mRNA and released the nascent peptides before the stop codon. The data indicate that protein translation of T. thermophilus was functionally reconstituted in vitro from purified components, and that these purified components were sufficient for in vitro synthesis of full-length proteins from natural mRNAs.
The translation by the reconstituted T. thermophilus system was examined at temperatures ranging from 37 to 75°C using mRNA encoding a highly thermostable Vent DNA polymerase (Vent DNAP). As shown in Figure 2B, the synthesis of Vent DNAP occurred efficiently from 37 to 60°C, but the yield decreased drastically at 65°C, and the reaction completely stopped at 70°C and above. Surprisingly, Vent DNAP was synthesized efficiently at a temperature as low as 37°C, resulting in nearly the same amount of Vent DNAP as that at 60°C, suggesting that the translational machinery of T. thermophilus is capable of protein synthesis at temperatures below the minimal growth temperature of 47°C (16). As another example, BstYI, a restriction endonuclease, was also produced at 37°C in a similar amount as that at 60°C (Supplementary Figure S1A, lanes 4 and 5). The synthesized BstYI at both 37°C and 60°C was active in restriction digestion assays, resulting in the same digestion pattern as that of the purified BstYI (Supplementary Figure S1B, compare lanes 6 and 8 with lane 4). As controls, aliquots from the translation reactions without BstYI mRNA were incubated with lambda DNA. No restriction digestion was observed (Supplementary Figure S1B, lanes 2 and 3).
To assess the mRNA stability, 32P-labeled BstYI mRNA was incubated for 2 h at 60°C in the translation reaction containing either the reconstituted T. thermophilus system or the cell extract of T. thermophilus. As shown in Figure 2C (left panel), a significant amount of BstYI mRNA remained full-length after 2 h incubation with the reconstituted T. thermophilus system. In contrast, most full-length BstYI mRNA was degraded after just 10 min incubation with the cell extract of T. thermophilus (Figure 2C, right panel). It is possible that the degradation of mRNA was caused by both hydrolysis at high temperatures and cleavage by nucleases. The data suggest that the amounts of nucleases were significantly reduced in the reconstituted T. thermophilus system, whereas nucleases could be a major reason for the instability of mRNA in the cell extract of T. thermophilus. Consistently, a much lower synthesis yield was observed in the cell extract of T. thermophilus than that in the reconstituted T. thermophilus system (Supplementary Figure S1A, compare lane 2 with lane 4).
To monitor protein translation at high temperatures in the reconstituted T. thermophilus system, a GFP mutant, named stGFP, was engineered from the superfolder GFP (15). The purified recombinant stGFP remained fully active (fluorescent) after incubation for 4 h at temperatures up to 70°C (Supplementary Figure S2), indicating that stGFP was sufficiently thermostable to serve as a reporter for in vitro protein synthesis. As shown in Figure 1A, increasing fluorescence was observed in the translation reactions containing stGFP mRNA at both 37°C (grey line) and 50°C (black line), whereas only background fluorescence was observed in the translation reactions without stGFP mRNA (dashed lines). The fluorescence intensity was largely proportional to the amount of the synthesized stGFP, as indicated by the western blot analysis of the aliquots taken at various times from the 50°C reaction (Figure 1A inset). Based on stGFP fluorescence, the translation reaction at 50°C stopped after ∼2 h, whereas the translation reaction at 37°C continued up to 4 h (Figure 1A). Due to a higher initial translation rate at 50°C than that at 37°C, similar amounts of stGFP were synthesized at 4 h at both temperatures. The data suggest that certain components in the translation reactions might lose their activity after a prolonged incubation (>2 h) at 50°C. Because the ribosome and elongation factors seemed to be functional up to 8 h at 60°C in the poly(Phe) assay (Supplementary Figure S8), it is possible that other components, such as stGFP mRNA, the aaRS and small molecules (GTP, ATP or phosphoenolpyruvate), could become increasingly unstable during the 50°C incubation.
