-
PDF
- Split View
-
Views
-
Cite
Cite
Mesut Muyan, Raymond W. Ruddon, Sheila E. Norton, Irving Boime, Elliott Bedows, Dissociation of Early Folding Events from Assembly of the Human Lutropin β-Subunit, Molecular Endocrinology, Volume 12, Issue 10, 1 October 1998, Pages 1640–1649, https://doi.org/10.1210/mend.12.10.0177
Close - Share Icon Share
Abstract
The human LH of the anterior pituitary is a member of the glycoprotein hormone family that includes FSH, TSH, and placental CG. All are noncovalently bound heterodimers that share a common α-subunit and β-subunits that confer biological specificity. LHβ and CGβ share more than 80% amino acid sequence identity; however, in transfected Chinese hamster ovary (CHO) cells, LHβ assembles with the α-subunit more slowly than does hCGβ, and only a fraction of the LHβ synthesized is secreted, whereas CGβ is secreted efficiently. To understand why the assembly and secretion of these related β-subunits differ, we studied the folding of LHβ in CHO cells transfected with either the LHβ gene alone, or in cells cotransfected with the gene expressing the common α-subunit, and compared our findings to those previously seen for CG. We found that the rate of conversion of the earliest detectable folding intermediate of LH, pβ1, to the second major folding form, pβ2, did not differ significantly from the pβ1-to-pβ2 conversion of CGβ, suggesting that variations between the intracellular fates of the two β-subunits cannot be explained by differences in the rates of their early folding steps. Rather, we discovered that unlike CGβ, where the folding to pβ2 results in an assembly-competent product, apparently greater than 90% of the LH pβ2 recovered from LHβ-transfected CHO cells was assembly incompetent, accounting for inefficient LHβ assembly with the α-subunit. Using the formation of disulfide (S-S) bonds as an index, we observed that, in contrast to CGβ, all 12 LHβ cysteine residues formed S-S linkages as soon as pβ2 was detected. Attempts to facilitate LH assembly with protein disulfide isomerase in vitro using LH pβ2 and excess urinary α-subunit as substrate were unsuccessful, although protein disulfide isomerase did facilitate CG assembly in this assay. Moreover, unlike CGβ, LHβ homodimers were recovered from transfected CHO cells. Taken together, these data suggest that differences seen in the rate and extent of LH assembly and secretion, as compared to those of CG, reflect conformational differences between the folding intermediates of the respectiveβ -subunits.
INTRODUCTION
The glycoprotein hormones, LH, human (h) CG, FSH, and TSH, are noncovalently bound heterodimers consisting of a common α-subunit and a distinct β-subunit that confers biological specificity (1). Human LHβ of the anterior pituitary and placental hCGβ are the most closely related β-subunits of this family, apparently having evolved from the same ancestral gene (2). LH- and hCGβ share more than 80% sequence identity, including 12 conserved cysteine residues that form 6 intramolecular disulfide (S-S) bonds (1). The structural similarities between LH and hCG account for the fact that they bind the same receptor and elicit the same biological response (3). However, despite these similarities, LHβ and hCGβ subunits exhibit dramatic differences in their rates of secretion as monomer and assembly with the common α-subunit. hCGβ is secreted and assembles with theα -subunit quantitatively, whereas the secretion and assembly of LHβ are inefficient. These intracellular characteristics of the subunits are observed in transfected Chinese hamster ovary (CHO) cells (4, 5), mouse C-127 mammary tumor cells (6), and somatotrope and corticotrope-derived GH3 and AtT-20 cells (7, 8), respectively. Previous studies have shown that the hCGβ subunit undergoes multiple maturation steps characterized by the formation of intramolecular S-S bonds to attain an assembly-competent conformation (9–11). Thus, variations in the rate and/or extent of folding of the LHβ subunit could be responsible for its inability to assemble and be secreted efficiently.
The reported S-S bond pairing of the ovine LHβ subunit between Cys residues 34–88, 38–57, 9–90, 23–72, 93–100, and 26–110 (12) is the same as that observed during the hCGβ kinetic folding pathway (10, 11, 13). However, the crystal structure of secreted hCGβ (14, 15) reveals S-S bonds formed between Cys residues 38–90 and 9–57 rather than between Cys residues 38–57 and 9–90 and implies that a S-S bond rearrangement occurs during the folding or processing of hCGβ. That the positions of the cysteine residues in hCGβ and LHβ are conserved (1) and both β-subunits assemble with a commonα -subunit suggest that the folding steps leading to formation of assembly-competent β-subunits are similar and that disulfide bond formation could be used as an index of LHβ folding, as it has been used for hCGβ folding. We have previously shown that folding of hCGβ from an early detectable precursor, pβ1, to an assembly-competent intermediate, pβ2, and assembly of hCG pβ2 with the common α-subunit, can be monitored by hCGβ S-S bond formation (for recent reviews see Refs. 16–18). The pairing order of these six hCGβ S-S bonds is the same whether wild-type CGβ folds in human choriocarcinoma (JAR) cells (10), where the hCGβ gene is eutopically expressed, in transfected CHO cells (11), or in vitro using purified hCGβ subunit as substrate (13). Intracellular hCGβ folding occurs in the presence or absence of the α-subunit (11).
To determine whether differences in the folding pattern between the hCG- and LH- β-subunits could account for the intracellular behavior of LHβ, we studied LHβ biosynthesis in CHO cells transfected with the human LHβ gene alone, or with the human glycoprotein hormoneα -subunit. We report here that, although the early folding steps for LHβ and hCGβ occur with similar kinetics in CHO cells, LH pβ2, in contrast to hCG pβ2, had minimal ability to assemble with the commonα -subunit. Greater that 90% of the LH pβ2 synthesized was assembly incompetent for at least 8 h after biosynthesis. Therefore, the rate-limiting step in the attainment of LHβ assembly competence does not appear to be the pβ1-to-pβ2 conversion step, but rather, the maturation of the LH pβ2 folding intermediate.
