-
PDF
- Split View
-
Views
-
Cite
Cite
Keng-See Chow, Kiew-Lian Wan, Mohd. Noor Mat Isa, Azlina Bahari, Siang-Hee Tan, K Harikrishna, Hoong-Yeet Yeang, Insights into rubber biosynthesis from transcriptome analysis of Hevea brasiliensis latex, Journal of Experimental Botany, Volume 58, Issue 10, July 2007, Pages 2429–2440, https://doi.org/10.1093/jxb/erm093
Close - Share Icon Share
Abstract
Hevea brasiliensis is the most widely cultivated species for commercial production of natural rubber (cis-polyisoprene). In this study, 10 040 expressed sequence tags (ESTs) were generated from the latex of the rubber tree, which represents the cytoplasmic content of a single cell type, in order to analyse the latex transcription profile with emphasis on rubber biosynthesis-related genes. A total of 3441 unique transcripts (UTs) were obtained after quality editing and assembly of EST sequences. Functional classification of UTs according to the Gene Ontology convention showed that 73.8% were related to genes of unknown function. Among highly expressed ESTs, a significant proportion encoded proteins related to rubber biosynthesis and stress or defence responses. Sequences encoding rubber particle membrane proteins (RPMPs) belonging to three protein families accounted for 12% of the ESTs. Characterization of these ESTs revealed nine RPMP variants (7.9–27 kDa) including the 14 kDa REF (rubber elongation factor) and 22 kDa SRPP (small rubber particle protein). The expression of multiple RPMP isoforms in latex was shown using antibodies against REF and SRPP. Both EST and quantitative reverse transcription-PCR (QRT-PCR) analyses demonstrated REF and SRPP to be the most abundant transcripts in latex. Besides rubber biosynthesis, comparative sequence analysis showed that the RPMPs are highly similar to sequences in the plant kingdom having stress-related functions. Implications of the RPMP function in cis-polyisoprene biosynthesis in the context of transcript abundance and differential gene expression are discussed.
Introduction
Latex is the cytoplasmic content of laticifers or latex vessels of Hevea brasiliensis, the cultivated tree species for commercial production of natural rubber. Laticifers form a ubiquitous network of tubes in the tree and is the major location of rubber biosynthesis (Gomez and Moir, 1979). The chemical composition of natural rubber is cis-polyisoprene, a high-molecular weight polymer formed from sequential condensation of isopentenyl diphosphate (IDP) units. Numerous classes of isoprenoids including Hevea cis-polyisoprene are produced from the plant isoprenoid biosynthesis pathway via IDP as a common intermediate (Kekwick, 1989). The mevalonate (MVA) pathway has been the conventionally studied pathway for isoprenoid biosynthesis since the 1950s (Fig. 1). Evidence supporting this cytosolic pathway of rubber formation was derived from a high level of incorporation of radiolabelled pathway intermediates such as mevalonate (Skilleter and Kekwick, 1971) and 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) (Hepper and Audley, 1969) into rubber. Only in more recent years has the plastidic 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (MEP) pathway been considered a possible alternative route for rubber biosynthesis. This pathway has been well characterized in bacterial and plant species (Lichtenthaler, 1999; Rodriguez-Concepcion and Boronat, 2002). The expression of 1-deoxy-D-xylulose 5-phosphate synthase (DXPS) in Hevea latex and leaves suggests that the MEP pathway exists in the laticifer (Ko et al., 2003) and therefore could provide an alternative means of generating IDP for cis-polyisoprene synthesis (Fig. 1).
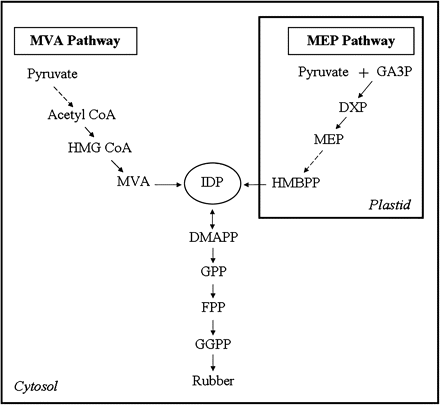
Biosynthesis of Hevea rubber. IDP for the biosynthesis of rubber may be contributed by the MVA and MEP pathways. Broken arrows indicate multiple pathway steps.
Short chain allylic diphosphates are synthesized from IDP by soluble trans-prenyltransferases as initiator molecules of rubber (Archer et al., 1963; Archer and Audley, 1987). A membrane-bound cis-prenyltransferase or rubber transferase is thought to switch subsequently the condensation of new IDP units from trans to cis configuration (Tanaka, 1989). Hitherto, there are numerous reports on the identification of Hevea rubber transferase (Archer and Cockbain, 1969; Archer and Audley, 1987; Light and Dennis, 1989; Cornish, 1993). However, evidence for the function of Hevea cis-prenyltransferase in generating high molecular weight rubber molecules was more recently reported (Asawatreratanakul et al., 2003). Nonetheless, a number of other proteins have been shown to participate in cis-polyisoprene biosynthesis. Initially, most attention was directed to the major membrane proteins of rubber particles, REF (rubber elongation factor) (Dennis and Light, 1989) and SRPP (small rubber particle protein) (Oh et al., 1999), which share 72% protein sequence similarity. Subsequently, cytosolic proteins identified were the rubber biosynthesis stimulator protein which corresponds to eIF-5A (Yusof et al., 2000; Chow et al., 2003) and a patatin-like inhibitor protein (Yusof et al., 1998). The numbers of proteins proposed to date support a mechanism of cis-polyisoprene synthesis that involves a complex of soluble and membrane-bound proteins. The site of synthesis of allylic diphosphate initiators and cis-polyisoprene is largely regarded to occur on the surface of pre-existing rubber particles (Archer et al., 1963; Archer and Audley, 1987). Additionally, it has been suggested that non-rubber particles of latex may be the site for rubber initiation (Tangpakdee et al., 1997; Wititsuwannakul et al., 2003).
In the era of high-throughput technologies for biology, expressed sequence tag (EST) sequencing has enabled laticifer gene expression to be surveyed on a large scale. In this report, an analysis is presented of the latex transcriptome based on a collection of 10 040 latex ESTs with emphasis on genes known to be related to rubber biosynthesis. The transcription profile of latex is described, especially of biosynthesis-related genes, and a group of rubber particle membrane proteins that are most abundantly expressed is characterized. The implications of these findings in Hevea cis-polyisoprene biosynthesis are also discussed.