The amount of stGFP synthesized in the reconstituted T. thermophilus system was sufficient to allow stGFP to be visualized on a Coomassie Blue-stained SDS–PAGE gel without [35S] methionine labeling (Figure 1B). Compared with the translation reaction without stGFP mRNA (Figure 1B, lane 5), a band corresponding to the MW of stGFP (29 kDa) was clearly visible in the translation reaction with stGFP mRNA (Figure 1B, lane 6). The translation reactions with and without stGFP mRNA were also analyzed by mass spectrometry, which correctly identified the synthesized stGFP (Supplementary Table S2 and Supplementary Data). Using the purified recombinant stGFP as a standard (Figure 1B, lanes 1–3), the amount of the synthesized stGFP in the translation reaction at 4 h was estimated to be ∼50 μg/ml. These data allowed the translation yield to be correlated to the observed fluorescence (Figure 1A). In addition, the specific activity of the in vitro synthesized stGFP (∼4800 fluorescent units/μg) was estimated to be 65% of that of the purified recombinant stGFP.
The effect of polyamines on protein translation in the reconstituted T. thermophilus system
Using stGFP as a convenient reporter, the effects of polyamines on the translation of T. thermophilus were examined in the reconstituted T. thermophilus system. The translation reactions were supplemented with various concentrations of polyamines, and the observed maximal fluorescence of stGFP was used to calculate the synthesis yields. As shown in Table 1, addition of putrescine in the absence of other polyamines resulted in no synthesis of stGFP at 50°C, whereas all other polyamines were required for the synthesis of stGFP at 50°C. Based on the maximal stGFP yields, the effectiveness of different polyamines seemed to be dependent on the number of nitrogen and carbon atoms in the polyamines (Table 1). The linear tetraamines (thermine and spermine) resulted in the highest yields, whereas higher concentrations of the shorter polyamines, and lower concentrations of the longer and branched polyamines, were needed in to reach the maximal yields. The aminopropyl group (3-carbon) seemed to be more effective than the aminobutyl group (4-carbon) in the polyamines. For instance, in terms of the maximal stGFP yield and the optimal concentration to reach the maximal yield, norspermidine was more effective than spermidine, which was in turn more effective than homospermidine (Table 1). Similarly, thermine was more effective than spermine, resulting in the highest yield of ∼60 μg/ml. The aforementioned data are largely consistent with previous studies in which the effects of polyamines were examined in the cell extract of T. thermophilus (11).
Polyamines have been shown to stabilize tRNA and the ternary complex of ribosome and mRNA and aminoacyl-tRNA at high temperatures (17,18). Because protein translation in the reconstituted T. thermophilus system could occur at 37°C, we asked the question whether polyamines were still necessary when the translation reaction was conducted at low temperatures. As shown in Supplementary Figure S3, the translation reactions at 37°C resulted in an increasing amount of stGFP in the presence of spermine but almost no stGFP in the absence of a polyamine (Supplementary Figure S3). The data suggest that T. thermophilus may intrinsically require polyamines for protein translation regardless of temperature.
