-
PDF
- Split View
-
Views
-
Cite
Cite
Jian-Min Chen, Zoltán Kukor, Cédric Le Maréchal, Miklós Tóth, Laurent Tsakiris, Odile Raguénès, Claude Férec, Miklós Sahin-Tóth, Evolution of Trypsinogen Activation Peptides, Molecular Biology and Evolution, Volume 20, Issue 11, November 2003, Pages 1767–1777, https://doi.org/10.1093/molbev/msg183
Close - Share Icon Share
Abstract
The activation peptide of mammalian trypsinogens contains a highly conserved tetra-aspartate sequence (D19-D20-D21-D22) preceding the K23-I24 scissile peptide bond, which is hydrolyzed as the first step in the activation process. Here, we examined the evolution and function of trypsinogen activation peptides through integrating functional characterization of disease-associated mutations with comparative genomic analysis. Activation properties of three chronic pancreatitis-associated activation peptide mutants (the novel D19A and the previously reported D22G and K23R) were simultaneously analyzed, for the first time, in the context of recombinant human cationic trypsinogen. A dramatic increase in autoactivation of cationic trypsinogen was observed in all three mutants, with D22G and K23R exhibiting the most marked increases. The physiological activator enteropeptidase activated the D19A mutant normally, activated the D22G mutant very poorly, and stimulated activation of the K23R mutant. The biochemical and structural data, taken together with a comprehensive sequence comparison, indicates that the tetra-aspartate sequence in mammalian trypsinogen activation peptides has evolved not only for optimal enteropeptidase recognition in the duodenum but also for efficient inhibition of trypsinogen autoactivation within the pancreas. Moreover, the use of lysine instead of arginine at the P1 position of activation peptides also has an advantageous effect against trypsinogen autoactivation. Finally, fixed substitutions in the key residues of the trypsinogen activation peptide may suggest the evolution of new functions unrelated to digestion, as found in the group III trypsinogens of cold-adapted fishes.
Introduction
Pancreatic trypsin (EC 3.4.21.4) is a member of the large and diverse serine peptidase family. It is characterized by a catalytic triad composed of a histidine, an aspartic acid, and a serine residue that specifically cleaves peptide bonds C-terminal to arginine or lysine. Trypsin plays a central role in pancreatic exocrine physiology because it acts as the trigger enzyme for the activation of all other pancreatic digestive zymogens, as well as its own inactive precursor, trypsinogen (reviewed in Halfon and Craik 1998).
Historically, trypsin(ogen) has been among the most extensively studied enzyme models of protein structure and function due to its availability in robust quantities from vertebrate pancreas. Bovine trypsin was purified by crystallization in the early 1930s (Northrop and Kunitz 1931), almost the entire amino acid sequence of bovine trypsinogen was determined by protein sequencing in the 1960s (Walsh et al. 1964), and the three-dimensional structure of bovine trypsin was solved in the 1970s (Huber et al. 1974; Stroud, Kay, and Dickerson 1974). The wealth of structural and functional information also made trypsin(ogen) a good model for studying gene and protein evolution (e.g., Hartley et al. 1965; Neurath 1984).
A number of early studies examined the role of the trypsinogen activation peptide, an N-terminal short sequence that is hydrolyzed as the first step in the activation process of trypsinogen. As early as in the 1950s, bovine trypsinogen was shown to be activated through cleavage of an N-terminal hexapeptide, Val-Asp-Asp-Asp-Asp-Lys-Ile, both by bovine trypsin (autoactivation) (Davie and Neurath 1955) and by its physiological activator, enteropeptidase (enterokinase) (Yamashina 1956). The unusual four aspartyl residues (Asp4) preceding the cleavage bond Lys23-Ile24 (human trypsinogen numbering is used throughout the text) were found to be strictly conserved in trypsinogen activation peptides of pig (Charles et al. 1963), sheep (Schyns, Bricteux-Gregoire, and Florkin 1969), and other mammals (e.g., Bricteux-Gregoire, Schyns, and Flokin 1972), including the human (Guy et al. 1976, 1978). However, this is not always the case in nonmammalian vertebrates (e.g., de Haen, Walsh, and Neurath 1977). More recently, a comparative analysis of trypsinogen activation peptides suggested that there might be a progressive increase in selective pressure for such acidic residues during the course of vertebrate evolution (Roach et al. 1997).
Nevertheless, the initial observation that the Asp4 sequence was conserved in the bovine, porcine, and ovine trypsinogen activation peptides stimulated studies on its role in the mechanism of trypsinogen activation. Qualitative studies using small synthetic peptides indicated that Asp4 slowed significantly the hydrolysis of the Lys23-Ile24 bond by bovine trypsin (Abita, Delagge, and Lazdunski 1969) but enhanced the action of enteropeptidase (Maroux, Baratti, and Desnuelle 1971). These results suggested that although Asp4 might play a protective role against occasional activation of the zymogen within the pancreas (Abita, Delagge, and Lazdunski 1969), it must constitute the signal for specific activation of trypsinogen by enteropeptidase in the duodenum (Maroux, Baratti, and Desnuelle 1971).
Recent advances in two different areas have provided unique opportunities for an in-depth evaluation of this important issue. On the one hand, two pancreatitis-associated mutations K23R (Ferec et al. 1999) and D22G (Teich et al. 2000) were identified in the activation peptide of human cationic trypsinogen (Online Mendelian Inheritance in Man [OMIM] 276000; http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM). On the other hand, a large number of trypsinogen sequences have been isolated from phyla and species that extend over the whole range of evolution from bacteria to mammals (reviewed in Halfon and Craik 1998). Whereas analyzing the effects of disease-associated mutations is helpful in understanding the evolutionary forces that have shaped gene products, comparative genomic analysis traces the evolutionary patterns and processes that affected those genes in the past. The two types of information are complementary, and their integration results in a clearer picture about gene evolution. This work represents such an attempt. We analyzed the functional consequences of a newly identified activation peptide mutation, D19A, as well as the previously found D22G and K23R mutations in recombinant human cationic trypsinogen. The functional data, together with a comprehensive sequence comparison, shed further light on the molecular evolution and biology of trypsinogen activation peptides.
Materials and Methods
Mutation Nomenclature
Mutations were named according to their actual position in the native, wild-type human preproenzyme (i.e., pretrypsinogen; see figure 1 for a brief introduction to the biogenesis of trypsin). A description of the new standard system with the translation initiator methionine numbered as 1 and its conversion to the conventional chymotrypsin numbering system can be found in figure 1 in Chen and Ferec (2000).
Plasmids and Mutagenesis
Construction of the pTrap-T7 expression plasmid harboring the wild-type human cationic trypsinogen gene was described previously (Sahin-Tóth 2000; Sahin-Tóth and Tóth 2000). Mutations D19A, D22G, and K23R were introduced by linker mutagenesis. Synthetic linkers carrying given mutations were ligated into the pTrap-T7 expression plasmid between restriction sites Nco I and EcoR I.
Expression and Purification of Trypsinogen
Small-scale expression and in vitro refolding of human cationic trypsinogen and mutants D19A, D22G, and K23R were carried out as reported previously (Sahin-Tóth 2000, 2001; Sahin-Tóth and Tóth 2000). Concentrations of zymogen solutions were measured from their ultraviolet absorbance using a calculated extinction coefficient of 36,160 M−1 cm−1 at 280 nm. The activation peptide sequence of recombinant zymogen preparations used in this study was Met-Ala-Pro-Phe-(Asp)4-Lys.
Trypsinogen Autoactivation
Aliquots of wild-type or mutant human cationic trypsinogen (2 μM final concentrations) were incubated at 37°C in 0.1 M Tris-HCl (pH 8.0) or 0.1 M Na-acetate buffer (pH 5.0), in the absence or presence of 1 mM CaCl2 in a final volume of 100 μl. At indicated times, 2.5 μl aliquots were removed for trypsin activity assays. Trypsin activity was determined using the synthetic chromogenic substrate N-CBZ-Gly-Pro-Arg-p-nitroanilide (Sigma, St. Louis, Mo.) at 140 μM final concentration. Kinetics of the chromophore release was followed at 405 nm in 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2, at 22°C. To prevent nonspecific binding of trypsinogen to tube walls at pH 5.0, bovine serum albumin (2 mg/ml) was included in the activation mixtures (see figure 4A in Kukor et al. 2002a).
Activation of Recombinant Trypsinogen with Enteropeptidase
Under conditions that are optimal to assay enteropeptidase activation, the trypsinogen mutants studied here exhibited a strong tendency for autoactivation. To minimize this confounding factor, enteropeptidase activation was determined by continuous monitoring of trypsin generation at dilute trypsinogen concentrations, as described previously (Sahin-Tóth 2000). Approximately 100 nM wild-type or mutant cationic trypsinogen (final concentrations) were mixed with 100 ng/ml enteropeptidase (final concentration) in a microplate well containing 200 μl of 0.1 M Tris-HCl (pH 8.0), 1 mM CaCl2, and 180 μM synthetic trypsin substrate N-CBZ-Gly-Pro-Arg-p-nitroanilide (final concentrations). The activation reaction was followed at 22°C by continuous monitoring of p-nitroanilide release at 405 nm as a measure of trypsin activity. Trypsinogen samples without added enteropeptidase exhibited a small increase in their absorbance readings over the 1 min period, and these values were subtracted from the absorbance readings of the enteropeptidase treated samples.