RESULTS
Early Events in hLHβ Folding
We have previously shown that the assembly and secretion of the LHβ -subunit with the α-subunit is markedly less efficient than that of the hCG β-subunit in transfected CHO cells (4, 5), mouse mammary tumor C-127 cells (6), and somatotrope-derived GH-3 and AtT-20 (7, 8) cells. These findings suggest that differences in the rate and extent of formation of assembly-competent forms could explain the differences in the intracellular fate of these two closely related β-subunits. To examine this issue, CHO cells transfected with only the LHβ gene were pulse labeled for 5 min with [35S]Cys and chased with unlabeled medium (Fig. 1). After a 5-min pulse and a 0-min chase, two major forms of LHβ were detected by nonreducing SDS-PAGE (Fig. 1A). The more slowly migrating of these two forms (Mr = 30,000) apparently represents the LHβ homodimer (LH β/β). There was no apparent precursor-product relationship in the formation of LH β/β since large amounts of the homodimer, representing equivalent percentages of total LHβ seen at later chase times, were recovered after chase periods of 0 min (Figs. 1, A and B). Also observed at 0 min of chase was an early form of LHβ that migrated with approximately half the apparent mol wt of LHβ /β, termed pβ1 (Mr = 17,000). LH pβ1 converted to a second intermediate, pβ2 (Mr = 24,000), with a t1/2 of 7–8 min (Fig. 1A), demonstrating that conversion of LHβ folding intermediates pβ1 to pβ2 was analogous to the conversion of hCG pβ1 to pβ2, which we have previously demonstrated occurs with a t1/2 of 4–5 min (10, 11).
![Pulse-Chase Kinetics of Early LHβ Folding Events Panel A, CHO cells expressing LHβ, but not the α-subunit, were pulse labeled for 5 min with [35S]Cys and chased for 0, 5, 15, or 30 min as indicated, precipitated with polyclonal antisera against LHβ, and assayed by nonreducing SDS-PAGE. Panels B and C represent the same experiment performed with CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 0.4 (panel B) or of 1.6 (panel C). Arrows to the right indicate the positions (from bottom to top) of LH pβ1, LHα, LH pβ2, and LH β/β (homodimer). Panels D–F show the electrophoretic patterns of LH subunits derived from aliquots of the samples shown in panels A–C, but run under reducing SDS-PAGE. Panel D, CHO cells expressing LHβ, but not the α-subunit. Panel E, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 0.4. Panel F, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 1.6. Positions of LHα and LHβ are indicated to the right of panel F. Quantitation of gel images was obtained from fluorograph images by BioImage analysis as described in Materials and Methods. Numbers to the left indicating mol wt markers (MW) are (from top): ovalbumin (Mr = 45,000), carbonic anhydrase (Mr = 29,000), and α-lactalbumin (Mr = 14,200).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/12/10/10.1210_mend.12.10.0177/2/m_mg1080177001.jpeg?Expires=1750559997&Signature=pt1sk5AfjKYiXyXRNo0aly6xfIIvbuGHCGk20IbM2EQ-HyXAOpDVB761mp9DaLY6yVgZt72tFyzhtbPRcb1X97hUMC8m7e5khC3UC76bULvYDbZWALmdRoDF4fTjDbYVvUrIegJBWqZHXGeWOVmZUXU7DzN8-qiEGtkqwqZgYVZT40LoQJbCSscOf~qkRI2HcFFR0-N5gmQFer8xy3TdPLztJ9yx-IqstocxfivweiPcT~FwV6-ZZFzC8CAz39Wfhhy-ikiNEO1IiS0dVZrEUNkle-slQ5j2Jg7QmgwPdqN1mcKb7rEN1yriz2lwG6ScvUtSdEZkn~6sjmHEccd9Zg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pulse-Chase Kinetics of Early LHβ Folding Events Panel A, CHO cells expressing LHβ, but not the α-subunit, were pulse labeled for 5 min with [35S]Cys and chased for 0, 5, 15, or 30 min as indicated, precipitated with polyclonal antisera against LHβ, and assayed by nonreducing SDS-PAGE. Panels B and C represent the same experiment performed with CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 0.4 (panel B) or of 1.6 (panel C). Arrows to the right indicate the positions (from bottom to top) of LH pβ1, LHα, LH pβ2, and LH β/β (homodimer). Panels D–F show the electrophoretic patterns of LH subunits derived from aliquots of the samples shown in panels A–C, but run under reducing SDS-PAGE. Panel D, CHO cells expressing LHβ, but not the α-subunit. Panel E, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 0.4. Panel F, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 1.6. Positions of LHα and LHβ are indicated to the right of panel F. Quantitation of gel images was obtained from fluorograph images by BioImage analysis as described in Materials and Methods. Numbers to the left indicating mol wt markers (MW) are (from top): ovalbumin (Mr = 45,000), carbonic anhydrase (Mr = 29,000), and α-lactalbumin (Mr = 14,200).
To assess whether the presence of the α-subunit would affect the kinetics of formation of LHβ folding intermediates, we examined cells expressing both LHβ and the α-subunit. It should be noted (see Fig. 1) that the intracellular α-subunit migrates with a Mr of 19,000 in either nonreducing (panels B and C) or reducing gels (panels E and F). On the other hand, nonreduced LH pβ1 (panels A–C) and reduced LHβ (panels D–F) migrate more rapidly than the α-subunit, while LH pβ2 (panels A–C) migrates more slowly than α. At anα /β-subunit ratio of either 0.4 (Fig. 1B) or 1.6 (Fig. 1C), the t1/2 of conversion of LH pβ1 to pβ2 was not altered. Conversion of LH pβ1 to pβ2 involves the formation of S-S bonds since these forms collapse to a single band under reducing conditions (Fig. 1D–F). These findings demonstrate that the rate of conversion of LH pβ1 to pβ2 did not differ significantly from that of hCGβ, even in the presence of α-subunit, and suggest that the differences between the intracellular fates of the two β-subunits cannot be explained by differences in the rate of their early folding events.