A minimal set of components sufficient for in vitro protein translation at high temperatures
We assessed the contribution of 13 TFs and ER to the overall protein synthesis by removing each or several components from the reconstituted T. thermophilus system synthesizing stGFP at 50°C. As shown in Figure 3, deletion of each initiation factor (ΔIF1, ΔIF2 or ΔIF3) resulted in <40% of the synthesis yield and deletion of three initiation factors together (ΔIFs) in ∼10%, suggesting that initiation factors were all active and contributed significantly to the overall synthesis of stGFP. As expected, EF-Tu and EF-G were absolutely essential, as deletion of each of them resulted in <5% of the synthesis yield. In contrast, the synthesis yield remained ∼60% without methionyl-tRNA formyltransferase or EF-Ts. The result was largely similar to that observed for the reconstituted E. coli translation system (12). Notably, the synthesis yield remained ∼80% in the absence of RF1 or RRF, suggesting that the ribosome termination and recycling were not efficient during the translation of stGFP. However, we could not rule out the possibility of contaminating RF1, RRF, methionyl-tRNA formyltransferase or EF-Ts, which would likely originate from the T. thermophilus ribosome that was directly purified from the cell extract of T. thermophilus. Of the four ER, PK played a major role in energy regeneration. Deletion of PK resulted in the lowest synthesis yield (<40%) compared with other ER, and deletion of all four ER resulted in only slightly further decrease in the synthesis yield (Figure 3). A significant contribution of the ER to the protein synthesis was also observed for BstYI (Supplementary Figure S1A, compare lane 4 with lane 3).
The functional compatibility of key translation components between T. thermophilus and E. coli
Molecular clock estimates suggest that T. thermophilus and E. coli last shared a common ancestor ∼3.2 billion years ago (19). We took advantage of having reconstituted protein translation from both T. thermophilus and E. coli and set out to test the functional compatibility of key translation components between these distantly related species. The reconstituted T. thermophilus system was primarily used to examine whether E. coli translation components could functionally replace their T. thermophilus counterparts. The translation reactions containing stGFP mRNA were conducted at 37°C rather than 50°C to ensure the activities of E. coli components. The relative yields of the synthesized stGFP served as a rough estimation of the extent of the evolutionary conservation of the translation components between T. thermophilus and E. coli. As shown in Figure 4A, replacing all three initiation factors (Tt IFs), EF-Tu and EF-Ts (Tt Tu/Ts) or ribosome (Tt ribosome) of T. thermophilus with the corresponding E. coli components (Ec IFs, Ec Tu/Ts and Ec ribosome) resulted in >80% of the synthesis yield relative to that of the complete T. thermophilus system (compare lanes 2, 4 and 10 with lane 11). Replacing T. thermophilus EF-G (Tt G) with its E. coli counterpart (Ec G) resulted in ∼50% of the synthesis yield (compare lane 8 with lane 11). As controls, no or low protein synthesis (<10%) was observed in the absence of IFs, Tu/Ts, G or ribosome (Figure 4A, lanes 1, 3, 5 and 9). The data suggest a high level of conservation and compatibility of initiation and elongation factors between T. thermophilus and E. coli.
Because EF-G was involved in both elongation and recycling steps of protein translation, the functional conservation of EF-G was further examined in conjunction with RRF in the reconstituted T. thermophilus system. The synthesis yield with Ec G alone could be significantly increased by the addition of Tt RRF (Figure 4A, compare lane 6 with 8), suggesting that Ec G was functionally compatible with Tt RRF. In fact, the cross-species combination, Ec G/Tt RRF, generated more proteins than the native pair, Ec G/Ec RRF (Figure 4A, compare lane 8 with 7). The data are in contrast to the previous study that suggested that Ec G could not functionally interact with Tt RRF to release Tt RRF or mRNA from the posttermination complex containing either Ec or Tt ribosome (20). The discrepancy could be due to the fact that the functional compatibility between Ec G and Tt RRF was examined by the final synthesis yield of stGFP after 4 h in our study, in comparison with the release assays that lasted only 15 min in the previous study (20).
Each Ec IF (Ec IF1, Ec IF2 and Ec IF3) was also examined individually for its ability to replace the corresponding Tt IF (Supplementary Figure S4). Replacing Tt IF1 or Tt IF2 with Ec IF1 or Ec IF2, respectively, resulted in no significant increase in the synthesis yield compared with that of IF deletions (Supplementary Figure S4, compare Ec IF1 with ΔIF1 and Ec IF2 with ΔIF2). In contrast, replacing Tt IF3 with Ec IF3 resulted in a significant increase in the synthesis yield (Supplementary Figure S4, compare Ec IF3 with Tt IFs), suggesting that the compatibility of initiation factors between T. thermophilus and E. coli observed previously (Figure 4A. lane 2) was largely due to the contribution of Ec IF3.