Acquisition of Trypsinogen Activation Peptide Sequences
Trypsinogen activation peptide sequences were mainly collated from major online protein databanks. The sequences were identified by searching with the keywords “trypsin” and “trypsinogen” separately in both GenBank and Swiss-Prot. In addition, several vertebrate activation peptide sequences were gathered through an extensive search of the literature.
To identify the functionally most relevant trypsinogen genes, target sequences were manually evaluated and selected for comparative analysis based upon the following criteria. First, only genes that have been physically identified at the mRNA and/or protein level were included. Second, if more than three trypsinogen genes were physically identified in a species, usually the first reported three were included for sequence comparison. Finally, all of the “fast-evolving” group III trypsinogen genes in cold-adapted fishes (Roach 2002 and references therein) were treated separately.
Uncertainties or mistakes in the designation of certain activation peptides were resolved or corrected by predicting the cleavage site for the removal of the signal peptide using the computer program SignalP version 2.0.b2 (Nielsen et al. 1997) and by multiple sequence alignments using ClustalW.
Results and Discussion
Identification of a New Pancreatitis-Associated Activation Peptide Mutation in the Human Cationic Trypsinogen
Chronic pancreatitis has long been presumed to be an autodigestive disease, in which prematurely activated trypsin within the pancreas plays a critical role in initiating pancreatic autodigestion. This notion received strong support when a missense mutation, R122H, in the human cationic trypsinogen was found to cause hereditary pancreatitis, a rare form of chronic pancreatitis (Whitcomb et al. 1996). The R122H mutation eliminates the primary autolysis cleavage site of trypsin that was postulated to act as an important “self-destruct” defensive mechanism against prematurely activated trypsin. In addition, the R122H mutation was shown to stimulate autoactivation of cationic trypsinogen (Sahin-Tóth and Tóth 2000). Similarly, further hereditary pancreatitis-associated mutations identified in the cationic trypsinogen are also believed to be “gain-of-function” mutations that may enhance trypsinogen autoactivation and/or inhibit trypsin degradation within the pancreas (for recent reviews, see Chen, Montier, and Férec 2001, Sahin-Tóth 2001, and Schneider and Whitcomb 2002).
In our routine screening for pancreatitis-associated genetic risk factors, a novel mutation termed D19A (GenBank accession number AY234116) was found in the activation peptide sequence of the human cationic trypsinogen gene from a 27-year-old white French patient with chronic pancreatitis. Details on patient information and mutation identification are available in Supplementary Material online. Figure 1 indicates the location of the D19A mutation and the two previously found D22G and K23R mutations in the trypsinogen activation peptide.
Functional Analysis of the D19A, D22G, and K23R Activation Peptide Mutants in the Context of Recombinant Human Cationic Trypsinogen
Early studies on the bovine trypsinogen activation peptide were performed using small synthetic peptides as models (Abita, Delagge, and Lazdunski 1969; Maroux, Baratti, and Desnuelle 1971). The same approach was used to analyze the two previously identified D22G and K23R mutations (Teich et al. 2000; Teich, Bodeker, and Keim 2002). Recently, methodology has been developed for the recombinant expression, in vitro refolding, and purification of human cationic trypsinogen. This type of recombinant trypsinogen preparation has been used in a growing number of studies that investigated the effects of hereditary pancreatitis-associated mutations (Sahin-Tóth 2000; Sahin-Tóth and Tóth 2000; Szilágyi et al. 2001; Kukor et al. 2002a, 2002b; Simon et al. 2002). It was further employed in the present study to analyze the functional effects of the three activation peptide mutations.
Effects of the Three Activation Peptide Mutations on Trypsinogen Autoactivation
We first analyzed the effects of the three activation peptide mutations on trypsinogen autoactivation. As shown in figure 2A, at pH 8.0, all three activation peptide mutations significantly increased autoactivation of human cationic trypsinogen. Increased activation was particularly striking with mutations D22G and K23R. Addition of 1 mM Ca2+ stimulated autoactivation of wild-type trypsinogen and mutant D19A (fig. 2B). In contrast, only a slight stimulation was evident with mutant K23R, whereas autoactivation of mutant D22G was marginally inhibited by the divalent cation. The effect of the mutations on autoactivation was also examined at pH 5.0 (fig. 2C), since hereditary pancreatitis-associated mutations studied previously caused increased autoactivation rates in an acidic milieu (Sahin-Tóth 2000, 2001; Sahin-Tóth and Tóth 2000; Szilágyi et al. 2001). Again, all three mutations increased autoactivation, although the stimulation by mutation D19A was less pronounced at this pH.
Our results on recombinant trypsinogens are complimentary with previous peptide studies. Model peptides that contain neutral residues in place of the Asp residues are cleaved by trypsin with increased efficiency (Delaage et al. 1967; Abita, Delagge, and Lazdunski 1969; Teich et al. 2000); chemical modifications that abolish the charge on the carboxylates increase trypsinogen activation by trypsin (Radhakrishnan, Walsh, and Neurath 1969); millimolar concentrations of Ca2+, which binds to the activation peptide and shields the negative charges, also increase trypsinogen activation (Radhakrishnan, Walsh, and Neurath 1969; Sahin-Tóth 2000; Sahin-Tóth and Tóth 2000). These observations, taken together, suggested that mutations that neutralize any of the Asp4 residues in the activation peptide are expected to cause increased autoactivation of trypsinogen. The more marked effect of the D22G mutation compared with the D19A mutation is readily explained by its proximity to the Lys23-Ile24 scissile bond.
Our finding that the K23R mutant trypsinogen exhibited dramatically increased autoactivation is consistent with the known Arg preference of trypsin. A model peptide carrying the K23R substitution was also cleaved by trypsin at an enhanced rate (Teich et al. 2000). The primary (S1) specificity pocket of trypsin interacts with positively charged side chains, such as Lys or Arg. Trypsin has a twofold to 10-fold preference for Arg over Lys at the P1 position of peptide substrates (Craik et al. 1985). The structural basis for this phenomenon is the stronger interactions of the Arg side chain with Asp194 (Asp189 in chymotrypsin numbering) at the bottom of the specificity pocket. Thus, the guanidinium group of a P1 Arg can form direct interactions with the carboxylate group of Asp194, whereas the contact between the amino group of a P1 Lys and Asp194 is mediated by a water molecule. In addition, the observation that Ca2+ does not cause significant further stimulation of autoactivation in the K23R mutant also suggests that the perfect geometry of the Arg23-Asp194, P1-S1 interaction counteracts the inhibitory effect of the tetra-aspartate residues.
In summary, functional characterization of the three pancreatitis-associated activation peptide mutations confirmed the hypothesis that the evolutionarily conserved, negatively charged tetra-aspartate sequence in mammalian trypsinogens protects against trypsinogen autoactivation within the pancreas (Abita, Delagge, and Lazdunski 1969). The use of Lys instead of Arg at the P1 position of human cationic trypsinogen activation peptide also proved to have such a protective effect.
Effects of the Three Activation Peptide Mutations on Trypsinogen Activation with Enteropeptidase
It is possible that the well-conserved Asp4 sequence in mammalian trypsinogen activation peptides is strongly selected for enteropeptidase recognition. Thus, we also analyzed the effects of the three activation peptide mutations on activation of trypsinogen by the physiological activator enteropeptidase. In these experiments, trypsinogen was incubated with bovine enteropeptidase in the presence of a synthetic trypsin substrate (N-CBZ-Gly-Pro-Arg-p-nitroanilide) and development of trypsin activity was continuously monitored by following release of the yellow p-nitroanilide. In this assay, trypsinogen concentrations were relatively low; thus, interference from autoactivation was less likely to occur. As shown in figure 3, at pH 8.0 in 1 mM Ca2+, the D19A mutation had no significant effect on enteropeptidase activation. In contrast, mutation K23R stimulated and mutation D22G almost completely inhibited enteropeptidase-mediated trypsinogen activation.
These results are largely consistent with an early study showing that cleavage by enteropeptidase requires two acidic residues at P2 and P3 in addition to a Lys or an Arg at P1. Replacement of Asp by alanine at P2 and P3 completely inhibited the action of enteropeptidase. An acidic residue at P4 was less essential, because its replacement by valine merely reduced the affinity of enteropeptidase for the peptide substrate (Maroux, Baratti, and Desnuelle 1971). The crystal structure of the enteropeptidase catalytic subunit provided insight into how the activation peptide of trypsinogen might interact with the activator enzyme (Lu et al. 1999). The critical interaction is a salt bridge formed between Lys99 of enteropeptidase (chymotrypsin numbering) and Asp-P2 and Asp-P4 of the trypsinogen activation peptide. In addition, Asp-P3 forms a hydrogen bond with Tyr174 on enteropeptidase. Thus, out of the four Asp residues, only three appear to be essential for enteropeptidase recognition. In complete agreement with these structural indications, the D22G mutant, which is unable to form the critical salt bridge, was very poorly activated by enteropeptidase and the D19A mutant had essentially no effect on enteropeptidase activation. In contrast, activation of K23R was increased, in all likelihood due to the better fit of the Arg side chain into the primary specificity pocket of enteropeptidase.