Role of pβ2 in LH-β Assembly
To ensure that the extent of heterodimer formation was not limited by the amount of α-subunit present in the following experiments, CHO cells overexpressing the common α-subunit relative to the LHβ subunit (i.e. at an α-/β-subunit ratio of 1.6) were used. The extent of LH heterodimer formation was assessed by immunoprecipitation with subunit-specific antisera. Precipitation of the α-subunit with β-antiserum, or the precipitation of theβ -subunit with α-antiserum, indicates heterodimer formation. Following pulse labeling, LH heterodimer precipitated with either α- or β-antisera contains radiolabeled (newly synthesized) α-subunit during initial chase periods; however, very little radiolabeled LHβ associated with labeled α-subunit is detected when the heterodimer is precipitated with α-antiserum (4–6). This is presumably due to the presence of a stable, preexisting nonradiolabeled intracellular pool of assembly-competent LHβ subunit that accumulates because nascent LHβ requires time to become assembly competent (4–6).
To identify all forms of LH subunits present in our cells, we performed Western blot analysis under nonreducing conditions of CHO cell lysates expressing both the LH α- and β-subunits (Fig. 2). Panel A shows that when polyclonal antiserum to the α-subunit was used, two bands appeared: a band of Mr = 19,000 was seen when intracellular lysates were probed (α-int; lane A1), while a heterogeneous band typical of secretedα -subunit (Mr = 22,000–26,000) was detected in the medium (α-sec; lane A2). When polyclonal antiserum to the LHβ -subunit was used, multiple bands appeared (panel B). In lysates, the most slowly migrating band (panel B, lane 1) is LH β/β homodimer, while a single LHβ band was detected as mature secreted LHβ (β-sec) (panel B, lane 2). In addition to LH β/β, lysates contained two bands that migrated at Mr = 22,000 and Mr = 20,000 (Fig. 2B, lane 1, designated pβ2-U (upper), and pβ2-L (lower), respectively]. Both of these bands are likely to be pβ2 forms, based on their apparent mol wts (pβ1 migrates at Mr = 17,000 (Fig. 1, A–C)).
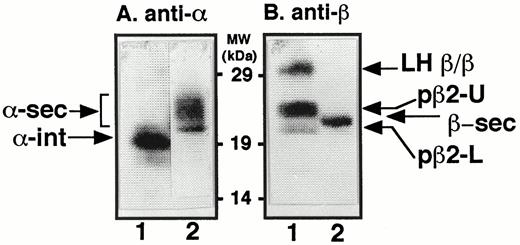
Western Blot Analysis of CHO Cells Expressing LHα and -β Subunits CHO cells expressing both α- and LH β-subunits were probed for the presence and gel migration loci of the LHα and -β bands. Intracellular lysates (lanes A1 and B1) or overnight serum-free conditioned medium (lanes A2 and B2) were run on 5–20% acrylamide gradient gels under nonreducing conditions and transferred onto polyvinylidene fluoride membranes. The membranes were then probed with either polyclonal antiserum to the α-subunit (panel A) or polyclonal antiserum to the LH β-subunit (panel B). Identified in panel A, lane 1, was intracellular α-subunit (α-int), and in panel A, lane 2, heterogeneous forms of secreted α-subunit (α-sec). In panel B, lane 1, three forms of LHβ were detected: (from top to bottom) LH β/β homodimer; pβ2-U (upper, the more slowly migrating of the LH-pβ2 forms), and pβ2-L (lower, the more rapidly migrating of the LH-pβ2 forms). Panel B, lane 2, shows the position of secreted LHβ (β-sec).
When CHO cells expressing only the LHβ subunit were pulse labeled for 5 min and chased for 30 min to 8 h, LHβ antiserum precipitated the pβ2-U and pβ2-L folding intermediates and LH β/β (Fig. 3A). The more predominant of the two pβ2 folding intermediates, pβ2-U, migrated more slowly than the lower band, pβ2-L, and contained more than 90% of the radiolabeled LH pβ2. When CHO cells expressing both the LHβ subunit and theα -subunit were pulse labeled and precipitated with LHβ antiserum (Fig. 3B), a small amount of pβ2-L, was detected. The appearance of LH pβ2-L is clearer after a 60-min chase, when changes in the migration rate of the α-subunit, presumably due to processing of the N-linked oligosaccharides, allows pβ2-L to be more readily distinguished from α. At these later chase times, α-subunit secretion occurred, which reduced the amount of intracellularα -subunit detected. Figure 3C shows that the minor folding form, pβ2-L, associates with the α-subunit. Here, CHO cells expressing both the LHβ subunit and the α-subunit were labeled and immunoprecipitated with antiserum against the α-subunit. Under these conditions the rapidly migrating LH pβ2-L, but not LH pβ2-U, was detected at chase times of 1–4 h as α-subunit processing proceeded, indicating that pβ2-L was not a spurious α-subunit band. Further verification that pβ2-L was a form of LHβ was obtained when we immunoprecipitated CHO cell lysates of cells expressing only theα -subunit with α-antisera and failed to detect its presence (Fig. 3D). Taken together, these data suggest that a form of LH pβ2 representing less than 10% of the total pβ2 in the cell was the only form of LH pβ2 competent to associate with the α-subunit and provide an explanation for why LHβ assembly is inefficient.