Materials and methods
Plant material
Latex was collected from 15-year-old H. brasiliensis trees (clone RRIM 600) which were in regular tapping from existing field trials in the Rubber Research Institute of Malaysia Experiment Station. Latex was collected directly into denaturing buffer and total RNA prepared according to the method of Kush et al. (1990). Total RNA samples for library construction and quantitative reverse transcription-PCRs (QRT-PCRs) originated from latex collections on different dates. Latex for isolation of rubber particle proteins was not collected in buffer but directly into chilled conical flasks.
Library construction and EST sequencing
Latex mRNA was purified from total RNA using the Poly(A) Quick® mRNA Isolation Kit (Stratagene). Construction of a latex cDNA library was performed using the ZAP-cDNA® Synthesis Kit and ZAP-cDNA® Gigapak® II Gold Cloning Kit (Stratagene). Latex cDNAs cloned in the Lambda Uni-Zap™ XR vector were amplified once before mass in vivo excision into pBluescript® SK phagemid clones. Individual bacterial colonies were picked into 96-well plates, and large-scale plasmid preparations were performed in a 96-well format using the Montage Plasmid Miniprep 96 Kit (Millipore). Individual plasmids containing latex cDNA inserts were subjected to 5′ end single-pass sequencing with the T3 primer (5′-ATT AAC CCT CAC TAA AGG GA-3′) and the BigDye® Terminator version 3.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing products were resolved and analysed on a 3730 DNA Analyzer (Applied Biosystems).
Sequence processing and analysis
Latex EST sequences were analysed using the StackPack version 2.2 package (Burke et al., 1999; Miller et al., 1999). In this pipeline, raw data from DNA sequencer chromatograms were quality trimmed at a threshold of phred 20 (Ewing and Green, 1998; Ewing et al., 1998) and vector-masked using Cross_match (http://www.phrap.org). Subsequently, edited files were assembled into high sequence identity groups using the D2_cluster (clusters), phrap (contigs), and craw (consensus sequences) algorithms. Each sequence assembly generates a consensus sequence, while individual ESTs with insufficient DNA identity to be assembled are known as singletons. The number of EST members of an assembly constitutes its EST frequency while the number of unique transcripts (UTs) is the total of the consensus sequences and singletons. Latex EST sequences have been deposited in dbEST at the National Centre for Biotechnology Information (NCBI) (accession nos EC600050–EC609910), and additional details are available at the Natural Rubber EST Database (NRESTdb) (http://genome.ukm.my/nrestdb/).
Functional classification was performed according to the Gene Ontology (GO) convention (Gene Ontology Consortium, 2000). The BLAST software (Altschul et al., 1997) was used for sequence similarity searching based on an E-value <1.0E-04. Protein sequence alignment and dendrogram analysis were performed using ClustalW (Thompson et al., 1994). Protein alignment output was displayed using BOXSHADE version 3.2 (the European Molecular Biology Network at http://www.ch.embnet.org/). General DNA and protein sequence analyses were carried out using bioinformatics tools of the National Biotechnology and Bioinformatics Network, Malaysia (http://cgat.ukm.my/tools/). Searches for ESTs matching cDNA sequences of REF (X56535) and SRPP (AF051317) were performed by BLASTN analysis of a locally set up latex EST database. For external sequence searches, databases used were those available at NCBI (http://www.ncbi.nlm.nih.gov/) and The Institute for Genomic Research (TIGR) (http://www.tigr.org/).
QRT-PCR analysis
All QRT-PCRs were performed using the Rotor-Gene 3000 Real Time Thermal Cycler (Corbett Research) and according to the recommendations of reagent kit manufacturers. RNA concentration and RNA integrity numbers (RINs) were determined using the Agilent 2100 Bioanalyser (Agilent Technologies). Primer pairs having a Tm range of 54–56 °C and flanking amplicon sizes of 100–200 bp were designed for 13 Hevea biosynthesis-related cDNAs and Hevea 18S cDNA (Table 3) using the Beacon Designer Version 4.0 (Premier Biosoft International). To determine primer efficiency, single-stranded cDNAs were first synthesized from latex total RNA (QuantiTect® Reverse Transcription Kit, Qiagen). Subsequently, PCRs containing an optimized mix of oligo(dT) and random primers (QuantiTect® SYBR® Green PCR Kit, Qiagen) were performed with each primer pair using a series of four 10-fold dilutions of single-stranded latex transcripts as templates. All reactions were performed in duplicate and a ‘no template’ control was included for each primer pair. Melting curve analysis was performed using the Rotor-Gene 3000 Software Version 1.7 (Corbett Research) to ensure that the PCR products did not include primer dimers or multiple products. Finally, the efficiency of each primer pair was confirmed following efficiency parameters given by the same software.
To determine the expression level of 13 Hevea biosynthesis-related gene transcripts, individual one-step QRT-PCRs were carried out using the QuantiTect® SYBR® Green RT-PCR Kit (Qiagen). Each reaction contained latex total RNA as template and a gene-specific primer pair. All reactions were performed in triplicate and ‘no template’ and ‘no reverse transcriptase’ controls were included for each primer pair. Because not all QRT-PCRs can be accommodated in one thermocycling experiment, reactions containing Hevea 18S primers were included in each set of QRT-PCRs. The entire relative expression analysis using all primer pairs was performed twice. Melting curve analysis was carried out to ensure that the QRT-PCR products did not include primer dimers or multiple products.
Transcript levels of genes of interest were calculated according to the relative quantification method of Pfaffl (2001). In this method, Hevea 18S was used as the normalizer to account for systematic error. Hevea 18S was also the calibrator used to calculate relative transcript levels by taking into account the percentage PCR efficiency of all genes generated by the Rotor-Gene 3000 Software Version 1.7 (Corbett Research).