To address the question whether Ec Ts could serve as an exchange factor for Tt Tu, the reconstituted E. coli system was used in which Ec Tu was replaced by Tt Tu. The reason for choosing the reconstituted E. coli system was to eliminate the possibility of contaminating native Tt Ts if the reconstituted T. thermophilus system was used (e.g. from the T. thermophilus ribosome). As shown in Figure 4B, addition of both Tt Tu and Tt Ts to the reconstituted E. coli system resulted in ∼30% of the synthesis yield (lane 6) relative to that of Ec Tu/Ts (lane 9), whereas addition of Tt Tu or Tt Ts alone resulted in no protein synthesis (lane 2 or 4), suggesting that the nucleotide exchange was absolutely necessary for Tt Tu to function with the E. coli translational machinery. However, when Ec Ts was added as the exchange factor for Tt Tu, no significant protein synthesis was observed (Figure 4B, lane 5). The data provide strong evidence that Ec Ts was not an effective exchange factor for Tt Tu. We could not make a definite conclusion on the other chimeric combination, i.e. Ec Tu and Tt Ts, using either the reconstituted E. coli or T. thermophilus system. Ec Tu alone resulted in >80% of the synthesis yield without Ts in the reconstituted E. coli system (Figure 4B, lane 7), possibly due to the ability of Ec Tu to function without Ts or the possibility of contaminating native Ts. Consequently, the data showing the effect of Tt Ts (if any) were not conclusive (Figure 4B, compare lane 8 with lane 7).
The functional conservation of resurrected ancient elongation factors in the reconstituted T. thermophilus system
The reconstituted T. thermophilus system was capable of carrying out protein translation at elevated temperatures and therefore provided a unique biological assay for resurrected ancient elongation factors, a majority of which have been predicted to come from ancient thermophilic species and shown to be thermostable (21).
The recombinant ancient elongation factors were purified to near homogeneity as indicated by the SDS-PAGE analyses (Supplementary Figure S7). The synthesis of stGFP was carried out at 50°C in the reconstituted T. thermophilus system in which each Tt Tu was replaced by ancient Tu. The functional conservation of an ancient Tu was measured by the relative synthesis yield. For a majority of the ancient Tu proteins, the relative synthesis yields correlated remarkably well with the evolutionary distances of the phylogenetic nodes to T. thermophilus (Figure 5 and Supplementary Figure S5). For instance, Tu 184, the nearest ancestor to Tt Tu, resulted in ∼85% of the synthesis yield relative to that of Tt Tu. In comparison, Tu 170 (last common ancestor of T. thermophilus and E. coli) resulted in ∼66% of the synthesis yield, whereas the most distant ancestor Tu 168 (last common ancestor of all bacteria) was only ∼34% (Figure 5 and Supplementary Figure S5). Two other distantly related ancestors (but more derived than the two ancestors 168 and 170), Tu 253 and Tu 317, also resulted in relatively low synthesis yields (47 and 32%, respectively). The Tu from Thermotoga maritima [a contemporary bacterium that diverged from T. thermophilus about 3.6 billion years ago (19)] resulted in the lowest synthesis yield (∼21%). The only exception to the observed trend was Tu 262, a distant ancestor of proteobacterial Tu proteins, which could effectively replace Tt Tu without a significant loss in protein synthesis (Figure 5 and Supplementary Figure S5).