![Pulse-Chase Kinetics of LH-β Assembly and Secretion Panel A, CHO cells expressing LHβ, but not the α-subunit, were pulse labeled for 5 min with [35S]Cys and chased for the times indicated, and intracellular or secreted material was precipitated with polyclonal antiserum against LHβ and assayed by nonreducing SDS-PAGE. Identified were LH β/β, and two LH pβ2 bands designated pβ2-U and pβ2-L. In addition, a small amount of LHβ subunit (β-sec) was found in the chase medium after 8 h. Panel B, CHO cells expressing both LHβ and the α-subunit at anα /β subunit ratio of 1.6 were labeled, chased, and immunoprecipitated with antisera against LHβ as in panel A. All bands seen in Fig. 3A were also recovered in panel B, and in addition, intracellular α-subunit (α) and heterogeneous secreted α-subunit (α-sec) were detected. Panel C, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 1.6 were labeled and chased as described for panel A and immunoprecipitated with antisera against the α-subunit. In addition to α-int and α-sec, intracellular LH pβ2-L was detected, along with secreted LH-β. Panel D, CHO cells expressing the α-subunit only were labeled and chased as described in panel A and immunoprecipitated with antiserum against the α-subunit. Note the absence of the Mr = 22,000 band of LH pβ2-L. The locus at which pβ2-U migrates is indicated in brackets (as [pβ2-U]) in panels C and D.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/12/10/10.1210_mend.12.10.0177/2/m_mg1080177003.jpeg?Expires=1750559997&Signature=TCAU2VeDXpvB9Ama-YdYUaxjdNpb510EReIvYn~P8S02u8K8tmzjNmNNc5KmuZGPqW16v-i-hu8PuLwxRTV1ilN38775vTGaHshMhH8GPYJ9-DeTDFprTAf99pj13sPLzq4HJnxP38SHXvNKFASOiExmcZkDI-cBEDFwq0C1u3lKD9Ut8hGB~qWEKyA2XieFpEczMHf9fbQY1u8pfQpERJxMtGSpFRuxtS00Cu18WsSfiAL-OC3W~YAnGySbpeRAjQ8it71AMqYXq-EpsqoN6aDoFYhOiOg0E1B34rx2CLqzWc1py0pqbiXf9pS7OJNTxfKMdaovbFGyiuhhgUyCVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pulse-Chase Kinetics of LH-β Assembly and Secretion Panel A, CHO cells expressing LHβ, but not the α-subunit, were pulse labeled for 5 min with [35S]Cys and chased for the times indicated, and intracellular or secreted material was precipitated with polyclonal antiserum against LHβ and assayed by nonreducing SDS-PAGE. Identified were LH β/β, and two LH pβ2 bands designated pβ2-U and pβ2-L. In addition, a small amount of LHβ subunit (β-sec) was found in the chase medium after 8 h. Panel B, CHO cells expressing both LHβ and the α-subunit at anα /β subunit ratio of 1.6 were labeled, chased, and immunoprecipitated with antisera against LHβ as in panel A. All bands seen in Fig. 3A were also recovered in panel B, and in addition, intracellular α-subunit (α) and heterogeneous secreted α-subunit (α-sec) were detected. Panel C, CHO cells expressing both LHβ and the α-subunit at an α/β subunit ratio of 1.6 were labeled and chased as described for panel A and immunoprecipitated with antisera against the α-subunit. In addition to α-int and α-sec, intracellular LH pβ2-L was detected, along with secreted LH-β. Panel D, CHO cells expressing the α-subunit only were labeled and chased as described in panel A and immunoprecipitated with antiserum against the α-subunit. Note the absence of the Mr = 22,000 band of LH pβ2-L. The locus at which pβ2-U migrates is indicated in brackets (as [pβ2-U]) in panels C and D.
Role of S-S Bond Formation in hLH-β Folding
To determine why LHβ undergoes such inefficient conversion to assembly-competent pβ2, we used formation of S-S bonds as an index of the extent of LHβ folding. We have previously shown that the conversion of hCG pβ1 to assembly-competent pβ2 can be monitored byβ -subunit S-S bond formation (10, 11). If any of the S-S bonds fail to form, an [35S]Cys-containing peptide is released from the disulfide-linked core following trypsin digestion, and these peptides can be detected by reversed-phase HPLC (10, 11, 13, 19–21). Moreover, these HPLC-derived tryptic maps of hCGβ reveal which S-S bonds are unformed. Since there is sufficient sequence identity between the hCGβ and LHβ subunits, we generated tryptic maps from both HPLC-purified LH pβ2 and hCG pβ2. When we compared these tryptic maps (Fig. 4), we failed to detect any peptides released from the S-S-linked core material of LH pβ2 (Fig. 4A). The radioactivity eluting in fractions 4–6 (Fig. 4A) coincides with the void volume of the column and does not contain LHβ peptides when rechromatographed and thus appears to be free[ 35S]Cys or some other degradation product (Refs. 10, 11 and data not shown). This suggests that all of the LHβ cysteine residues were paired as soon as pβ1-to-pβ2 conversion was detected. By contrast, in hCGβ, peptides 96–104 (peaks 1a and b) and 105–114 (peak 2) are released from assembly-competent pβ2 (Fig. 4B; and Refs. 10, 11, 13). The release of these two peptides indicates that S-S bonds 93–100 and 26–110 remain unformed when hCG pβ1-to-pβ2 conversion occurs (10, 11, 13), which is consistent with the observation that these two bonds form coincident with or shortly after hCGβ assembly with the α-subunit (10). In the case of LHβ, however, it appears that all free thiols were converted to S-S bonds as LHβ folded from pβ1 to pβ2.