Integration of Functional Characterization of Disease-Associated Mutations with Comparative Sequence Analysis of Trypsinogen Activation Peptides
Table 1 is a summary of currently available trypsinogen activation peptide sequences we collated from major databanks and original publications. Since trypsinogens found in bacteria and fungi do not possess a “typical” activation peptide, only two representative sequences, one from the bacterium Streptomyces griseus and one from the mold Fusarium oxysporum, were included in the table. In addition, although multiple trypsinogen genes are present in several insects, nearly all insect trypsinogen activation peptides terminate in Gly at P2 and Arg at P1; therefore, only one sequence from Drosophila melanogaster was listed.
Stepwise Evolution of Trypsinogen Activation Peptides from Bacteria to Mammals
Since trypsin was first isolated from the cattle, it has been identified in a wide range of species, including mammals, birds, amphibians, true fishes, dogfish, sea lamprey, urochordates, crustaceans, insects, fungi, and bacteria (table 1). The fact that homology between the bacterial Streptomyces griseus trypsin and mammalian trypsins is higher than between trypsin and related enzymes within Streptomyces griseus has once led to a hypothesis that a gene transfer has taken place from a mammal to a bacterium (Hartley 1970, 1979). However, later structural and phylogenetic analysis established that there was a continuous evolutionary divergence of trypsin from a common ancestor of both prokaryotes and eukaryotes and thus resolved the question of the origin of trypsin in prokaryotes (Rypniewski et al. 1994 and references therein). Apparently, this is also the case in the molecular evolution of trypsinogen activation peptides, as will be discussed in the following sections.
Lys in the P1 Position Has Evolved to Minimize Trypsinogen Autoactivation
As shown in table 1, trypsinogen activation peptides in bacteria and fungi contain no Lys or Arg residues at the P1 position. Consequently, they are not capable of autocatalyzing their own activation. Although these trypsins are significantly different from the pancreatic trypsins of mammals, they can hydrolyze synthetic peptide substrates having arginine or lysine at the P1 position and can be inhibited by typical trypsin inhibitors (e.g., Lee, Kang, and Kim 1998).
All other species, with the exception of the sea lamprey, have evolved the autoactivating property within trypsinogen, characterized by the presence of Lys or Arg as the P1 residue in their activation peptides. At the present time, it is not clear why trypsinogens adapted the ability to autoactivate, although one may speculate that this may be a mechanism initially evolved for activating trypsinogen. In support of this view is the observation that in fungi, insects, and crustaceans, trypsinogen activation peptides lack an acidic residue at P2 (table 1) and thus may not be recognized by enteropeptidase.
Nevertheless, based upon the absence of an Arg-P1 residue in the vast majority of trypsinogen activation peptides (table 1), the biochemical and structural data demonstrating the preference of trypsin for Arg over Lys, and the association of the K23R mutation in the human cationic trypsinogen with pancreatitis, it seems reasonable to conclude that Lys at P1 has evolved to minimize autoactivation within the pancreas. In contrast, the selective use of Lys at P1 is clearly not critical for enteropeptidase recognition, since an activation peptide having Arg at P1 is more readily activated by enteropeptidase. This evolutionary scenario suggests that even a minor increase in trypsinogen autoactivation within the pancreas may be potentially dangerous, whereas a small decrease in affinity for enteropeptidase is inconsequential due to its high catalytic activity and relative abundance in the duodenum.
An intriguing exception in the evolution of trypsinogen activation peptides is observed in the sea lamprey, one of the most primitive vertebrates. Strikingly, all of its five trypsinogen activation peptides end in a histidine (see table 1), a residue after which trypsin or enteropeptidase cannot cleave. As suggested by Roach et al. (1997), this exception may be associated with the sea lamprey's life cycle, during which adults can go months to years without eating. Therefore, there may exist a different, tightly controlled mechanism for trypsinogen activation in the sea lamprey. Alternatively, these trypsinogens may not be activated, indicating a possible divergence from the digestive function of trypsins, analogous to the group III trypsinogens of cold-adapted fishes (discussed later).
The Asp4 Sequence in Mammalian Trypsinogen Activation Peptides Has Evolved to Inhibit Autoactivation and to Enhance Enteropeptidase Cleavage
The comprehensive comparative analysis of trypsinogen activation peptides performed in this study revealed how the Asp4 sequence strictly conserved in mammalian trypsinogen activation peptides has evolved progressively. As indicated in table 1, the insect and crustacean activation peptides do not possess an acidic residue immediately before Lys-P1 or Arg-P1. The two urochordates, tunicate and star ascidian, have an Asp residue at P2. Additional Asp residues have gradually evolved during the course of vertebrate evolution: two in the sea lamprey; three or four in fishes, amphibians, and birds; and strictly four in the mammals. The adaptation and subsequent increase of the number of Asp residues preceding the Lys23-Ile24 cleavage bond is associated with a progressively decreased tendency to autoactivate, as evidenced by available biochemical and structural data on wild-type, D19A, and D22G mutant trypsinogens.
An interesting exception to the rule is the presence of the Asp4 motif in the primitive vertebrate dogfish. We could not exclude the possibility that the common ancestor of the dogfish and Osteicthyes possessed the tetra-aspartyl sequence in its trypsinogen activation peptide. Nevertheless, based upon the general evolutionary pattern of trypsinogen activation peptides from bacteria to mammals, we believe that the presence of the Asp4 sequence in the dogfish is a coincidence. Alternatively, this may be due to convergent evolution resulting from common selective pressures. Additional trypsinogen activation peptide sequences from the most vertebrate-like invertebrates such as hagfish and Amphioxus and other species of Chondrichthyes may help to clarify this issue.
Fixed Substitutions in Key Residues of Trypsinogen Activation Peptides May Suggest the Evolution of New Functions
The pancreatitis-associated activation peptide mutations are rare alleles in the population that are constantly selected against. However, fixed substitutions in the key residues of the trypsinogen activation peptide may suggest the evolution of new functions unrelated to digestion.
Vertebrate trypsins have been classified into two groups according to their genomic location and, to a lesser degree, their charge at physiologic pH. Group I trypsins are usually, but not always, anionic at physiologic pH, whereas group II trypsins are usually, but not always, cationic at physiologic pH (Roach 2002 and references therein). However, some trypsin sequences determined from fishes that spend all or part of their lives at temperatures near 0°C cannot be classified into either of these two groups and were designated as group III (table 2). Principal component analysis of amino acid compositions, molecular trees, and multidimensional scaling of molecular sequence distances indicated that group III trypsins have evolved recently from other vertebrate trypsins. The increased rate of evolution in these trypsins, relative to group I and group II trypsins, might be correlated with the development of a new function—possibly extreme cold adaptation (Roach 2002 and references therein).
The pattern of fixed sequence changes in key residues of the activation peptide in group III trypsinogens correlates perfectly with the evolutionary relationship between these trypsins and the other fish trypsins and also between themselves (see figures 1, 2, and 3 in Roach 2002). As shown in table 2, the group III trypsinogen activation peptides are characterized by the presence of an Arg at P1 and a deletion or a substitution of Asp at P2, which distinguishes them from the other fish trypsinogens, whose activation peptides terminate with an invariable Lys at P1 and contain at least three acidic residues immediately before P1 (table 1). The deletion of a well-conserved Asp residue in the Japanese flounder trypsinogen versus the substitution of Asp by Gly at P2 in the other five species (table 2) is consistent with the separation of the former trypsin from the five clustered trypsins in multidimensionally scaled trypsin pairwise molecular distances (see figure 3 in Roach 2002). In addition, the fact that the activation peptides in group III trypsinogens have two adjacent acidic residues supports the view that group III trypsins have a common ancestor with other vertebrate trypsins.
Interestingly, the key substitutions in the group III trypsinogen activation peptides are Asp to Gly at P2 and Lys to Arg at P1 with respect to the other fish trypsinogens and the mammalian trypsinogens. Since Gly-P2 and Arg-P1 are present only in insect and salmon louse trypsinogens (table 1), and the D22G and K23R mutations in the human cationic trypsinogen have been associated with pancreatitis, it appears that group III trypsinogens are dangerous to the organism. We believe that concomitant evolutionary changes within the trypsin sequence might have counteracted the potentially deleterious effects of the new activation peptides. As a result, the group III “trypsins,” whose primary structure is notably different from those of the other fish trypsins, might function differently than the classic pancreatic trypsins. Therefore, it is important to make a distinction between rare alleles that encode functional digestive trypsinogens and fixed alleles that may have evolved new functions, possibly unrelated to digestion.