The ancient Tu proteins were also assayed for their ability to participate in protein synthesis in the reconstituted E. coli system (Supplementary Figure S10). A reciprocal trend was observed when compared with the trend in the T. thermophilus system (compare Figure 5 and Supplementary Figure S10). For instance, Tu 317, the nearest ancestor to Ec Tu, displayed near 80% of the synthesis yield in the E. coli system, whereas this same ancestor had only 32% in the T. thermophilus system. Conversely, Tu 184, the nearest ancestor to Tt Tu, displayed much higher activity (∼85%) in the T. thermophilus system than that (∼65%) in the E. coli system. Ancient Tu proteins representing evolutionary transitions between nodes 184 and 317 had largely intermediate functionalities (Figure 5, Supplementary Figure S5 and Supplementary Data). A similar trend followed for the modern Tu proteins in the two systems. Tt Tu, with Ec Ts or Tt Ts, displayed an activity of 1.7% or ∼30% in the E. coli system (at 37°C), respectively (Figure 4B), whereas Ec Tu (with Ec Ts or Tt Ts) had an activity of only ∼50% in the T. thermophilus system (at 50°C) (data not shown). Tu from Thermotoga maritima was not able to participate very well in the E. coli system (Supplementary Figure S10, no activity).
Despite of the reciprocal trends that correlated relatively well with the evolutionary distances, we cannot rule out the possibility that some differences in the activities of modern and ancient Tu proteins were caused by partial folding of the purified proteins. Though expressed and purified under the same conditions (and used in the same concentrations), the exact differences in the specific activities of these Tu proteins in protein synthesis may need to be determined by other indirect methods, such as biophysical analyses or guanosine diphosphate and aminoacyl-tRNA-binding assays.
DISCUSSION
Protein translation has been extensively characterized using E. coli as a model organism (1,2). Through in vitro reconstitution of protein translation with purified components, we demonstrated biochemically that the translation in thermophilic T. thermophilus involves essentially the same key components as those of mesophilic E. coli, and that the TFs (IFs, EFs, RF and RRF) and 20 aminoacyl tRNA synthetases are sufficient for T. thermophilus ribosome to translate active proteins at temperatures up to 65°C. At the maximal yield of ∼60 μg/ml, the reconstituted T. thermophilus system synthesized significantly less protein than the reconstituted E. coli system (∼200 μg/ml) (Asahara and Chong, unpublished), but more protein than the cell extract-based protein synthesis system, such as that of T. thermophilus (∼20 μg/ml) (22) or Thermococcus kodakaraensis (6.5 μg/ml) (23). However, at the yield of 60 μg/ml, the reconstituted T. thermophilus system produced an average of one stGFP molecule per ribosome under the current conditions. There may be several possible reasons for such a low efficiency in protein synthesis. First, the specific activity of the ribosome might be low. Only a portion of the ribosome molecules could be involved in translation; Second, the efficiency in ribosome termination and recycling might be low. This notion is supported by the fact that the protein synthesis in the reconstituted T. thermophilus system decreased only slightly on deletion of RF1 or RRF (Figure 3). In contrast, removal of RF1 or RRF resulted in a significant decrease in protein synthesis in the reconstituted E. coli system (Asahara and Chong, unpublished). In addition, T. thermophilus does not have RF3, whose function in E. coli is to accelerate the dissociation of RF1 or RF2 from ribosome after peptide release (24). It is therefore possible that the reconstituted T. thermophilus system might lack a factor(s) with a similar function as E. coli RF3. However, addition of E. coli RF3 to the reconstituted T. thermophilus system had a slightly negative effect on the synthesis of stGFP (data not shown). Third, the ribosome could stall during elongation due to a lack of sufficient aminoacyl-tRNAs. The codon usages between T. thermophiles and E. coli are quite different as showed by the significant difference in the GC contents of their genomes (>60% for T. thermophiles and ∼50% for E. coli). However, the relative amounts of aaRSs and tRNAs used in the reconstituted T. thermophilus system were entirely based on those of the reconstituted E. coli system. Further optimization of the ratios of aaRSs and tRNAs in the reconstituted T. thermophilus system may relieve potential bottlenecks during elongation, thereby enhancing ribosome termination and recycling.