![Tryptic Maps of Glycoprotein Hormone β-Subunits Panel A shows the HPLC-derived peptide map produced following tryptic cleavage of CHO cell LH pβ2 recovered after a pulse labeling period of 5 min with [35S]Cys and a chase of 6 min. The radioactive material that eluted in fractions 4–6 is void volume material that does not contain LHβ peptides. The broad peak eluting between 85 and 102 min represents disulfide-linked (core) peptides of LHβ. Panel B shows the tryptic maps of hGC pβ2 generated under conditions identical to those described in Fig. 4A. In addition to the disulfide-linked core peptides, this profile reveals a doublet (peaks 1a and 1b), corresponding to peptide 96–104 (unformed disulfide bond 93–100) and peak 2, containing peptide 105–114 (unformed disulfide bond 26–110) (10, 11 ). These data show that[ 35S]Cys-labeled peptides analogous to those released from the S-S-linked core of hCG pβ2 are not released from the S-S-linked core of LH pβ2 and suggest that all thiols are disulfide linked in LH pβ2 as soon as it is detectable.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/12/10/10.1210_mend.12.10.0177/2/m_mg1080177004.jpeg?Expires=1750559997&Signature=hKBqvn9NE1OuUGq~iPGPhgInYvFvEsVOUTS1JQAaXnqVxcWp-jw53ZH8ibwNWHMUDZFXyPe0Eu5yabeoqQa~ufR4Ls4QIGsfmx1ZOT-ss61KNwtE8Br08vVnW7jBHHr9sVlsrjinRNebickJ7mleEsi~W0CrQC9V1Lum9PDshDJtpWW8dip5SrFD0OULfqWXG4NFNdNROzcTYY0HLEIxXD1AUUsllyoxp~T6RaMdTjr5lOQHCiFTXnwpkYfYShy5KdAeVSwP07dQ0ebLYM7Tf5XbfRoit8d7gs6sAxT8XMjniT3~opCI1wEifJoUK3On68ZYPaQsGzvfeeYBiLIHsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Tryptic Maps of Glycoprotein Hormone β-Subunits Panel A shows the HPLC-derived peptide map produced following tryptic cleavage of CHO cell LH pβ2 recovered after a pulse labeling period of 5 min with [35S]Cys and a chase of 6 min. The radioactive material that eluted in fractions 4–6 is void volume material that does not contain LHβ peptides. The broad peak eluting between 85 and 102 min represents disulfide-linked (core) peptides of LHβ. Panel B shows the tryptic maps of hGC pβ2 generated under conditions identical to those described in Fig. 4A. In addition to the disulfide-linked core peptides, this profile reveals a doublet (peaks 1a and 1b), corresponding to peptide 96–104 (unformed disulfide bond 93–100) and peak 2, containing peptide 105–114 (unformed disulfide bond 26–110) (10, 11 ). These data show that[ 35S]Cys-labeled peptides analogous to those released from the S-S-linked core of hCG pβ2 are not released from the S-S-linked core of LH pβ2 and suggest that all thiols are disulfide linked in LH pβ2 as soon as it is detectable.
When the 26–110 S-S bond of uncombined hCGβ is formed, the subunit cannot assemble with the common α-subunit (13) because this is the last S-S bond to form during hCGβ folding (10, 11, 13) and forms a“ seatbelt” (14) that stabilizes native hCG following heterodimer assembly; preformation of the 26–100 bond inhibits CGβ assembly with the α-subunit (13). We, therefore, examined whether the 26–110 S-S bond of LHβ had already formed, preventing LH assembly. To do this, we used an in vitro assembly assay (13). We have previously demonstrated that protein disulfide isomerase is capable of reducing the 26–110 and 93–100 S-S bonds of hCGβ, thereby enhancing its in vitro assembly with the α-subunit under appropriate redox conditions (13). Similarly, we determined whether protein disulfide isomerase was capable of enhancing LH assembly. Using HPLC-purified pβ2 (see Materials and Methods) derived from CHO cells expressing either the LHβ or CGβ subunits, we examined the ability of the respective pβ2 subunits to assemble with a large molar excess of purified urinary α-subunit in vitro (Fig. 5). Under conditions where hCG pβ2 efficiently assembled with the α-subunit (Fig. 5A; also Ref. 13), no heterodimer containing LH pβ2 was detected (Fig. 5B). This result supports the hypothesis that there is some structural constraint other than reduction of the 26–110 S-S bond in LH pβ2 that must be overcome before the LHβ subunit can form heterodimer. Conceivably, these constraints might make the LHβ 26–110 S-S bond insusceptible to protein disulfide isomerase.