Latex total protein preparation and immunodetection
Field-collected latex was mixed with Triton X-100 solution (one part of latex to four parts of 0.125% Triton X-100), followed by stirring at 4 °C for 1 h. The mixture was centrifuged at 44 000 g for 1 h at 4 °C to recover the supernatant (serum phase), which was then diluted with an equal volume of phosphate-buffered saline (PBS). SDS-PAGE was carried out on the Triton X-100 extract under reducing conditions (in the presence of β-mercaptoethanol) on 15% gels (Laemmli, 1970). The separated proteins were western blotted onto a nitrocellulose membrane and an immuno-detection procedure was performed that allowed REF and SRPP to be differentially detected on the same membrane. The blotted membrane was first blocked with 5% non-fat milk in PBS and then incubated for 90 min with PBS-milk containing a monoclonal antibody against SRPP and polyclonal antibodies against REF. (There was no significant cross-reactivity between the two antibodies and their antigenic proteins.) After three washes with PBS-milk, the nitrocellulose membrane was incubated for 1 h with anti-rabbit IgG conjugated to horseradish peroxidase, which served as the secondary antibody to REF. Following another three washes with PBS-milk, the nitrocellulose membrane was incubated in freshly prepared peroxidase substrate solution (diaminobenzidine tetrahydrochloride/H2O2) in PBS to display the REF protein bands on the membrane. After adequate development of the brown coloured REF protein bands, the nitrocellulose membrane was washed with excess distilled water to stop the enzyme reaction before re-equilibration in PBS-milk. The nitrocellulose membrane was then incubated for another hour with anti-mouse IgG conjugated to alkaline phosphatase, which served as the secondary antibody to SRPP. After three cycles of washing with PBS-milk, the nitrocellulose membrane was incubated for 10 min in TBS before being immersed in alkaline phosphate substrate solution (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium) to generate the purple protein bands that denoted the presence of SRPP.
Results
Bioinformatic analysis of EST sequences
A total of 10 193 sequences were generated from 5′ end single-pass sequencing of random latex cDNA clones. Quality editing and subsequent removal of vector sequences resulted in 10 040 latex ESTs (Table 1) where this number is the denominator for calculations referring to the proportion of total latex ESTs. The StackPack pipeline generated 1380 consensus sequences from progressive assembly of 10 040 EST sequences. Addition of consensus sequences and singletons thus produced 3441 UTs or non-redundant mRNA transcripts of genes expressed in latex (Table 1). The sequence redundancy rate for 10 040 latex ESTs in this study was calculated to be 65.7%. To investigate the redundancy factor in EST sequencing, StackPack analysis was performed for a randomly selected pool of 1000 latex ESTs followed by the same analysis but with sequential additions of 1000 ESTs until the final total of 10 040 sequences. As shown in Fig. 2, the redundancy rate for the ESTs generated herein has reached a steady rate of approximately 70% although total redundancy would be reached eventually.
| No. of sequences | (%) | |
| Raw sequences | 10 193 | |
| Quality trimmed sequences (phred20) | 10 040 | |
| Clustering analysis: | ||
| Clusters | 1127 | |
| Contigs | 1321 | |
| Consensus sequences | 1380 | |
| Singletons | 2061 | |
| Unique transcripts (UTs) | 3441a | (34.3)b |
| Redundant ESTs | 6599 | (65.7)b |
| Functional analysis: | ||
| UTs with GO match | 902 | (26.2)c |
| Molecular function | 736 | |
| Biological process | 543 | |
| Cellular component | 349 | |
| UTs without GO match | 2539 | (73.8)c |
| No. of sequences | (%) | |
| Raw sequences | 10 193 | |
| Quality trimmed sequences (phred20) | 10 040 | |
| Clustering analysis: | ||
| Clusters | 1127 | |
| Contigs | 1321 | |
| Consensus sequences | 1380 | |
| Singletons | 2061 | |
| Unique transcripts (UTs) | 3441a | (34.3)b |
| Redundant ESTs | 6599 | (65.7)b |
| Functional analysis: | ||
| UTs with GO match | 902 | (26.2)c |
| Molecular function | 736 | |
| Biological process | 543 | |
| Cellular component | 349 | |
| UTs without GO match | 2539 | (73.8)c |
EST sequences were assembled into clusters and contigs followed by generation of consensus sequences (StackPack version 2.2). UTs were classified according to the Gene Ontology (GO) functional categories: molecular function, biological process, and cellular component, where each included UTs classified in more than one category.
Number of unique transcripts is the total of consensus sequences and singletons.
Percentage calculated from a total of 10 040 ESTs.
Percentage calculated from a total of 3441 UTs.
| No. of sequences | (%) | |
| Raw sequences | 10 193 | |
| Quality trimmed sequences (phred20) | 10 040 | |
| Clustering analysis: | ||
| Clusters | 1127 | |
| Contigs | 1321 | |
| Consensus sequences | 1380 | |
| Singletons | 2061 | |
| Unique transcripts (UTs) | 3441a | (34.3)b |
| Redundant ESTs | 6599 | (65.7)b |
| Functional analysis: | ||
| UTs with GO match | 902 | (26.2)c |
| Molecular function | 736 | |
| Biological process | 543 | |
| Cellular component | 349 | |
| UTs without GO match | 2539 | (73.8)c |
| No. of sequences | (%) | |
| Raw sequences | 10 193 | |
| Quality trimmed sequences (phred20) | 10 040 | |
| Clustering analysis: | ||
| Clusters | 1127 | |
| Contigs | 1321 | |
| Consensus sequences | 1380 | |
| Singletons | 2061 | |
| Unique transcripts (UTs) | 3441a | (34.3)b |
| Redundant ESTs | 6599 | (65.7)b |
| Functional analysis: | ||
| UTs with GO match | 902 | (26.2)c |
| Molecular function | 736 | |
| Biological process | 543 | |
| Cellular component | 349 | |
| UTs without GO match | 2539 | (73.8)c |
EST sequences were assembled into clusters and contigs followed by generation of consensus sequences (StackPack version 2.2). UTs were classified according to the Gene Ontology (GO) functional categories: molecular function, biological process, and cellular component, where each included UTs classified in more than one category.
Number of unique transcripts is the total of consensus sequences and singletons.
Percentage calculated from a total of 10 040 ESTs.
Percentage calculated from a total of 3441 UTs.
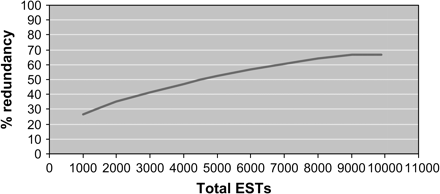
Redundancy rate of gene discovery. The rate of sequence redundancy was obtained for latex ESTs in increments of 1000 sequences analysed using the StackPack version 2.2 pipeline.