Conclusion
In summary, we reported a new pancreatitis-associated activation peptide mutation, D19A, in the human cationic trypsinogen. This new mutation, as well as the previously found D22RG and K23R mutations, was, for the first time, functionally evaluated in the context of recombinant human cationic trypsinogen. The functional data extended the previous observations on the role of the well-conserved Asp4 sequence in mammalian trypsinogen activation peptides and, more importantly, clarified the driving force behind this strong natural selection. Incorporation of this information into a comprehensive sequence comparison provided further insights into the molecular evolution and biology of trypsinogen activation peptides. Our most important findings indicate that the Asp4 sequence in the mammalian trypsinogen activation peptides has evolved for both efficient inhibition of trypsinogen autoactivation within the pancreas and optimal enteropeptidase recognition in the duodenum. The use of Lys at the P1 position has also an advantageous effect against trypsinogen autoactivation. Furthermore, we proposed that fixed substitutions in the key residues of the trypsinogen activation peptide may suggest the evolution of new functions. Finally, more information on the structure, function, and evolution of enteropeptidase is essential for a full picture of the molecular evolution of trypsinogen activation peptides, the evolution of the P1 and P2 positions in particular.
Diethard Tautz, Associate Editor

Biogenesis of pancreatic trypsins, exemplified by the human cationic trypsin. Pretrypsinogen is synthesized on ribosomes attached to the pancreatic acinar cell's rough endoplasmic reticulum (RER). The removal of the signal peptide (italics) takes place cotranslationally in the RER. Trypsinogen is then transported to the Golgi complex, concentrated and stored within secretory granules, secreted into the pancreatic duct, and ultimately discharged into the duodenal space, where it is activated by enteropeptidase. The signal-peptidase and enteropeptidase proteolytic cleavage sites are indicated by vertical arrows. The amino acid residues of the activation peptide are shaded in black and numbered P1, P2, P3, etc., from the scissile bond toward the N terminus (Berger and Schechter 1970). The figure also shows the positions of a novel mutation (D19A) identified in this study and the two previously reported D22G and K23R activation peptide mutations
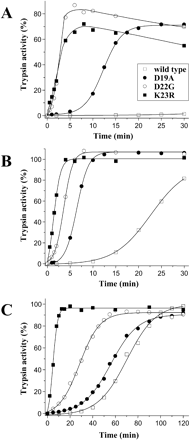
Effect of activation peptide mutations on autoactivation of human cationic trypsinogen. Approximately 2 μM wild-type or mutant trypsinogen (final concentration in a final volume of 100 μl) was incubated at 37°C, in 0.1 M Tris-HCl (pH 8.0) with no Ca2+ added (A) or with 1 mM CaCl2 (B) or in 0.1 M Na-acetate buffer (pH 5.0) in the presence of 2 mg/ml bovine serum albumin (C). Aliquots of 2.5 μl were withdrawn from reaction mixtures at indicated times and trypsin activity was determined with the synthetic substrate N-CBZ-Gly-Pro-Arg-p-nitroanilide. Activity was expressed as percentage of the potential total activity, as determined on similar zymogen samples activated with enteropeptidase
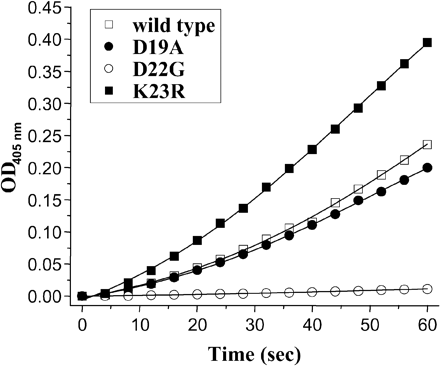
Effect of activation peptide mutations on activation of human cationic trypsinogen with enteropeptidase. Approximately 100 nM wild-type or mutant trypsinogen (final concentration) was activated with 100 ng/ml bovine enteropeptidase (final concentration) as described in Materials and Methods. The activation reaction was followed by continuous monitoring of p-nitroanilide release at 405 nm as a measure of trypsin activity
| Species . | Activation Peptide Sequences . | GenBank or Swiss-Prot Accession Number . | Reference . | |||
|---|---|---|---|---|---|---|
| Mammals | ||||||
| Human (Homo sapiens) | ||||||
| Cationic | APFDDDDK | —a | Guy et al. 1978 | |||
| P07477 | Emi et al. 1986 | |||||
| Anionic | APFDDDDK | — | Guy et al. 1976 | |||
| P07478 | Emi et al. 1986 | |||||
| Mesotrypsinogen | VPFDDDDK | BAA08257 | Nyaruhucha, Kito, and Fukuoka 1997 | |||
| Cow (Bos taurus) | ||||||
| Cationic | FPVDDDDKb | — | Davie and Neurath 1955 | |||
| P00760 | Okajima et al. 1994c | |||||
| Anionic | FPSDDDDK | — | Louvard and Puigserver 1974 | |||
| Q29463 | Le Huerou et al. 1990 | |||||
| Goat (Capra hircus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971a | |||
| Sheep (Ovis aries) | FPVDDDDK | — | Schyns, Bricteux-Gregoire, and Florkin 1969 | |||
| Roe deer (Capreolus capreolus) | FPVDDDDK | — | Bricteux-Gregoire 1970 | |||
| Red deer (Cervus elaphus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971b | |||
| Dromedary (Camelus dromedarius) | VPIDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971c | |||
| Pig (Sus scrofa) | ||||||
| Cationic | FPTDDDDK | P00761 | Charles et al. 1963 | |||
| Anionic | FPTDDDDK | — | Louvard and Puigserver 1974 | |||
| Lesser rorqual (Balaenoptera acutorostrata) | FPIDDDDK | A61328 | Bricteux-Gregoire et al. 1975 | |||
| Elephant seal (Mirounga leonina L.) | FPTDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1974 | |||
| Dog (Canis familiaris) | ||||||
| Cationic | FPIDDDDK | P06871 | Pinsky, LaForge, and Scheele 1985 | |||
| Anionic | TPTDDDDK | P06872 | Pinsky, LaForge, and Scheele 1985 | |||
| Cat (Felis catus) | FPIDDDDK | P81071 | Steiner, Medinger, and Williams 1997 | |||
| Norway rat (Rattus norvegicus) | ||||||
| I | FPLEDDDK | P00762 | MacDonald, Stary, and Swift 1982 | |||
| II | FPVDDDDK | P00763 | MacDonald, Stary, and Swift 1982 | |||
| III | LPLDDDDDK | P08426 | Fletcher et al. 1987 | |||
| Mouse | FPVDDDDK | P07146 | Stevenson, Hagenbuchle, and Wellauer 1986 | |||
| Horse (Equus caballus) | SSTDDDDK | — | Harris and Hofmann 1969 | |||
| Birds | ||||||
| Chicken (Gallus gallus) | ||||||
| 1 | FPISDEDDDK | Q90627 | Wang et al. 1995 | |||
| 2 | FPISDEDDDK | Q90628 | Wang et al. 1995 | |||
| 3 | FPGGADDDK | Q90629 | Wang et al. 1995 | |||
| Ostritch (Struthioniformes) | VPGDADDDK | AAB35161 | Bodley et al. 1995 | |||
| Amphibians | ||||||
| African clawed frog (Xenopus laevis) | ||||||
| I | FDDDK | P19799 | Shi and Brown 1990 | |||
| II | FEDDDK | P70059 | Wang et al. 1996c | |||
| Fishes | ||||||
| African lungfish (Protopterus aethiopicus) | ||||||
| A | LPLEDDK | — | de Haen, Walsh, and Neurath 1977 | |||
| B | FPIEEDK | P35051 | Hermodson et al. 1971 | |||
| Maori cod (Paranotothenia magellanica) | FATEDDK | S49489 | Genicot et al. 1996 | |||
| Winter flounder (Pseudopleuronectes americanus) | FALEDDK | AAC32752 | Douglas and Gallant 1998 | |||
| Japanese flounder (Paralichthys olivaceus) | ||||||
| 1 | FAMEDDK | BAA82362 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| 2 | FATEDDK | BAA82363 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| Pufferfish (Takifugu rubripes) | PIDEDDK | TRU25747 | Roach et al. 1997 | |||
| Atlantic cod (Gadus morhua) | ||||||
| I | VFAEEDK | S39047 | Gudmundsdottir et al. 1993 | |||
| X | VFAEEDK | S39048 | Gudmundsdottir et al. 1993 | |||
| Atlantic salmon (Salmo salar) | ||||||
| I | FATEDDK | P35031 | Male et al. 1995 | |||
| II | FATEDDK | P35032 | Male et al. 1995 | |||
| III | PIDDEDDK | P35033 | Male et al. 1995 | |||
| Zebrafish (Danio rerio) | APLGDDDDK | AAO16212 | Wan and Gong 2002c | |||
| Japanese anchovy (Engraulis japonicus) | ||||||
| 1 | FAEDDK | BAB40329 | Watabe, Ahsan, and Funabara 2000c | |||
| 2 | FAEDDK | BAB40330 | Watabe, Ahsan, and Funabara 2000c | |||