The cell extract of T. thermophilus has been shown to synthesize polyphenylalanine in the presence of spermine at temperatures up to 80°C (17). However, the translation reaction in the reconstituted T. thermophilus system failed to occur above 65°C (Figure 2B), a temperature near optimal for the growth of T. thermophilus. Representing the minimal translational machinery of T. thermophilus, the reconstituted T. thermophilus system is likely to lack certain cellular components that allow protein translation possible at temperatures above 65°C. Such components may include endogenous complex polyamines, cellular chaperones and some unknown factors. Despite their thermophilic origin, recombinant T. thermophilus proteins could become increasingly unstable at high temperatures under in vitro conditions. For instance, the recombinant alanyl-tRNA synthetase from T. thermophilus purified from E. coli was shown to be active only up to 65°C (25).
The ribosome and certain aaRS of T. thermophilus have been shown to exhibit significantly lower activities below 40°C than at higher temperatures, based on the poly(Phe) assay (11,26) and the aminoacylation assay (25,27), respectively. In our study, the synthesis of stGFP in the reconstituted T. thermophilus system started at an earlier time point and proceeded with a higher initial rate at 50°C compared with that at 37°C (Figure 1A), suggesting that the T. thermophilus components were indeed more active at higher temperatures. Surprisingly, the in vitro translation of T. thermophilus proceeded efficiently at 37°C, a temperature well below the optimal growth temperature of ∼73°C for T. thermophilus (Figure 2B, 1A and Supplementary Figure S1A). These data suggest the possibility that the ribosome and other essential translation components of T. thermophilus may have evolved from ancestors that had to function under a wide range of temperatures, similar to the range in growth temperatures of hyperthermophiles living in hydrothermal vents.
The availability of the reconstituted systems from both T. thermophilus and E. coli presented us a unique opportunity to examine the evolutionary conservation of the translational machineries from two phylogenetically distant species. A similar attempt has been made previously using the cell extract of E. coli and purified initiation factors and ribosomes from T. thermophilus (28). However, the nature of cell extract-based experiments limited the study to only initiation factors and ribosomes (28). In this previous study, it was found that all three IFs together could be equally exchanged from one species to another without a significant loss in protein synthesis and regardless of the sources of ribosomes (28). However, our study provided a more detailed picture regarding the compatibility of IFs. In our study, we found that Ec IF1 or Ec IF2 could not restore the synthesis activity by replacing Tt IF1 or Tt IF2, respectively, and only Ec IF3 could effectively substitute Tt IF3 in the reconstituted T. thermophilus system (Supplementary Figure S4).
Compared with monomeric Ec Ts (282 residues), Tt Ts (196 residues) is considerably shorter and functions as a dimer. Crystal structures have revealed that the complex of Tt Tu/Ts is a heterotetramer, whereas the complex of Ec Tu/Ts is a heterodimer (29,30). Because Tt Ts dimer formation is required for its function as a nucleotide exchange factor (31), it would be expected that the monomeric Ec Ts would not function effectively with Tt Tu. However, based on the crystal structures, Tt Tu and Tt Ts interact through almost the same bipartite interface as that between Ec Tu and Ec Ts (29,30), and based on in vivo studies, Tt Tu has been suggested to participate in protein synthesis in E. coli (32). A remaining question had been whether Ec Ts could be an effective exchange factor for Tt Tu. In this study, we provided evidence that Ec Ts was not an effective exchange factor for Tt Tu.
Thermusthermophilus is a member of the Thermus-Deinococcus group and possibly represents an intermediate in the transition between gram-positive and gram-negative bacteria (33). Compared with that of E. coli, the translational machinery of T. thermophilus may be more similar to those of ancient bacteria, therefore being more suitable for functional studies of resurrected ancient elongation factors (21,34). It is known that the elongation factor Tu participates in multiple cellular networks that include translation, chaperones/protein folding (35), cytoskeletal and others (36). The reconstituted translation system removes many of these networks by only retaining a majority of the protein synthesis network. This provided us with a unique opportunity to separate some of the evolutionary constraints that shape protein structure (e.g. thermostability) from the constraints that promote coevolution among components in a network.