Transcription profile analysis
The latex transcriptome was examined by EST frequency and putative gene function of UTs. The EST frequency of 3441 UTs ranged from one to 820 where more than half (53.5%) of the ESTs was represented by 360 UTs of frequency range 5–820 (data not shown). Functional analysis revealed that 26.2% of the 3441 UTs can be assigned known gene identities according to the GO convention (Table 1). To gain further insights into laticifer gene expression, a subset of highly expressed ESTs (frequency 15–820), which consists of 58 UTs or 31.9% of total latex ESTs, were looked at in more detail. Their gene identities based on BLASTX searching of the non-redundant GenBank database were manually curated for functional classes as indicated in Table 2. All except four UTs showed hits to sequences from plant species. Six exceptionally abundant ESTs having a frequency of 110–820 were noted (Table 2). Clearly, there is a dominance of ESTs involved in rubber biosynthesis (REF and SRPP) within these six abundant genes. In total, five REF and SRPP protein isoforms are found in Table 2. Even so, not all proteins involved in rubber biosynthesis are found, the only other ones being a patatin-like biosynthesis inhibitor (frequency 31), cis-prenyltransferase (frequency 20), and HMG CoA synthase (frequency 15). Genes for other biosynthesis pathway proteins such as HMG CoA reductase and the rubber biosynthesis stimulator protein were present but at EST frequencies lower than 15 and therefore not listed in Table 2. Overall, ESTs encoding genes involved in rubber biosynthesis rank as most abundant, constituting about half of the number of ESTs in Table 2. ESTs related to stress and defence responses also feature prominently. The abundant ones include ASR-like proteins (frequency 66 and 17), prohevein (frequency 63), and heat shock proteins (frequencies 50 and 20). REF and SRPP also show significant matches with stress-related proteins (see the section on comparative sequence analysis). Therefore, by including REF and SRPP, stress and defence response proteins are evidently very abundantly expressed in the laticifer.
| EST frequency | Protein matches | Species | GenBank accession no. | Functional class |
| 820 | Rubber elongation factor protein (REF) | Hevea brasiliensis | P15252 | RB, S |
| 229 | Small rubber particle protein (SRPP) | Hevea brasiliensis | O82803 | RB, S |
| 156 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 139 | Rubber elongation factor (REF) | Hevea brasiliensis | P15252 | RB, S |
| 127 | C3HC4-type RING zinc finger protein | Hevea brasiliensis | AAP46154 | T |
| 110 | REP1 protein of yeast plasmid partitioning system | Saccharomyces cerevisiae | S40417 | X |
| 71 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_567195 | T |
| 70 | Expressed protein | Arabidopsis | NP_566643 | U |
| 69 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 66 | ASR-like protein | Hevea brasiliensis | AAP46155 | S |
| 64 | Rubber elongation factor (REF) | Hevea brasiliensis | AAR11448 | RB, S |
| 63 | No hits found | – | U | |
| 63 | Pro-hevein precursor | Hevea brasiliensis | P02877 | D, RB |
| 55 | Unknown protein | Arabidopsis | NP_178443 | U |
| 51 | MFP1 attachment factor | Glycine max | AAF63659 | T |
| 50 | Low molecular weight heat-shock protein | Corylus avellana | AAD15628 | S |
| 46 | Translationally controlled tumour protein (TCTP) | Hevea brasiliensis | Q9ZSW9 | X |
| 42 | Protease inhibitor protein | Hevea brasiliensis | AAP46156 | D |
| 39 | Chorismate synthase | Arabidopsis | NP_564534 | M |
| 39 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 37 | ADP-ribosylation factor | Oryza sativa | NP_915954 | M |
| 37 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66432 | RB, S |
| 36 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 32 | Heavy metal-associated domain-containing protein | Arabidopsis | NP_177261 | D |
| 31 | Patatin-like latex allergen/rubber biosynthesis inhibitor | Hevea brasiliensis | T10765 | RB |
| 31 | Acyl-CoA-binding protein | Panax ginseng | BAB85987 | M |
| 28 | Hexameric polyubiquitin | Arabidopsis | AAA68878 | PT |
| 28 | Galactosyl-1-phosphate transferase | Salmonella enterica | AAC44096 | X |
| 28 | Unknown protein | Homo sapiens | AAL55824 | U |
| 24 | Chloroplast precursor of ferredoxin III protein | Zea mays | P27788 | M |
| 23 | Polyubiquitin | Petroselinum crispum | CAA45621 | PT |
| 23 | Nuclear transport factor | Arabidopsis | NP_174118 | M |
| 22 | Phosphate-responsive family protein | Arabidopsis | NP_565409 | M |
| 22 | Hydroxyproline-rich glycoprotein family protein | Arabidopsis | NP_565899 | D |
| 21 | Nuclear transport factor | Arabidopsis | NP_189151 | M |
| 20 | Cis-prenyltransferase | Hevea brasiliensis | BAB71776 | RB |
| 20 | Cyclophilin | Ricinus communis | CAC80550 | D |
| 20 | 18.5 kDa class I heat shock protein | Glycine max | P05478 | S |
| 20 | Iron–sulphur cluster assembly complex protein | Arabidopsis | NP_193953 | M |
| 20 | DnaJ protein | Hevea brasiliensis | AAD12055 | S |
| 20 | Fibre protein | Gossypium barbadense | AAN77145 | X |
| 20 | Unknown protein | Arabidopsis | BAC42514 | U |
| 19 | Eukaryotic translation initiation factor 4A | Nicotiana tabacum | Q40465 | PT |
| 18 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_193376 | T |
| 18 | Glycosyl transferase family protein | Arabidopsis | NP_181493 | D |