| Japanese eel (Anguilla japonica) | VALDDDK | Q8QGW3 | Kurokawa et al. 2001c | |||
| Primitive vertebrates | ||||||
| Spiny dogfish (Squalus acanthias) | APDDDDK | — | Bradshaw et al. 1970 | |||
| P00764 | Titani et al. 1975 | |||||
| Sea lamprey (Petromyzon marinus) | ||||||
| A1 | APYMYEDH | AAB65411 | Roach et al. 1997 | |||
| A2 | APYMYEDH | AAB69654 | Roach et al. 1997 | |||
| A3 | APYMYEDH | AAB69655 | Roach et al. 1997 | |||
| B1 | APYMYEDH | AAB69656 | Roach et al. 1997 | |||
| B2 | APYMYEDH | AAB69657 | Roach et al. 1997 | |||
| Urochordates | ||||||
| Tunicate (Boltenia villosa) | AVNADK | AAB69653 | Roach et al. 1997 | |||
| Star ascidian (Botryllus schlosseri) | ||||||
| 1 | FCGANADK | O01309 | Pancer et al. 1996 | |||
| 2 | LYGANADK | O01310 | Pancer et al. 1996 | |||
| Crustaceans | ||||||
| Red king crab (Pacifastacus camtschaticus) | APSRKPTFRRGLNK | AF461036 | Rebrikov 2001c | |||
| Signal crayfish (Pacifastacus leniusculus) | APSRRLRFPPNNYK | CAA10915 | Hernandez-Cortes et al. 1999 | |||
| Pacific white shrimp (Litopenaeus vannamei)d | ||||||
| APSRKPTFRRGLNK | CAA60129 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75310 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75311 | Klein et al. 1996 | ||||
| Salmon louse (Lepeophtheirus salmonis) | ||||||
| Type 1 | VPPQIKYSESFMKVKSMRHRFGGR | AAL71875 | Johnson et al. 2002 | |||
| Type 2 | VPRIKYSDSFMKVKSMRHRFGGR | AAL71876 | Johnson et al. 2002 | |||
| Type 3 | VPRIKYSESFMKVKSMRHRFGGR | AAL71877 | Johnson et al. 2002 | |||
| Insects | ||||||
| Fruit fly (Drosophilamelanogaster)e | LLPQLDGR | M96372 | Wang, Magoulas, and Hickey 1999 | |||
| Fungi | ||||||
| Fusarium oxysporume | APQEIPN | P35049 | Rypniewski et al. 1993 | |||
| Bacteria | ||||||
| Streptomyces griseuse | APNP | P00775 | Kim et al. 1991 | |||
| Species . | Activation Peptide Sequences . | GenBank or Swiss-Prot Accession Number . | Reference . | |||
|---|---|---|---|---|---|---|
| Mammals | ||||||
| Human (Homo sapiens) | ||||||
| Cationic | APFDDDDK | —a | Guy et al. 1978 | |||
| P07477 | Emi et al. 1986 | |||||
| Anionic | APFDDDDK | — | Guy et al. 1976 | |||
| P07478 | Emi et al. 1986 | |||||
| Mesotrypsinogen | VPFDDDDK | BAA08257 | Nyaruhucha, Kito, and Fukuoka 1997 | |||
| Cow (Bos taurus) | ||||||
| Cationic | FPVDDDDKb | — | Davie and Neurath 1955 | |||
| P00760 | Okajima et al. 1994c | |||||
| Anionic | FPSDDDDK | — | Louvard and Puigserver 1974 | |||
| Q29463 | Le Huerou et al. 1990 | |||||
| Goat (Capra hircus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971a | |||
| Sheep (Ovis aries) | FPVDDDDK | — | Schyns, Bricteux-Gregoire, and Florkin 1969 | |||
| Roe deer (Capreolus capreolus) | FPVDDDDK | — | Bricteux-Gregoire 1970 | |||
| Red deer (Cervus elaphus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971b | |||
| Dromedary (Camelus dromedarius) | VPIDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971c | |||
| Pig (Sus scrofa) | ||||||
| Cationic | FPTDDDDK | P00761 | Charles et al. 1963 | |||
| Anionic | FPTDDDDK | — | Louvard and Puigserver 1974 | |||
| Lesser rorqual (Balaenoptera acutorostrata) | FPIDDDDK | A61328 | Bricteux-Gregoire et al. 1975 | |||
| Elephant seal (Mirounga leonina L.) | FPTDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1974 | |||
| Dog (Canis familiaris) | ||||||
| Cationic | FPIDDDDK | P06871 | Pinsky, LaForge, and Scheele 1985 | |||
| Anionic | TPTDDDDK | P06872 | Pinsky, LaForge, and Scheele 1985 | |||
| Cat (Felis catus) | FPIDDDDK | P81071 | Steiner, Medinger, and Williams 1997 | |||
| Norway rat (Rattus norvegicus) | ||||||
| I | FPLEDDDK | P00762 | MacDonald, Stary, and Swift 1982 | |||
| II | FPVDDDDK | P00763 | MacDonald, Stary, and Swift 1982 | |||
| III | LPLDDDDDK | P08426 | Fletcher et al. 1987 | |||
| Mouse | FPVDDDDK | P07146 | Stevenson, Hagenbuchle, and Wellauer 1986 | |||
| Horse (Equus caballus) | SSTDDDDK | — | Harris and Hofmann 1969 | |||
| Birds | ||||||
| Chicken (Gallus gallus) | ||||||
| 1 | FPISDEDDDK | Q90627 | Wang et al. 1995 | |||
| 2 | FPISDEDDDK | Q90628 | Wang et al. 1995 | |||
| 3 | FPGGADDDK | Q90629 | Wang et al. 1995 | |||
| Ostritch (Struthioniformes) | VPGDADDDK | AAB35161 | Bodley et al. 1995 | |||
| Amphibians | ||||||
| African clawed frog (Xenopus laevis) | ||||||
| I | FDDDK | P19799 | Shi and Brown 1990 | |||
| II | FEDDDK | P70059 | Wang et al. 1996c | |||
| Fishes | ||||||
| African lungfish (Protopterus aethiopicus) | ||||||
| A | LPLEDDK | — | de Haen, Walsh, and Neurath 1977 | |||
| B | FPIEEDK | P35051 | Hermodson et al. 1971 | |||
| Maori cod (Paranotothenia magellanica) | FATEDDK | S49489 | Genicot et al. 1996 | |||
| Winter flounder (Pseudopleuronectes americanus) | FALEDDK | AAC32752 | Douglas and Gallant 1998 | |||
| Japanese flounder (Paralichthys olivaceus) | ||||||
| 1 | FAMEDDK | BAA82362 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| 2 | FATEDDK | BAA82363 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| Pufferfish (Takifugu rubripes) | PIDEDDK | TRU25747 | Roach et al. 1997 | |||
| Atlantic cod (Gadus morhua) | ||||||
| I | VFAEEDK | S39047 | Gudmundsdottir et al. 1993 | |||
| X | VFAEEDK | S39048 | Gudmundsdottir et al. 1993 | |||
| Atlantic salmon (Salmo salar) | ||||||
| I | FATEDDK | P35031 | Male et al. 1995 | |||
| II | FATEDDK | P35032 | Male et al. 1995 | |||
| III | PIDDEDDK | P35033 | Male et al. 1995 | |||
| Zebrafish (Danio rerio) | APLGDDDDK | AAO16212 | Wan and Gong 2002c | |||
| Japanese anchovy (Engraulis japonicus) | ||||||
| 1 | FAEDDK | BAB40329 | Watabe, Ahsan, and Funabara 2000c | |||
| 2 | FAEDDK | BAB40330 | Watabe, Ahsan, and Funabara 2000c | |||
| Japanese eel (Anguilla japonica) | VALDDDK | Q8QGW3 | Kurokawa et al. 2001c | |||
| Primitive vertebrates | ||||||
| Spiny dogfish (Squalus acanthias) | APDDDDK | — | Bradshaw et al. 1970 | |||
| P00764 | Titani et al. 1975 | |||||
| Sea lamprey (Petromyzon marinus) | ||||||
| A1 | APYMYEDH | AAB65411 | Roach et al. 1997 | |||
| A2 | APYMYEDH | AAB69654 | Roach et al. 1997 | |||
| A3 | APYMYEDH | AAB69655 | Roach et al. 1997 | |||
| B1 | APYMYEDH | AAB69656 | Roach et al. 1997 | |||
| B2 | APYMYEDH | AAB69657 | Roach et al. 1997 | |||
| Urochordates | ||||||
| Tunicate (Boltenia villosa) | AVNADK | AAB69653 | Roach et al. 1997 | |||
| Star ascidian (Botryllus schlosseri) | ||||||
| 1 | FCGANADK | O01309 | Pancer et al. 1996 | |||
| 2 | LYGANADK | O01310 | Pancer et al. 1996 | |||
| Crustaceans | ||||||
| Red king crab (Pacifastacus camtschaticus) | APSRKPTFRRGLNK | AF461036 | Rebrikov 2001c | |||
| Signal crayfish (Pacifastacus leniusculus) | APSRRLRFPPNNYK | CAA10915 | Hernandez-Cortes et al. 1999 | |||
| Pacific white shrimp (Litopenaeus vannamei)d | ||||||
| APSRKPTFRRGLNK | CAA60129 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75310 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75311 | Klein et al. 1996 | ||||
| Salmon louse (Lepeophtheirus salmonis) | ||||||
| Type 1 | VPPQIKYSESFMKVKSMRHRFGGR | AAL71875 | Johnson et al. 2002 | |||
| Type 2 | VPRIKYSDSFMKVKSMRHRFGGR | AAL71876 | Johnson et al. 2002 | |||
| Type 3 | VPRIKYSESFMKVKSMRHRFGGR | AAL71877 | Johnson et al. 2002 | |||
| Insects | ||||||
| Fruit fly (Drosophilamelanogaster)e | LLPQLDGR | M96372 | Wang, Magoulas, and Hickey 1999 | |||
| Fungi | ||||||
| Fusarium oxysporume | APQEIPN | P35049 | Rypniewski et al. 1993 | |||
| Bacteria | ||||||
| Streptomyces griseuse | APNP | P00775 | Kim et al. 1991 | |||
aDetermined by protein sequencing. No accession number is available.