![In Vitro Assembly of Glycoprotein Hormone pβ2-Subunits with α-Subunits [35S]Cys-labeled LH pβ2 and hCG pβ2 were purified by HPLC as described in Materials and Methods and tested for their respective abilities to assemble with a vast molar excess of unlabeled urinary α-subunit. Assays were performed in the presence of glutathione to maintain appropriate redox conditions as previously described (Ref. 13; and Materials and Methods), and protein disulfide isomerase (Takara) was included in each reaction. Incubations were carried out at 37 C, and reactions were stopped by the addition of iodoacetate at the times indicated. Samples were analyzed by nonreducing SDS-PAGE at 4 C followed by autoradiography. The data reveal that LH pβ2 (panel B) was assembly incompetent under assay conditions (13 ) where hCG pβ2 (panel A) was assembly competent. (The faint band that appears at Mr = 30,000 at all time points of panel B likely reflects minor contamination of the LH pβ2 substrate with LH β/β.)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/mend/12/10/10.1210_mend.12.10.0177/2/m_mg1080177005.jpeg?Expires=1750559997&Signature=3HKlR4GvHU77qqc3Y-79dg6hATqjaXK4emTLCOZWouL9SBSVCc0j2ua3iUe3nWf0HcXQ4cs5z6BCxfrqZW4r6vDvVmpTeqb0T4D~xOCBvAof07VXHDfAJO-VnURFq8RkKz1Izr4JYG8CrOBCzEkkxvji~5FNXPicYqC9xvvY5OJeVzwl~6GivJyrbkF~IF6lXfIu0T8mJWyOY6ztBcTHdme5clwYY8lDEZ-S2~b1zlAernfJOeBATTalsHDb-f39XnRQCja7EeXXTZcl0dlMWsxL-Kll-Eeo3Ik~jmE0Vc3e1hePWLpMlshgP8MW62Iiy~8z1ohkpkjnuYjPbv-gxg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
In Vitro Assembly of Glycoprotein Hormone pβ2-Subunits with α-Subunits [35S]Cys-labeled LH pβ2 and hCG pβ2 were purified by HPLC as described in Materials and Methods and tested for their respective abilities to assemble with a vast molar excess of unlabeled urinary α-subunit. Assays were performed in the presence of glutathione to maintain appropriate redox conditions as previously described (Ref. 13; and Materials and Methods), and protein disulfide isomerase (Takara) was included in each reaction. Incubations were carried out at 37 C, and reactions were stopped by the addition of iodoacetate at the times indicated. Samples were analyzed by nonreducing SDS-PAGE at 4 C followed by autoradiography. The data reveal that LH pβ2 (panel B) was assembly incompetent under assay conditions (13 ) where hCG pβ2 (panel A) was assembly competent. (The faint band that appears at Mr = 30,000 at all time points of panel B likely reflects minor contamination of the LH pβ2 substrate with LH β/β.)
As mentioned above, when lysates of CHO cells expressing LHβ were precipitated with LHβ antiserum, a band migrating with a Mr = 30,000 was seen by nonreducing SDS-PAGE (Fig. 1, A and B, and Fig. 3, A and B). There appeared to be no precursor-product relationship between LH β/β and other LHβ folding intermediates as large amounts of the homodimer representing equivalent percentages of total LHβ seen at later chase times were recovered following chase periods of 0 min (Fig. 1, A and B). These forms persisted within the cell for chase periods of up to 8 h (Fig. 3, A and B). Moreover, since LH β/β comigrated with LHβ monomer (Mr = 17,000) under reducing conditions (Fig. 1, D and E), it appears that LHβ /β is formed by intermolecular S-S bonds. For these reasons, and because the tryptic digestion of LH β/β produced a peptide map similar to those seen when intermolecular S-S bond containing homodimers and multimers of hCG-β were analyzed (Ref. 19 and data not shown), we have concluded that this material represents a homodimeric form of the LHβ subunit.
High levels of LH β/β were detected in LHβ transfected CHO cells either lacking (Figs. 1A and 3A) or underexpressing (Fig. 1B) theα -subunit relative to LHβ (α/β subunit ratio = 0.4). However, when the α-subunit was overexpressed (α/β subunit ratio = 1.6), very little LH β/β was seen, especially at early chase times (Figs. 1C and 3B). Thus, it appears that the α-subunit is affecting the kinetics of formation and the amount of LH β/β formed. LH β/β was not detected in the media following chase periods of up to 8 h (Figs. 3, A and B), demonstrating that, like unassembled LHβ subunits (Fig. 3A), secretion of LH β/β was inefficient.
DISCUSSION
Although hCGβ and hLHβ subunits share extensive amino acid sequence identity (1), their kinetics and extent of assembly and secretion in a variety of cell lines, which include pituitary-derived GH3 and AtT-20 cells (4–8), differ significantly. Here we addressed why these two closely related molecules have different fates by comparing the folding kinetics of LHβ to that of hCGβ. The folding pathway of hCGβ has been well studied (16, 17); it folds efficiently from an early detectable precursor, pβ1, to an assembly-competent intermediate, pβ2.
By contrast, conversion of LH pβ1 to pβ2 did not produce an assembly-competent subunit. Rather, for LHβ, the folding and assembly steps appeared to require additional conformational changes, possibly involving S-S bond rearrangement before becoming assembly competent. In contrast to hCGβ, all 12 LHβ cysteine residues appeared to be involved in S-S linkages as soon as pβ1-to-pβ2 conversion was detected. This conclusion was based on the observation that no peptides were released from LHβ S-S linked core protein following tryptic digestion (Fig. 4A). While we cannot rule out the possibility that tryptic cleavage sites were buried due to collapse of the hydrophobic domains of the LHβ subunit, this seems unlikely because the purified form of LHβ subjected to trypsin had been highly denatured by exposure to SDS and 6 m guanidinium hydrochloride during the extraction and purification procedures before trypsin treatment.
It is not clear why LHβ folds and assembles differently from hCGβ. While the two molecules share extensive homology, they have very different C-terminal amino acid sequences (1). hCGβ has a 31-amino acid hydrophilic C-terminal peptide that contains four O-linked glycans. Unlike hCGβ, LHβ possesses a seven-amino acid hydrophobic C terminus. The hydrophobicity conferred by the LHβ C-terminal heptapeptide, together with hydrophobic amino acid residues localized at the amino terminus of the molecule, appear capable of serving as nucleation sites for LHβ aggregation soon after the nascent polypeptide is synthesized (4, 5). This is consistent with previous studies showing that an interaction of the hydrophobic LHβ C terminus with other LHβ residues is critical in delayed secretion and assembly of the LHβ subunit (5). This hypothesis is also supported by the detection of a homodimeric species of LHβ, LH β/β. There is apparently no precursor-product relationship between the LH β/β form and the assembly-competent subunit since it was detected following a 0-min chase. LH β/β was detected intracellularly in all CHO cell clones, regardless of the expression level of LHβ (data not shown) following chase periods of up to 8 h. Because of this intracellular stability, LH β/β may represent a dead-end nonproductive product or an assembly-incompetent storage form of LHβ, a fraction of which can be rescued when sufficient amounts ofα -subunit are present to drive assembly.