| 18 | Unknown protein | Arabidopsis | AAM64350 | U |
| 17 | Expressed protein | Arabidopsis | AAM15078 | U |
| 17 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66433 | RB, S |
| 17 | Elongation factor 1-alpha | Manihot esculenta | O49169 | PT |
| 17 | ASR-like protein | Hevea brasiliensis | AAP46158 | S |
| 17 | Hypothetical protein | Arabidopsis | T05538 | U |
| 17 | Caffeic acid 3-O-methyltransferase | Rosa chinensis | Q8GU25 | M |
| 16 | No apical meristem (NAM) family protein | Arabidopsis | NP_196822 | S, T |
| 16 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_191037 | T |
| 15 | Thioredoxin h | Ricinus communis | Q43636 | M |
| 15 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_568946 | T |
| 15 | Hypothetical protein | Bacteroides thetaiotaomicron | NP_813385 | U |
| 15 | HMG CoA synthase | Hevea brasiliensis | AAL18930 | RB |
| EST frequency | Protein matches | Species | GenBank accession no. | Functional class |
| 820 | Rubber elongation factor protein (REF) | Hevea brasiliensis | P15252 | RB, S |
| 229 | Small rubber particle protein (SRPP) | Hevea brasiliensis | O82803 | RB, S |
| 156 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 139 | Rubber elongation factor (REF) | Hevea brasiliensis | P15252 | RB, S |
| 127 | C3HC4-type RING zinc finger protein | Hevea brasiliensis | AAP46154 | T |
| 110 | REP1 protein of yeast plasmid partitioning system | Saccharomyces cerevisiae | S40417 | X |
| 71 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_567195 | T |
| 70 | Expressed protein | Arabidopsis | NP_566643 | U |
| 69 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 66 | ASR-like protein | Hevea brasiliensis | AAP46155 | S |
| 64 | Rubber elongation factor (REF) | Hevea brasiliensis | AAR11448 | RB, S |
| 63 | No hits found | – | U | |
| 63 | Pro-hevein precursor | Hevea brasiliensis | P02877 | D, RB |
| 55 | Unknown protein | Arabidopsis | NP_178443 | U |
| 51 | MFP1 attachment factor | Glycine max | AAF63659 | T |
| 50 | Low molecular weight heat-shock protein | Corylus avellana | AAD15628 | S |
| 46 | Translationally controlled tumour protein (TCTP) | Hevea brasiliensis | Q9ZSW9 | X |
| 42 | Protease inhibitor protein | Hevea brasiliensis | AAP46156 | D |
| 39 | Chorismate synthase | Arabidopsis | NP_564534 | M |
| 39 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 37 | ADP-ribosylation factor | Oryza sativa | NP_915954 | M |
| 37 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66432 | RB, S |
| 36 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 32 | Heavy metal-associated domain-containing protein | Arabidopsis | NP_177261 | D |
| 31 | Patatin-like latex allergen/rubber biosynthesis inhibitor | Hevea brasiliensis | T10765 | RB |
| 31 | Acyl-CoA-binding protein | Panax ginseng | BAB85987 | M |
| 28 | Hexameric polyubiquitin | Arabidopsis | AAA68878 | PT |
| 28 | Galactosyl-1-phosphate transferase | Salmonella enterica | AAC44096 | X |
| 28 | Unknown protein | Homo sapiens | AAL55824 | U |
| 24 | Chloroplast precursor of ferredoxin III protein | Zea mays | P27788 | M |
| 23 | Polyubiquitin | Petroselinum crispum | CAA45621 | PT |
| 23 | Nuclear transport factor | Arabidopsis | NP_174118 | M |
| 22 | Phosphate-responsive family protein | Arabidopsis | NP_565409 | M |
| 22 | Hydroxyproline-rich glycoprotein family protein | Arabidopsis | NP_565899 | D |
| 21 | Nuclear transport factor | Arabidopsis | NP_189151 | M |
| 20 | Cis-prenyltransferase | Hevea brasiliensis | BAB71776 | RB |
| 20 | Cyclophilin | Ricinus communis | CAC80550 | D |
| 20 | 18.5 kDa class I heat shock protein | Glycine max | P05478 | S |
| 20 | Iron–sulphur cluster assembly complex protein | Arabidopsis | NP_193953 | M |
| 20 | DnaJ protein | Hevea brasiliensis | AAD12055 | S |
| 20 | Fibre protein | Gossypium barbadense | AAN77145 | X |
| 20 | Unknown protein | Arabidopsis | BAC42514 | U |
| 19 | Eukaryotic translation initiation factor 4A | Nicotiana tabacum | Q40465 | PT |
| 18 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_193376 | T |
| 18 | Glycosyl transferase family protein | Arabidopsis | NP_181493 | D |
| 18 | Unknown protein | Arabidopsis | AAM64350 | U |
| 17 | Expressed protein | Arabidopsis | AAM15078 | U |
| 17 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66433 | RB, S |
| 17 | Elongation factor 1-alpha | Manihot esculenta | O49169 | PT |
| 17 | ASR-like protein | Hevea brasiliensis | AAP46158 | S |
| 17 | Hypothetical protein | Arabidopsis | T05538 | U |
| 17 | Caffeic acid 3-O-methyltransferase | Rosa chinensis | Q8GU25 | M |
| 16 | No apical meristem (NAM) family protein | Arabidopsis | NP_196822 | S, T |
| 16 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_191037 | T |
| 15 | Thioredoxin h | Ricinus communis | Q43636 | M |
| 15 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_568946 | T |
| 15 | Hypothetical protein | Bacteroides thetaiotaomicron | NP_813385 | U |
| 15 | HMG CoA synthase | Hevea brasiliensis | AAL18930 | RB |
UTs are listed according to the EST frequency, and are shown with the corresponding protein match, species, GenBank accession number, and functional class of the match. Protein function was classified with reference to known laticifer processes wherever possible. RB, rubber biosynthesis; S, stress-related responses; D, defence; T, transcription-related; PT, protein turnover; M, metabolism (various); X, unusual; U, unknown.