bInitally identified as VDDDDK by protein sequencing (Davie and Neurath 1955).
cDirect submission to databanks, not included in Literature Cited.
dFormerly Penaeus vannamei.
eRepresentative sequence (refer to the text).
| Species . | Activation Peptide Sequences . | GenBank or Swiss-Prot Accession Number . | Reference . | |||
|---|---|---|---|---|---|---|
| Mammals | ||||||
| Human (Homo sapiens) | ||||||
| Cationic | APFDDDDK | —a | Guy et al. 1978 | |||
| P07477 | Emi et al. 1986 | |||||
| Anionic | APFDDDDK | — | Guy et al. 1976 | |||
| P07478 | Emi et al. 1986 | |||||
| Mesotrypsinogen | VPFDDDDK | BAA08257 | Nyaruhucha, Kito, and Fukuoka 1997 | |||
| Cow (Bos taurus) | ||||||
| Cationic | FPVDDDDKb | — | Davie and Neurath 1955 | |||
| P00760 | Okajima et al. 1994c | |||||
| Anionic | FPSDDDDK | — | Louvard and Puigserver 1974 | |||
| Q29463 | Le Huerou et al. 1990 | |||||
| Goat (Capra hircus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971a | |||
| Sheep (Ovis aries) | FPVDDDDK | — | Schyns, Bricteux-Gregoire, and Florkin 1969 | |||
| Roe deer (Capreolus capreolus) | FPVDDDDK | — | Bricteux-Gregoire 1970 | |||
| Red deer (Cervus elaphus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971b | |||
| Dromedary (Camelus dromedarius) | VPIDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971c | |||
| Pig (Sus scrofa) | ||||||
| Cationic | FPTDDDDK | P00761 | Charles et al. 1963 | |||
| Anionic | FPTDDDDK | — | Louvard and Puigserver 1974 | |||
| Lesser rorqual (Balaenoptera acutorostrata) | FPIDDDDK | A61328 | Bricteux-Gregoire et al. 1975 | |||
| Elephant seal (Mirounga leonina L.) | FPTDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1974 | |||
| Dog (Canis familiaris) | ||||||
| Cationic | FPIDDDDK | P06871 | Pinsky, LaForge, and Scheele 1985 | |||
| Anionic | TPTDDDDK | P06872 | Pinsky, LaForge, and Scheele 1985 | |||
| Cat (Felis catus) | FPIDDDDK | P81071 | Steiner, Medinger, and Williams 1997 | |||
| Norway rat (Rattus norvegicus) | ||||||
| I | FPLEDDDK | P00762 | MacDonald, Stary, and Swift 1982 | |||
| II | FPVDDDDK | P00763 | MacDonald, Stary, and Swift 1982 | |||
| III | LPLDDDDDK | P08426 | Fletcher et al. 1987 | |||
| Mouse | FPVDDDDK | P07146 | Stevenson, Hagenbuchle, and Wellauer 1986 | |||
| Horse (Equus caballus) | SSTDDDDK | — | Harris and Hofmann 1969 | |||
| Birds | ||||||
| Chicken (Gallus gallus) | ||||||
| 1 | FPISDEDDDK | Q90627 | Wang et al. 1995 | |||
| 2 | FPISDEDDDK | Q90628 | Wang et al. 1995 | |||
| 3 | FPGGADDDK | Q90629 | Wang et al. 1995 | |||
| Ostritch (Struthioniformes) | VPGDADDDK | AAB35161 | Bodley et al. 1995 | |||
| Amphibians | ||||||
| African clawed frog (Xenopus laevis) | ||||||
| I | FDDDK | P19799 | Shi and Brown 1990 | |||
| II | FEDDDK | P70059 | Wang et al. 1996c | |||
| Fishes | ||||||
| African lungfish (Protopterus aethiopicus) | ||||||
| A | LPLEDDK | — | de Haen, Walsh, and Neurath 1977 | |||
| B | FPIEEDK | P35051 | Hermodson et al. 1971 | |||
| Maori cod (Paranotothenia magellanica) | FATEDDK | S49489 | Genicot et al. 1996 | |||
| Winter flounder (Pseudopleuronectes americanus) | FALEDDK | AAC32752 | Douglas and Gallant 1998 | |||
| Japanese flounder (Paralichthys olivaceus) | ||||||
| 1 | FAMEDDK | BAA82362 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| 2 | FATEDDK | BAA82363 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| Pufferfish (Takifugu rubripes) | PIDEDDK | TRU25747 | Roach et al. 1997 | |||
| Atlantic cod (Gadus morhua) | ||||||
| I | VFAEEDK | S39047 | Gudmundsdottir et al. 1993 | |||
| X | VFAEEDK | S39048 | Gudmundsdottir et al. 1993 | |||
| Atlantic salmon (Salmo salar) | ||||||
| I | FATEDDK | P35031 | Male et al. 1995 | |||
| II | FATEDDK | P35032 | Male et al. 1995 | |||
| III | PIDDEDDK | P35033 | Male et al. 1995 | |||
| Zebrafish (Danio rerio) | APLGDDDDK | AAO16212 | Wan and Gong 2002c | |||
| Japanese anchovy (Engraulis japonicus) | ||||||
| 1 | FAEDDK | BAB40329 | Watabe, Ahsan, and Funabara 2000c | |||
| 2 | FAEDDK | BAB40330 | Watabe, Ahsan, and Funabara 2000c | |||
| Japanese eel (Anguilla japonica) | VALDDDK | Q8QGW3 | Kurokawa et al. 2001c | |||
| Primitive vertebrates | ||||||
| Spiny dogfish (Squalus acanthias) | APDDDDK | — | Bradshaw et al. 1970 | |||
| P00764 | Titani et al. 1975 | |||||
| Sea lamprey (Petromyzon marinus) | ||||||
| A1 | APYMYEDH | AAB65411 | Roach et al. 1997 | |||
| A2 | APYMYEDH | AAB69654 | Roach et al. 1997 | |||
| A3 | APYMYEDH | AAB69655 | Roach et al. 1997 | |||
| B1 | APYMYEDH | AAB69656 | Roach et al. 1997 | |||
| B2 | APYMYEDH | AAB69657 | Roach et al. 1997 | |||
| Urochordates | ||||||
| Tunicate (Boltenia villosa) | AVNADK | AAB69653 | Roach et al. 1997 | |||
| Star ascidian (Botryllus schlosseri) | ||||||
| 1 | FCGANADK | O01309 | Pancer et al. 1996 | |||
| 2 | LYGANADK | O01310 | Pancer et al. 1996 | |||
| Crustaceans | ||||||
| Red king crab (Pacifastacus camtschaticus) | APSRKPTFRRGLNK | AF461036 | Rebrikov 2001c | |||
| Signal crayfish (Pacifastacus leniusculus) | APSRRLRFPPNNYK | CAA10915 | Hernandez-Cortes et al. 1999 | |||
| Pacific white shrimp (Litopenaeus vannamei)d | ||||||
| APSRKPTFRRGLNK | CAA60129 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75310 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75311 | Klein et al. 1996 | ||||
| Salmon louse (Lepeophtheirus salmonis) | ||||||
| Type 1 | VPPQIKYSESFMKVKSMRHRFGGR | AAL71875 | Johnson et al. 2002 | |||
| Type 2 | VPRIKYSDSFMKVKSMRHRFGGR | AAL71876 | Johnson et al. 2002 | |||
| Type 3 | VPRIKYSESFMKVKSMRHRFGGR | AAL71877 | Johnson et al. 2002 | |||
| Insects | ||||||
| Fruit fly (Drosophilamelanogaster)e | LLPQLDGR | M96372 | Wang, Magoulas, and Hickey 1999 | |||
| Fungi | ||||||
| Fusarium oxysporume | APQEIPN | P35049 | Rypniewski et al. 1993 | |||
| Bacteria | ||||||
| Streptomyces griseuse | APNP | P00775 | Kim et al. 1991 | |||
| Species . | Activation Peptide Sequences . | GenBank or Swiss-Prot Accession Number . | Reference . | |||
|---|---|---|---|---|---|---|
| Mammals | ||||||
| Human (Homo sapiens) | ||||||
| Cationic | APFDDDDK | —a | Guy et al. 1978 | |||
| P07477 | Emi et al. 1986 | |||||
| Anionic | APFDDDDK | — | Guy et al. 1976 | |||
| P07478 | Emi et al. 1986 | |||||
| Mesotrypsinogen | VPFDDDDK | BAA08257 | Nyaruhucha, Kito, and Fukuoka 1997 | |||
| Cow (Bos taurus) | ||||||
| Cationic | FPVDDDDKb | — | Davie and Neurath 1955 | |||
| P00760 | Okajima et al. 1994c | |||||
| Anionic | FPSDDDDK | — | Louvard and Puigserver 1974 | |||
| Q29463 | Le Huerou et al. 1990 | |||||
| Goat (Capra hircus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971a | |||
| Sheep (Ovis aries) | FPVDDDDK | — | Schyns, Bricteux-Gregoire, and Florkin 1969 | |||