By decreasing LH β/β formation (aggregation), and facilitating folding and assembly, the α-subunit, when overexpressed in CHO cells, appears to be acting in a chaperone-like manner, presumably by binding to LHβ subunits before they bind each other. Since LHβ is not assembly competent following 0 min of chase, the α-subunit seems to bind assembly-incompetent LHβ at one epitope accessible in assembly-incompetent LHβ and at a different epitope, found only in assembly-competent LHβ, during heterodimer formation. This mechanism explains why LHβ need not be assembly competent to bind α. It is likely that the endoplasmic reticulum chaperones play a role in facilitating the folding and assembly of LHβ. We previously identified hCGβ-chaperone complexes that facilitate folding and assembly of CG in transfected CHO cells (22). The endoplasmic reticulum chaperones, BiP, ERp72, GRp94 (22), calreticulin, and calnexin (E. Bedows, unpublished), associate with hCGβ as pβ1 folds into assembly-competent pβ2. Because of the large degree of amino acid identity between CGβ and LHβ subunits, and the hydrophobic nature of the LHβ C terminus, molecular chaperone intervention likely determines how and when LHβ folding intermediates proceed along their kinetic folding pathway.
The intracellular behavior of these two gonadotropin β-subunits may reflect their respective biological roles. Secretion of hCG from the placenta is primarily constitutive to maintain the corpus luteum, whereas secretion of LH from the pituitary is pulsatile and regulated by LHRH levels (for a review see Ref. 23). Since unassembled LHβ is not secreted efficiently, the ability of the pituitary to build up stores of free LHβ may assist secretion of large quantities of the hormone from the anterior pituitary during the LH surge before ovulation (4). There remain several unanswered questions about the mechanisms for the selective retention of LHβ in the endoplasmic reticulum. Cellular factors including chaperone association (24–26) and the presence of intracellular retention signals (27) may influence how quickly secretory proteins such as LH are allowed to exit the endoplasmic reticulum. It will be important to explore how these factors differentially control the folding, assembly, and secretion of the glycoprotein hormone β-subunits with their commonα -subunit.
MATERIALS AND METHODS
Cell Culture
CHO cells transfected with wild-type hLHβ (4) or hCGβ (5, 11) genes alone or cotransfected with the wild-type glyco-protein hormone α gene, were grown in F-12 medium supplemented with 5% FBS, the neomycin analog G-418 (GIBCO, Grand Island, NY), and antibiotics (19, 28).
Metabolic Labeling of Cells with Radioactive Substrates
CHO cells grown to 90–95% confluency in 100-mm plastic dishes were pulse labeled for the times indicated in the text with l-[35S]cysteine (∼1100 Ci/mmol; Du Pont-New England Nuclear, Boston, MA), at a concentration of 200–300 μCi/ml, in serum-free medium lacking cysteine (19). All pulse incubations were carried out as described previously (19), and the cells were incubated for the chase times indicated in the text. Cells were harvested by rinsing with cold PBS and immediately lysed in 5 ml PBS containing detergents (1.0% Triton X-100, 0.5% sodium deoxycholate, and 0.1% SDS); protease inhibitors (20 mm EDTA and 2 mm phenylmethanesulfonyl fluoride); and 50 mm iodoacetic acid (pH 8.0), to trap the free sulfhydryl groups of the β folding intermediates. Cell lysates were incubated 20–30 min at 22 C in the dark, followed by disruption through a 22-ga needle (three times), centrifuged for 1 h at 100,000 × g, and immunoprecipitated (see below) or frozen at −70 C for further use.
Immunoprecipitation of Cell Lysates and Culture Media
The immunoreactive forms of LHβ or hCGβ were immunoprecipitated with a rabbit (4) or goat (19) polyclonal antiserum that recognizes the folding intermediates of both β-subunits. Because of the sequence identity between them, efficient precipitation of both subunits was observed. All immunoprecipitations were carried out for 16 h at 4 C with rotation in the dark. Immune complexes were precipitated with Protein A-Sepharose (Sigma Chemical Co., St. Louis, MO) and prepared for SDS-PAGE or reversed-phase HPLC as described below.
SDS-PAGE, Fluorography, and Western Blot Analysis
Radiolabeled LH or CG forms that adsorbed to Protein A-Sepharose beads were eluted with 2 × concentrated SDS gel sample buffer (125 mm Tris-HCl, pH 6.8, containing 2% SDS, 20% glycerol, and 40 μg/ml bromophenol blue). Samples run under reducing conditions were boiled for 4 min in sample buffer containing 2%β -mercaptoethanol, while samples run under nonreducing conditions were boiled for 4 min in sample buffer lacking β-mercaptoethanol. The washed samples, including the Protein A-Sepharose beads, were applied to polyacrylamide gradient slab gels (5–20%) that were run by the method of Laemmli (29). Gels were rinsed in water, dried in vacuo on filter paper, and exposed to x-ray film. Fluorographs were photographed with a Kodak CCD (charged caption device) camera (BioImage 110S System, Genomic Solutions Inc., Ann Arbor, MI). Quantitation of gel images was obtained by transferring photographed fluorograph images to a Sun SPARCstation 1+ computer and analyzed using BioImage Whole Band software and printed on a Seiko Instruments USA Inc. (San Jose, CA) CH-5504 color printer.