| EST frequency | Protein matches | Species | GenBank accession no. | Functional class |
| 820 | Rubber elongation factor protein (REF) | Hevea brasiliensis | P15252 | RB, S |
| 229 | Small rubber particle protein (SRPP) | Hevea brasiliensis | O82803 | RB, S |
| 156 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 139 | Rubber elongation factor (REF) | Hevea brasiliensis | P15252 | RB, S |
| 127 | C3HC4-type RING zinc finger protein | Hevea brasiliensis | AAP46154 | T |
| 110 | REP1 protein of yeast plasmid partitioning system | Saccharomyces cerevisiae | S40417 | X |
| 71 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_567195 | T |
| 70 | Expressed protein | Arabidopsis | NP_566643 | U |
| 69 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 66 | ASR-like protein | Hevea brasiliensis | AAP46155 | S |
| 64 | Rubber elongation factor (REF) | Hevea brasiliensis | AAR11448 | RB, S |
| 63 | No hits found | – | U | |
| 63 | Pro-hevein precursor | Hevea brasiliensis | P02877 | D, RB |
| 55 | Unknown protein | Arabidopsis | NP_178443 | U |
| 51 | MFP1 attachment factor | Glycine max | AAF63659 | T |
| 50 | Low molecular weight heat-shock protein | Corylus avellana | AAD15628 | S |
| 46 | Translationally controlled tumour protein (TCTP) | Hevea brasiliensis | Q9ZSW9 | X |
| 42 | Protease inhibitor protein | Hevea brasiliensis | AAP46156 | D |
| 39 | Chorismate synthase | Arabidopsis | NP_564534 | M |
| 39 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 37 | ADP-ribosylation factor | Oryza sativa | NP_915954 | M |
| 37 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66432 | RB, S |
| 36 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 32 | Heavy metal-associated domain-containing protein | Arabidopsis | NP_177261 | D |
| 31 | Patatin-like latex allergen/rubber biosynthesis inhibitor | Hevea brasiliensis | T10765 | RB |
| 31 | Acyl-CoA-binding protein | Panax ginseng | BAB85987 | M |
| 28 | Hexameric polyubiquitin | Arabidopsis | AAA68878 | PT |
| 28 | Galactosyl-1-phosphate transferase | Salmonella enterica | AAC44096 | X |
| 28 | Unknown protein | Homo sapiens | AAL55824 | U |
| 24 | Chloroplast precursor of ferredoxin III protein | Zea mays | P27788 | M |
| 23 | Polyubiquitin | Petroselinum crispum | CAA45621 | PT |
| 23 | Nuclear transport factor | Arabidopsis | NP_174118 | M |
| 22 | Phosphate-responsive family protein | Arabidopsis | NP_565409 | M |
| 22 | Hydroxyproline-rich glycoprotein family protein | Arabidopsis | NP_565899 | D |
| 21 | Nuclear transport factor | Arabidopsis | NP_189151 | M |
| 20 | Cis-prenyltransferase | Hevea brasiliensis | BAB71776 | RB |
| 20 | Cyclophilin | Ricinus communis | CAC80550 | D |
| 20 | 18.5 kDa class I heat shock protein | Glycine max | P05478 | S |
| 20 | Iron–sulphur cluster assembly complex protein | Arabidopsis | NP_193953 | M |
| 20 | DnaJ protein | Hevea brasiliensis | AAD12055 | S |
| 20 | Fibre protein | Gossypium barbadense | AAN77145 | X |
| 20 | Unknown protein | Arabidopsis | BAC42514 | U |
| 19 | Eukaryotic translation initiation factor 4A | Nicotiana tabacum | Q40465 | PT |
| 18 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_193376 | T |
| 18 | Glycosyl transferase family protein | Arabidopsis | NP_181493 | D |
| 18 | Unknown protein | Arabidopsis | AAM64350 | U |
| 17 | Expressed protein | Arabidopsis | AAM15078 | U |
| 17 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66433 | RB, S |
| 17 | Elongation factor 1-alpha | Manihot esculenta | O49169 | PT |
| 17 | ASR-like protein | Hevea brasiliensis | AAP46158 | S |
| 17 | Hypothetical protein | Arabidopsis | T05538 | U |
| 17 | Caffeic acid 3-O-methyltransferase | Rosa chinensis | Q8GU25 | M |
| 16 | No apical meristem (NAM) family protein | Arabidopsis | NP_196822 | S, T |
| 16 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_191037 | T |
| 15 | Thioredoxin h | Ricinus communis | Q43636 | M |
| 15 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_568946 | T |
| 15 | Hypothetical protein | Bacteroides thetaiotaomicron | NP_813385 | U |
| 15 | HMG CoA synthase | Hevea brasiliensis | AAL18930 | RB |
| EST frequency | Protein matches | Species | GenBank accession no. | Functional class |
| 820 | Rubber elongation factor protein (REF) | Hevea brasiliensis | P15252 | RB, S |
| 229 | Small rubber particle protein (SRPP) | Hevea brasiliensis | O82803 | RB, S |
| 156 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 139 | Rubber elongation factor (REF) | Hevea brasiliensis | P15252 | RB, S |
| 127 | C3HC4-type RING zinc finger protein | Hevea brasiliensis | AAP46154 | T |
| 110 | REP1 protein of yeast plasmid partitioning system | Saccharomyces cerevisiae | S40417 | X |
| 71 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_567195 | T |
| 70 | Expressed protein | Arabidopsis | NP_566643 | U |
| 69 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 66 | ASR-like protein | Hevea brasiliensis | AAP46155 | S |
| 64 | Rubber elongation factor (REF) | Hevea brasiliensis | AAR11448 | RB, S |
| 63 | No hits found | – | U | |
| 63 | Pro-hevein precursor | Hevea brasiliensis | P02877 | D, RB |
| 55 | Unknown protein | Arabidopsis | NP_178443 | U |
| 51 | MFP1 attachment factor | Glycine max | AAF63659 | T |
| 50 | Low molecular weight heat-shock protein | Corylus avellana | AAD15628 | S |
| 46 | Translationally controlled tumour protein (TCTP) | Hevea brasiliensis | Q9ZSW9 | X |
| 42 | Protease inhibitor protein | Hevea brasiliensis | AAP46156 | D |
| 39 | Chorismate synthase | Arabidopsis | NP_564534 | M |
| 39 | Latex abundant protein | Hevea brasiliensis | AAP46157 | U |
| 37 | ADP-ribosylation factor | Oryza sativa | NP_915954 | M |
| 37 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66432 | RB, S |
| 36 | Rubber elongation factor (REF) | Hevea brasiliensis | AAP57419 | RB, S |
| 32 | Heavy metal-associated domain-containing protein | Arabidopsis | NP_177261 | D |
| 31 | Patatin-like latex allergen/rubber biosynthesis inhibitor | Hevea brasiliensis | T10765 | RB |
| 31 | Acyl-CoA-binding protein | Panax ginseng | BAB85987 | M |
| 28 | Hexameric polyubiquitin | Arabidopsis | AAA68878 | PT |
| 28 | Galactosyl-1-phosphate transferase | Salmonella enterica | AAC44096 | X |
| 28 | Unknown protein | Homo sapiens | AAL55824 | U |
| 24 | Chloroplast precursor of ferredoxin III protein | Zea mays | P27788 | M |
| 23 | Polyubiquitin | Petroselinum crispum | CAA45621 | PT |
| 23 | Nuclear transport factor | Arabidopsis | NP_174118 | M |
| 22 | Phosphate-responsive family protein | Arabidopsis | NP_565409 | M |