| Roe deer (Capreolus capreolus) | FPVDDDDK | — | Bricteux-Gregoire 1970 | |||
| Red deer (Cervus elaphus) | FPVDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971b | |||
| Dromedary (Camelus dromedarius) | VPIDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1971c | |||
| Pig (Sus scrofa) | ||||||
| Cationic | FPTDDDDK | P00761 | Charles et al. 1963 | |||
| Anionic | FPTDDDDK | — | Louvard and Puigserver 1974 | |||
| Lesser rorqual (Balaenoptera acutorostrata) | FPIDDDDK | A61328 | Bricteux-Gregoire et al. 1975 | |||
| Elephant seal (Mirounga leonina L.) | FPTDDDDK | — | Bricteux-Gregoire, Schyns, and Florkin 1974 | |||
| Dog (Canis familiaris) | ||||||
| Cationic | FPIDDDDK | P06871 | Pinsky, LaForge, and Scheele 1985 | |||
| Anionic | TPTDDDDK | P06872 | Pinsky, LaForge, and Scheele 1985 | |||
| Cat (Felis catus) | FPIDDDDK | P81071 | Steiner, Medinger, and Williams 1997 | |||
| Norway rat (Rattus norvegicus) | ||||||
| I | FPLEDDDK | P00762 | MacDonald, Stary, and Swift 1982 | |||
| II | FPVDDDDK | P00763 | MacDonald, Stary, and Swift 1982 | |||
| III | LPLDDDDDK | P08426 | Fletcher et al. 1987 | |||
| Mouse | FPVDDDDK | P07146 | Stevenson, Hagenbuchle, and Wellauer 1986 | |||
| Horse (Equus caballus) | SSTDDDDK | — | Harris and Hofmann 1969 | |||
| Birds | ||||||
| Chicken (Gallus gallus) | ||||||
| 1 | FPISDEDDDK | Q90627 | Wang et al. 1995 | |||
| 2 | FPISDEDDDK | Q90628 | Wang et al. 1995 | |||
| 3 | FPGGADDDK | Q90629 | Wang et al. 1995 | |||
| Ostritch (Struthioniformes) | VPGDADDDK | AAB35161 | Bodley et al. 1995 | |||
| Amphibians | ||||||
| African clawed frog (Xenopus laevis) | ||||||
| I | FDDDK | P19799 | Shi and Brown 1990 | |||
| II | FEDDDK | P70059 | Wang et al. 1996c | |||
| Fishes | ||||||
| African lungfish (Protopterus aethiopicus) | ||||||
| A | LPLEDDK | — | de Haen, Walsh, and Neurath 1977 | |||
| B | FPIEEDK | P35051 | Hermodson et al. 1971 | |||
| Maori cod (Paranotothenia magellanica) | FATEDDK | S49489 | Genicot et al. 1996 | |||
| Winter flounder (Pseudopleuronectes americanus) | FALEDDK | AAC32752 | Douglas and Gallant 1998 | |||
| Japanese flounder (Paralichthys olivaceus) | ||||||
| 1 | FAMEDDK | BAA82362 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| 2 | FATEDDK | BAA82363 | Suzuki, Srivastava, and Kurokawa 2002 | |||
| Pufferfish (Takifugu rubripes) | PIDEDDK | TRU25747 | Roach et al. 1997 | |||
| Atlantic cod (Gadus morhua) | ||||||
| I | VFAEEDK | S39047 | Gudmundsdottir et al. 1993 | |||
| X | VFAEEDK | S39048 | Gudmundsdottir et al. 1993 | |||
| Atlantic salmon (Salmo salar) | ||||||
| I | FATEDDK | P35031 | Male et al. 1995 | |||
| II | FATEDDK | P35032 | Male et al. 1995 | |||
| III | PIDDEDDK | P35033 | Male et al. 1995 | |||
| Zebrafish (Danio rerio) | APLGDDDDK | AAO16212 | Wan and Gong 2002c | |||
| Japanese anchovy (Engraulis japonicus) | ||||||
| 1 | FAEDDK | BAB40329 | Watabe, Ahsan, and Funabara 2000c | |||
| 2 | FAEDDK | BAB40330 | Watabe, Ahsan, and Funabara 2000c | |||
| Japanese eel (Anguilla japonica) | VALDDDK | Q8QGW3 | Kurokawa et al. 2001c | |||
| Primitive vertebrates | ||||||
| Spiny dogfish (Squalus acanthias) | APDDDDK | — | Bradshaw et al. 1970 | |||
| P00764 | Titani et al. 1975 | |||||
| Sea lamprey (Petromyzon marinus) | ||||||
| A1 | APYMYEDH | AAB65411 | Roach et al. 1997 | |||
| A2 | APYMYEDH | AAB69654 | Roach et al. 1997 | |||
| A3 | APYMYEDH | AAB69655 | Roach et al. 1997 | |||
| B1 | APYMYEDH | AAB69656 | Roach et al. 1997 | |||
| B2 | APYMYEDH | AAB69657 | Roach et al. 1997 | |||
| Urochordates | ||||||
| Tunicate (Boltenia villosa) | AVNADK | AAB69653 | Roach et al. 1997 | |||
| Star ascidian (Botryllus schlosseri) | ||||||
| 1 | FCGANADK | O01309 | Pancer et al. 1996 | |||
| 2 | LYGANADK | O01310 | Pancer et al. 1996 | |||
| Crustaceans | ||||||
| Red king crab (Pacifastacus camtschaticus) | APSRKPTFRRGLNK | AF461036 | Rebrikov 2001c | |||
| Signal crayfish (Pacifastacus leniusculus) | APSRRLRFPPNNYK | CAA10915 | Hernandez-Cortes et al. 1999 | |||
| Pacific white shrimp (Litopenaeus vannamei)d | ||||||
| APSRKPTFRRGLNK | CAA60129 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75310 | Klein et al. 1996 | ||||
| APSRKPTFRRGLNK | CAA75311 | Klein et al. 1996 | ||||
| Salmon louse (Lepeophtheirus salmonis) | ||||||
| Type 1 | VPPQIKYSESFMKVKSMRHRFGGR | AAL71875 | Johnson et al. 2002 | |||
| Type 2 | VPRIKYSDSFMKVKSMRHRFGGR | AAL71876 | Johnson et al. 2002 | |||
| Type 3 | VPRIKYSESFMKVKSMRHRFGGR | AAL71877 | Johnson et al. 2002 | |||
| Insects | ||||||
| Fruit fly (Drosophilamelanogaster)e | LLPQLDGR | M96372 | Wang, Magoulas, and Hickey 1999 | |||
| Fungi | ||||||
| Fusarium oxysporume | APQEIPN | P35049 | Rypniewski et al. 1993 | |||
| Bacteria | ||||||
| Streptomyces griseuse | APNP | P00775 | Kim et al. 1991 | |||
aDetermined by protein sequencing. No accession number is available.
bInitally identified as VDDDDK by protein sequencing (Davie and Neurath 1955).
cDirect submission to databanks, not included in Literature Cited.
dFormerly Penaeus vannamei.
eRepresentative sequence (refer to the text).
Activation Peptides of “Fast-Evolving” Group III Trypsinogen Genes in Cold-Adapted Fishes.
| Species . | Activation Peptide Sequences . | GenBank or SwissProt Accession Number . | Reference . |
|---|---|---|---|
| Japanese flounder (Paralichthys olivaceus) | APTDDR | BAA82364 | Suzuki, Srivastava, and Kurokawa 2002 |
| Atlantic cod (Gadus morhua) | VPREDGR | Q8JFQ7 | Spilliaert and Gudmundsdottir 1999 |
| Winter flounder (Pseudopleuronectes americanus) | VPREDGR | AAC32751 | Douglas and Gallant 1998 |
| Plaice (Pleuronectes platessa) | VPREDGR | P35034 | Leaver and George 1990a |
| Black rockcod (Notothenia coriiceps) | VPREDGR | AAD30107 | Cheng and Chen 1999 |
| Giant Antarctic toothfish (Dissostichus mawsoni) | VPREDGR | AAB57728 | Chen, DeVries, and Cheng 1997 |
| Species . | Activation Peptide Sequences . | GenBank or SwissProt Accession Number . | Reference . |
|---|---|---|---|
| Japanese flounder (Paralichthys olivaceus) | APTDDR | BAA82364 | Suzuki, Srivastava, and Kurokawa 2002 |
| Atlantic cod (Gadus morhua) | VPREDGR | Q8JFQ7 | Spilliaert and Gudmundsdottir 1999 |
| Winter flounder (Pseudopleuronectes americanus) | VPREDGR | AAC32751 | Douglas and Gallant 1998 |
| Plaice (Pleuronectes platessa) | VPREDGR | P35034 | Leaver and George 1990a |
| Black rockcod (Notothenia coriiceps) | VPREDGR | AAD30107 | Cheng and Chen 1999 |
| Giant Antarctic toothfish (Dissostichus mawsoni) | VPREDGR | AAB57728 | Chen, DeVries, and Cheng 1997 |
Note.—Pufferfish has two group III trypsinogens (International Fugu Sequencing Consortium, unpublished data), as noted by Roach (2002).
aDirect submission to databanks.