Western blot analysis was performed using aliquots (50 μl) of media or cell lysates from CHO cells expressing LHβ and the commonα -subunit following a 24-h incubation in conditioned media lacking serum and were resolved by SDS-PAGE on a 5–20% gradient gel under nonreducing conditions. Gels were transferred to nitrocellulose membranes and probed with either α-antiserum or hCGβ antiserum. Proteins were detected by the Tropix (Bedford, MA) chemiluminescent detection system.
Purification of β-Subunit Folding Intermediates and HPLC Analysis
The hCGβ and LHβ folding intermediates pβ1 and pβ2 were purified by a two-step process (immunoprecipitation followed by C4 reversed-phase HPLC) as described by Huth et al. (10). Briefly, pβ1 and pβ2 were immunoprecipitated from cell lysates with polyclonal antisera, and immunocomplexes were precipitated with Protein A-Sepharose beads. To dissociate precipitated immunocomplexes, pellets were treated with 6 m guanidine-HCl (pH 3) (Pierce, Rockford, IL; sequencing grade) for 16 h at room temperature with 100 μg of myoglobin (Sigma) as carrier. Following low-speed centrifugation to remove Protein-A Sepharose beads, the guanidine eluates were injected onto a Vydac 300Å C4 reversed-phase column (Hesparia Separations Group, Hesparia, CA) equilibrated with 0.1% trifluoroacetic acid (TFA) and eluted using an acetonitrile gradient as previously described (10). Fractions containing LHβ or hCGβ forms were concentrated by vacuum centrifugation and pooled for tryptic analysis.
Tryptic Digestions and HPLC Purification of Tryptic Peptides
Nonreduced LHβ or hCGβ forms were digested for 16 h at 37 C in silanized polypropylene tubes containing 100 μg myoglobin, 0.03% diphenylcarbamyl chloride-treated Trypsin (Sigma), 5 mm CaCl2, and 100 mm Tris-HCl, pH 8. The digestion was continued with the addition of two sequential aliquots of 50 μg trypsin (0.06% final concentration) for 2 h. Tryptic digests of β-subunits were injected onto a Brownlee 300 Å C8 reversed-phase column (Applied Biosystems, Foster City, CA) equilibrated with 0.1% TFA (10, 11). The column was eluted isocratically for 3 min with 0.1% TFA followed by a 0.32%/min acetonitrile gradient in 0.1% TFA for 100 min. The column was washed with 80% acetonitrile, 0.1% TFA for 5 min and then reequilibrated in 0.1% TFA. The flow rate was 1.0 ml/min. One-minute fractions were collected in silanized polypropylene tubes. Tubes into which S-S-linked peptides eluted contained 5 μg myoglobin as carrier. Samples were concentrated by vacuum centrifugation and stored at −20 C.
Identification of Peptides Following Tryptic Digestion
Fully folded hCGβ contains 6 disulfide bonds and 13 Arg and Lys residues that are arranged such that all of the Cys-containing tryptic peptides remain attached to each other as a result of their covalent disulfide bridges (9, 10). If, however, particular disulfide bonds are not formed in a given hCGβ folding intermediate, specific Cys-containing tryptic peptides are released from the S-S-linked CGβ core. For example, if the 26–110 bond is unformed, then CGβ peptide 105–114 (containing Cys-110) would be released. The pattern of tryptic-released peptides, distinguished from the disulfide-linked peptides by HPLC, reveals incomplete bond formation (10). By lysing cells in the presence of the alkylating agent iodoacetate, the Cys residues of the unformed hCGβ S-S bonds are trapped. The alkylated folding intermediates are then resolved by C8 reversed-phase HPLC (10). Identification of HPLC peptide peaks was made by comparing elution times of peaks generated from wild-type CGβ tryptic digests (10, 11) that had been verified by microsequencing. Amino acid sequence analysis revealed whether the Cys-containing peptides had been alkylated (indicating that the S-S bond had not been formed in the intact molecule).
In Vitro Assembly of LH- and CGα and -β Subunits
In vitro assembly reactions were performed by a modification of the procedure described by Huth et al. (13). To generate the pβ2 used in the experiment shown in Fig. 5, CHO cells expressing either CGβ or LHβ were pulse labeled for 5 min with[ 35S]Cys and chased with unlabeled medium for 20 min for CGβ or 30 min for LHβ, followed by lysis in PBS containing EDTA, phenylmethanesulfonyl fluoride, and the detergent mixture described above, but lacking alkylating agent. Respective CG and LH pβ2 subunits were purified by immunoprecipitation followed by reversed-phase HPLC. HPLC-derived fractions containing radiolabeled pβ2 substrate to be used in CG or LH assembly reactions were concentrated under vacuum. Each reaction contained a final concentration of 1 μm urinary hCGα, 150,000 cpm (∼2 ng) [35S]cysteine-labeled pβ2, 1.7 mm reduced glutathione, 0.27 mm oxidized glutathione, 1 mm EDTA, and 20 mm sodium phosphate (pH 7.8); a final concentration of 17.5 μm bovine liver protein disulfide isomerase (Takara Biochemical Inc., Berkeley, CA) was also included in the assay. The reactions were incubated at 37 C, and aliquots were withdrawn at the times indicated and terminated with a solution of 900 mm iodoacetate containing 450 mm Tris-HCl (pH 8.7). Samples were mixed with ice-cold nonreducing electrophoresis buffer and analyzed by SDS-PAGE at 4 C.
This work was supported in part by NIH Grants HD-23398 and CA-32949, by American Cancer Society Institutional Grant IRG-165G and NCI Cancer Center Support Grant P30CA3627.
Current address: University of Rochester Medical Center, Department of Biochemistry, Box 607, 601 Elmwood, Rochester, New York 14620.
Current address: Corporate Office of Science and Technology, Johnson & Johnson, 410 George Street, New Brunswick, New Jersey 08901.