| 22 | Hydroxyproline-rich glycoprotein family protein | Arabidopsis | NP_565899 | D |
| 21 | Nuclear transport factor | Arabidopsis | NP_189151 | M |
| 20 | Cis-prenyltransferase | Hevea brasiliensis | BAB71776 | RB |
| 20 | Cyclophilin | Ricinus communis | CAC80550 | D |
| 20 | 18.5 kDa class I heat shock protein | Glycine max | P05478 | S |
| 20 | Iron–sulphur cluster assembly complex protein | Arabidopsis | NP_193953 | M |
| 20 | DnaJ protein | Hevea brasiliensis | AAD12055 | S |
| 20 | Fibre protein | Gossypium barbadense | AAN77145 | X |
| 20 | Unknown protein | Arabidopsis | BAC42514 | U |
| 19 | Eukaryotic translation initiation factor 4A | Nicotiana tabacum | Q40465 | PT |
| 18 | Basic helix–loop–helix (bHLH) family protein | Arabidopsis | NP_193376 | T |
| 18 | Glycosyl transferase family protein | Arabidopsis | NP_181493 | D |
| 18 | Unknown protein | Arabidopsis | AAM64350 | U |
| 17 | Expressed protein | Arabidopsis | AAM15078 | U |
| 17 | Small rubber particle protein (SRPP) | Hevea brasiliensis | AAO66433 | RB, S |
| 17 | Elongation factor 1-alpha | Manihot esculenta | O49169 | PT |
| 17 | ASR-like protein | Hevea brasiliensis | AAP46158 | S |
| 17 | Hypothetical protein | Arabidopsis | T05538 | U |
| 17 | Caffeic acid 3-O-methyltransferase | Rosa chinensis | Q8GU25 | M |
| 16 | No apical meristem (NAM) family protein | Arabidopsis | NP_196822 | S, T |
| 16 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_191037 | T |
| 15 | Thioredoxin h | Ricinus communis | Q43636 | M |
| 15 | RNA recognition motif (RRM)-containing protein | Arabidopsis | NP_568946 | T |
| 15 | Hypothetical protein | Bacteroides thetaiotaomicron | NP_813385 | U |
| 15 | HMG CoA synthase | Hevea brasiliensis | AAL18930 | RB |
UTs are listed according to the EST frequency, and are shown with the corresponding protein match, species, GenBank accession number, and functional class of the match. Protein function was classified with reference to known laticifer processes wherever possible. RB, rubber biosynthesis; S, stress-related responses; D, defence; T, transcription-related; PT, protein turnover; M, metabolism (various); X, unusual; U, unknown.
Seven UTs matching proteins involved in transcriptional and post-transcriptional activity were identified (Table 2). Three of these are major classes of plant transcription factors: the C3HC4 Zn finger (frequency 127), basic helix–loop–helix (bHLH) (frequencies 71 and 18), and no apical meristem (NAM; frequency 16) protein families. The NAM family of proteins is particularly interesting as the laticifer is not known to be associated with apical meristematic growth. Other matches with functions not known to be relevant to the laticifer are the abundant replication protein of the Saccharomyces cerevisiae plasmid partitioning system (frequency 110), a previously isolated Hevea translationally controlled tumour protein, TCTP (frequency 46), a bacterial enzyme for assembling Gram-negative bacteria cell wall components (frequency 28), and a cotton fibre protein (frequency 20).
Overall, there are 11 UTs classified as unknown in Table 2. All of them show hits to unknown or hypothetical proteins in plant and non-plant species, except for one (frequency 63) which displayed no hit with any public database sequence. Among the unknown matches, the latex abundant protein (frequency 156 and 39) is the most highly expressed unknown EST and has previously been reported to match with an Arabidopsis hypothetical protein and not to affect IDP incorporation in rubber biosynthesis assays (Shin et al., 1999).
Expression of rubber biosynthesis pathway genes
Not all proteins in the MVA and MEP rubber biosynthesis pathways were detected among the highly expressed ESTs in Table 2. To detect these gene transcripts, a BLASTN search of the latex EST collection was first made for 13 proteins of these two pathways listed in Table 3. The search results showed that REF (1150 matches) and SRPP (375 matches) were distinctly more abundant than the other genes which showed 1–31 matches. ESTs for four genes, mevalonate kinase, phosphomevalonate kinase, FPP synthase, and IDP isomerase, were probably not captured in this collection due to very low expression.
| Rubber biosynthesis pathway genes | GenBank accession no. | EST frequency | Relative latex transcript level based on Pfaffl ratio | Mean CP±SD |
| REF | X56535 | 1150 | 426 | 9.10±0.80 |
| SRPP | AF051317 | 375 | 2932 | 5.51±0.28 |
| Cis-prenyl-transferase | AB061234 | 31 | 41 | 13.71±2.62 |
| Patatin-like inhibitor | AF113546 | 31 | 11 | 14.38±0.72 |
| HMG CoA synthase | AY534617 | 15 | 7 | 14.79±0.10 |
| HMG CoA reductase | X54659 | 10 | 4 | 16.21±1.12 |
| Rubber biosynthesis stimulator protein | AF516353 | 3 | 4 | 16.96±1.99 |
| GGPP synthase | AB055496 | 3 | 1 | 16.71±0.01 |
| DXPS | U27099 | 1 | 13 | 13.64±0.28 |
| Mevalonate kinase | AF429384 | 0 | 1 | 18.63±0.42 |
| Phosphomevalonate kinase | AF429385 | 0 | 17 | 13.17±0.03 |
| FPP synthase | Z49786 | 0 | 10 | 13.86±0.32 |
| IDP isomerase | BD301623 | 0 | 1 | 19.13±0.60 |
| 18S rRNA | AY496880 | NA | 10 000 | 4.14±0.45 |
| Rubber biosynthesis pathway genes | GenBank accession no. | EST frequency | Relative latex transcript level based on Pfaffl ratio | Mean CP±SD |
| REF | X56535 | 1150 | 426 | 9.10±0.80 |
| SRPP | AF051317 | 375 | 2932 | 5.51±0.28 |
| Cis-prenyl-transferase | AB061234 | 31 | 41 | 13.71±2.62 |
| Patatin-like inhibitor | AF113546 | 31 | 11 | 14.38±0.72 |
| HMG CoA synthase | AY534617 | 15 | 7 | 14.79±0.10 |
| HMG CoA reductase | X54659 | 10 | 4 | 16.21±1.12 |
| Rubber biosynthesis stimulator protein | AF516353 | 3 | 4 | 16.96±1.99 |
| GGPP synthase | AB055496 | 3 | 1 | 16.71±0.01 |
| DXPS | U27099 | 1 | 13 | 13.64±0.28 |
| Mevalonate kinase | AF429384 | 0 | 1 | 18.63±0.42 |
| Phosphomevalonate kinase | AF429385 | 0 | 17 | 13.17±0.03 |
| FPP synthase | Z49786 | 0 | 10 | 13.86±0.32 |
| IDP isomerase | BD301623 | 0 | 1 | 19.13±0.60 |
| 18S rRNA | AY496880 | NA | 10 000 | 4.14±0.45 |
Expression levels are based on EST frequency by BLASTN search and relative transcript level by QRT-PCR analysis of latex total RNA. BLASTN searches were performed using rubber biosynthesis-related cDNAs available for Hevea in all cases, except for DXPS where the Arabidopsis cDNA was used. Primer pairs for QRT-PCRs were designed for Hevea rubber biosynthesis-related cDNA targets including Hevea DXPS that was detected by the Arabidopsis DXPS. Transcript levels were calculated relative to Hevea 18S according to the Pfaffl mathematical model (2001) where CP is the cycle threshold. NA, not applicable; SD, standard deviation.