Activation Peptides of “Fast-Evolving” Group III Trypsinogen Genes in Cold-Adapted Fishes.
| Species . | Activation Peptide Sequences . | GenBank or SwissProt Accession Number . | Reference . |
|---|---|---|---|
| Japanese flounder (Paralichthys olivaceus) | APTDDR | BAA82364 | Suzuki, Srivastava, and Kurokawa 2002 |
| Atlantic cod (Gadus morhua) | VPREDGR | Q8JFQ7 | Spilliaert and Gudmundsdottir 1999 |
| Winter flounder (Pseudopleuronectes americanus) | VPREDGR | AAC32751 | Douglas and Gallant 1998 |
| Plaice (Pleuronectes platessa) | VPREDGR | P35034 | Leaver and George 1990a |
| Black rockcod (Notothenia coriiceps) | VPREDGR | AAD30107 | Cheng and Chen 1999 |
| Giant Antarctic toothfish (Dissostichus mawsoni) | VPREDGR | AAB57728 | Chen, DeVries, and Cheng 1997 |
| Species . | Activation Peptide Sequences . | GenBank or SwissProt Accession Number . | Reference . |
|---|---|---|---|
| Japanese flounder (Paralichthys olivaceus) | APTDDR | BAA82364 | Suzuki, Srivastava, and Kurokawa 2002 |
| Atlantic cod (Gadus morhua) | VPREDGR | Q8JFQ7 | Spilliaert and Gudmundsdottir 1999 |
| Winter flounder (Pseudopleuronectes americanus) | VPREDGR | AAC32751 | Douglas and Gallant 1998 |
| Plaice (Pleuronectes platessa) | VPREDGR | P35034 | Leaver and George 1990a |
| Black rockcod (Notothenia coriiceps) | VPREDGR | AAD30107 | Cheng and Chen 1999 |
| Giant Antarctic toothfish (Dissostichus mawsoni) | VPREDGR | AAB57728 | Chen, DeVries, and Cheng 1997 |
Note.—Pufferfish has two group III trypsinogens (International Fugu Sequencing Consortium, unpublished data), as noted by Roach (2002).
aDirect submission to databanks.
We are grateful to the two anonymous reviewers for their helpful criticism. C.F. thanks Isabelle Quéré for technical assistance. This work was supported by the Institut National de la Santé et de la Recherche Médicale and the Projet Hospitalier de Recherche Clinique, France (C.F.) and NIH grant DK58088, USA (M.S.-T).
Literature Cited
Abita, J. P., M. Delaage, and M. Lazdunski.
Berger, A., and I. Schechter.
Bodley, M. D., R. J. Naude, W. Oelofsen, and A. Patthy.
Bradshaw, R. A., H. Neurath, R. W. Tye, K. A. Walsh, and W. P. Winter.
Bricteux-Gregoire, S.
Bricteux-Gregoire, S., R. Schyns, and M. Florkin.
Bricteux-Gregoire, S., R. Schyns, and M. Florkin.
Bricteux-Gregoire, S., R. Schyns, and M. Florkin.
Bricteux-Gregoire, S., R. Schyns, and M. Florkin.
Bricteux-Gregoire, S., R. Schyns, and M. Florkin.
Bricteux-Gregoire, S., R. Schyns, M. Florkin, M. Emmens, G. W. Welling, and J. J. Beintema.
Charles, M., M. Rovery, A. Guidoni, and P. Desnuelle.
Chen, J. M., and C. Ferec.
Chen, J. M., T. Montier, and C. Ferec.
Chen, L., A. L. DeVries, and C. H. Cheng.
Craik, C. S., C. Largman, T. Fletcher, S. Roczniak, P. J. Barr, R. Fletterick, and W. J. Rutter.
Davie, E., and H. Neurath.
de Haen, C., K. A. Walsh, and H. Neurath.
Delaage, M., P. Desnuelle, M. Lazdunski, E. Bricas, and J. Savrda.
Douglas, S. E., and J. W. Gallant.
Emi, M., Y. Nakamura, M. Ogawa, T. Yamamoto, T. Nishide, T. Mori, and K. Matsubara.
Ferec, C., O. Raguenes, and R. Salomon, et al. (15 co-authors).
Fletcher, T. S., M. Alhadeff, C. S. Craik, and C. Largman.
Genicot, S., F. Rentier-Delrue, D. Edwards, J. VanBeeumen, and C. Gerday.
Gudmundsdottir, A., E. Gudmundsdottir, S. Oskarsson, J. B. Bjarnason, A. K. Eakin, and C. S. Craik.
Guy, O., D. C. Bartelt, J. Amic, E. Colomb, and C. Figarella.
Guy, O., D. Lombardo, D. C. Bartelt, J. Amic, and C. Figarella.
Halfon, S., and C. S. Craik.
Harris, C. I., and T. Hofmann.
Hartley, B. S.
Hartley, B. S.
Hartley, B. S., J. R. Brown, D. L. Kauffman, and L. B. Smillie.
Hermodson, M. A., R. W. Tye, G. R. Reeck, H. Neurath, and K. A. Walsh.
Hernandez-Cortes, P., L. Cerenius, F. Garcia-Carreno, and K. Soderhall.
Huber, R., D. Kukla, W. Bode, P. Schwager, K. Bartels, J. Deisenhofer, and W. Steigemann.
Johnson, S. C., K. V. Ewart, J. A. Osborne, D. Delage, N. W. Ross, and H. M. Murray.
Kim, J. C., S. H. Cha, S. T. Jeong, S. K. Oh, and S. M. Byun.
Klein, B., G. Le Moullac, D. Sellos, and A. Van Wormhoudt.
Kukor, Z., J. Mayerle, B. Kruger, M. Tóth, P. M. Steed, W. Halangk, M. M. Lerch, and M. Sahin-Tóth.
Kukor, Z., M. Tóth, G. Pál, and M. Sahin-Tóth.
Lee K. J., S. G. Kang, and I. S. Kim.
Le Huerou, I., C. Wicker, P. Guilloteau, R. Toullec, and A. Puigserver.
Louvard, M. N., and A. Puigserver.
Lu, D., K. Futterer, S. Korolev, X. Zheng, K. Tan, G. Waksman, and J. E. Sadler.
MacDonald, R. J., S. J. Stary, and G. H. Swift.
Male, R., J. B. Lorens, A. O. Smalas, and K. R. Torrissen.
Maroux, S., J. Baratti, and P. Desnuelle.
Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne.
Northrop, J. H., and M. Kunitz.
Nyaruhucha, C. N., M. Kito, and S. I. Fukuoka.
Pancer, Z., J. Leuck, B. Rinkevich, R. Steffen, I. Muller, and W. E. Muller.
Pinsky, S. D., K. S. LaForge, and G. Scheele.
Radhakrishnan, T. M., K. A. Walsh, and H. Neurath.
Roach, J. C., K. Wang, L. Gan, and L. Hood.
Rypniewski, W. R., S. Hastrup, C. Betzel, M. Dauter, Z. Dauter, G. Papendorf, S. Branner, and K. S. Wilson.
Rypniewski, W. R., A. Perrakis, C. E. Vorgias, and K. S. Wilson.
Sahin-Tóth, M.
Sahin-Tóth, M.
Sahin-Tóth, M., and M. Tóth.
Schneider, A., and D. C. Whitcomb.
Schyns, R., S. Bricteux-Gregoire, and M. Florkin.
Shi, Y. B., and D. D. Brown.
Simon, P., F. U. Weiss, M. Sahin-Tóth, M. Parry, O. Nayler, B. Lenfers, J. Schnekenburger, J. Mayerle, W. Domschke, and M. M. Lerch.
Spilliaert, R., and A. Gudmundsdottir.
Steiner, J. M., T. L. Medinger, and D. A. Williams.
Stevenson, B. J., O. Hagenbuchle, and P. K. Wellauer.
Stroud, R. M., L. M. Kay, and R. E. Dickerson.
Suzuki, T., A. S. Srivastava, and T. Kurokawa.
Szilágyi, L., E. Kenesi, G. Katona, G. Kaslik, G. Juhász, and L. Gráf.
Teich, N., H. Bodeker, and V. Keim.
Teich, N., J. Ockenga, A. Hoffmeister, M. Manns, J. Mossner, and V. Keim.
Titani, K., L. H. Ericsson, H. Neurath, and K. A. Walsh.
Walsh, K. A., D. L. Kauffman, K. S. V. Sampath Kumar, and H. Neurath.
Wang, K., L. Gan, I. Lee, and L. Hood.
Wang, S., C. Magoulas, and D. Hickey.
Whitcomb, D. C., M. C. Gorry, and R. A. Preston, et al. (15 co-authors).