For instance, Tt Tu has a melting temperature of 76°C (34), most similar to that of the ancient elongation factor Tu 168 (at 73°C, Supplementary Figure S5). However, this ancient protein could only generate ∼34% of protein synthesis in the reconstituted T. thermophilus system compared with Tt Tu (Figure 5). Conversely, the ancient elongation factor Tu 184 could generate 85% of protein synthesis despite having a melting temperature >10°C lower than Tt Tu (Figure 5 and Supplementary Figure S5). This suggests that the ability of Tu 184 to interact with other components of the T. thermophilus translation machinery supersedes its diminished stability compared to Tu 168. This scenario is supported by the fact that Tu 184 has greater overall sequence identity to Tt Tu than Tu 168 does to Tt Tu.
Our study may also provide experimental evidence that ancient proteins represent evolutionary intermediates between modern biomolecular systems. Despite the observation that modern Tu proteins from T. thermophilus and E. coli were not able to be highly interoperable between their respective systems, many ancient Tu proteins displayed intermediate functionalities in the T. thermophilus and E. coli systems (Figure 5, Supplementary Figure S5 and Supplementary Data), This notion suggests that the reconstituted systems may benefit from the use of ancient components, and that synthetic biology in general could benefit from evolutionary studies to expand the functionality of biomolecular properties (37).
Because Tt Ts was functional as the exchange factor for many ancient Tu proteins that replaced Tt Tu in the reconstituted T. thermophilus system (Figure 5), it is tempting to speculate that ancient Ts, like Tt Ts, might function as a dimer and form a heterotetrameric complex with an ancient Tu. Such a higher-order complex could serve to stabilize the interactions between Tu and Ts at elevated temperatures.
The reconstituted E. coli translation system has been proven useful for basic research of cotranslational folding and ribosome pausing (38–42). The reconstituted T. thermophilus system may provide additional advantages for such studies. For instance, by allowing the translation to occur at a wide range of temperatures between 37 and 60°C, the reconstituted T. thermophilus system presents a unique opportunity to study the effect of temperature on protein folding. Such an approach can be applied to proteins of thermophilic origins that cannot be expressed in active forms in mesophilic hosts (systems) possibly due to the requirement of high temperatures for correct folding (43,44). The reconstituted T. thermophilus system may address the question whether temperature is the only factor affecting protein folding.
Like the reconstituted E. coli system, the reconstituted T. thermophilus system would also be useful for in vitro protein evolution (45). There is currently no effective in vitro protein evolution method to select proteins for enhanced thermostability (46). The reconstituted T. thermophilus system contains far less nucleases and proteases than a T. thermophilus cell extract-based system, making mRNA and synthesized proteins more stable. Such a cleaner cell-free protein synthesis system may provide an advantage in applying in vitro protein engineering methods, such as ribosome display and in vitro compartmentalization (45,47), for the selection of protein variants with enhanced thermostability.
FUNDING
New England Biolabs (to S.C.); National Institute of Health [GM086930-01 to S.C.]; NASA Exobiology [NNX08AO12G to E.A.G.]. Funding for open access charge: New England Biolabs, Inc.
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank Drs C. Noren, W. Jack and H. Noller for comments on the manuscript, Dr Y. Zheng for assistance with bioinformatics of Thermus genome sequences, Dr Y. Terui for providing us with polyamines, Drs C. Takemoto and L. Lancaster for providing us with purified T. thermophilus ribosomes as positive controls, Dr M. Cole and J. Kratzer for assistance with ancient EFs, Dr Jack Benner and Colleen O’Neill for mass spectrometry analyses and Dr Donald Comb for encouragement.




Comments