| Rubber biosynthesis pathway genes | GenBank accession no. | EST frequency | Relative latex transcript level based on Pfaffl ratio | Mean CP±SD |
| REF | X56535 | 1150 | 426 | 9.10±0.80 |
| SRPP | AF051317 | 375 | 2932 | 5.51±0.28 |
| Cis-prenyl-transferase | AB061234 | 31 | 41 | 13.71±2.62 |
| Patatin-like inhibitor | AF113546 | 31 | 11 | 14.38±0.72 |
| HMG CoA synthase | AY534617 | 15 | 7 | 14.79±0.10 |
| HMG CoA reductase | X54659 | 10 | 4 | 16.21±1.12 |
| Rubber biosynthesis stimulator protein | AF516353 | 3 | 4 | 16.96±1.99 |
| GGPP synthase | AB055496 | 3 | 1 | 16.71±0.01 |
| DXPS | U27099 | 1 | 13 | 13.64±0.28 |
| Mevalonate kinase | AF429384 | 0 | 1 | 18.63±0.42 |
| Phosphomevalonate kinase | AF429385 | 0 | 17 | 13.17±0.03 |
| FPP synthase | Z49786 | 0 | 10 | 13.86±0.32 |
| IDP isomerase | BD301623 | 0 | 1 | 19.13±0.60 |
| 18S rRNA | AY496880 | NA | 10 000 | 4.14±0.45 |
| Rubber biosynthesis pathway genes | GenBank accession no. | EST frequency | Relative latex transcript level based on Pfaffl ratio | Mean CP±SD |
| REF | X56535 | 1150 | 426 | 9.10±0.80 |
| SRPP | AF051317 | 375 | 2932 | 5.51±0.28 |
| Cis-prenyl-transferase | AB061234 | 31 | 41 | 13.71±2.62 |
| Patatin-like inhibitor | AF113546 | 31 | 11 | 14.38±0.72 |
| HMG CoA synthase | AY534617 | 15 | 7 | 14.79±0.10 |
| HMG CoA reductase | X54659 | 10 | 4 | 16.21±1.12 |
| Rubber biosynthesis stimulator protein | AF516353 | 3 | 4 | 16.96±1.99 |
| GGPP synthase | AB055496 | 3 | 1 | 16.71±0.01 |
| DXPS | U27099 | 1 | 13 | 13.64±0.28 |
| Mevalonate kinase | AF429384 | 0 | 1 | 18.63±0.42 |
| Phosphomevalonate kinase | AF429385 | 0 | 17 | 13.17±0.03 |
| FPP synthase | Z49786 | 0 | 10 | 13.86±0.32 |
| IDP isomerase | BD301623 | 0 | 1 | 19.13±0.60 |
| 18S rRNA | AY496880 | NA | 10 000 | 4.14±0.45 |
Expression levels are based on EST frequency by BLASTN search and relative transcript level by QRT-PCR analysis of latex total RNA. BLASTN searches were performed using rubber biosynthesis-related cDNAs available for Hevea in all cases, except for DXPS where the Arabidopsis cDNA was used. Primer pairs for QRT-PCRs were designed for Hevea rubber biosynthesis-related cDNA targets including Hevea DXPS that was detected by the Arabidopsis DXPS. Transcript levels were calculated relative to Hevea 18S according to the Pfaffl mathematical model (2001) where CP is the cycle threshold. NA, not applicable; SD, standard deviation.
Subsequently, QRT-PCR analysis was used to investigate the in vivo transcription of biosynthesis-related Hevea genes in latex RNA. The latex total RNA used had a RIN of 7.5 indicating highly intact RNA (Fleige et al., 2006) which is suitable for QRT-PCR analysis. As shown in Table 3, the abundance of all target transcripts amplified from the latex RNA sample was lower than that of the Hevea 18S gene transcript. In considering the number of ESTs and the relative transcript levels, both expression profiles showed REF and SRPP as the most abundant proteins of rubber biosynthesis. It was noted, nevertheless, that while a large number of REF ESTs was recorded, more gene transcripts were detected for SRPP by QRT-PCR (Table 3). Transcripts of the four genes not found in the EST collection (mevalonate kinase, phosphomevalonate kinase, FPP synthase, and IDP isomerase) were detected using QRT-PCR analysis. This confirmed the presence of these gene transcripts in latex but indicated that their absence as ESTs was due to limitation of the depth of EST generation in this study.
Rubber particle membrane protein (RPMP) isoforms
Observation of the high abundance of REF and SRPP led to the investigation of the ESTs for these membrane proteins. To do this, a BLASTN search of latex UTs was performed using published cDNA sequences of REF (X56535) and SRPP (AF051317) from which 37 UTs were identified. Predicted translation frames of the consensus sequences showed that 14 of these UTs contained REF and SRPP proteins including variant sequences. A total of nine full-length protein isoforms were observed among the 14 UTs based on the predicted protein translations of their consensus sequences (Table 4). Subsequently, the authenticity of their amino acid sequences was confirmed by double-stranded sequencing of a representative EST from each consensus.
| REF/SRPP protein isoform code based on N-terminus | No. of UTs | EST frequency of UTs | Molecular weight (kDa) | Available Hevea sequences in GenBank (accession no.) |
| MAEEV | 2 | 229, 7 | 22.4 | SRPP cDNA (AF051317) |
| MAEGEG | 1 | 17 | 27.0 | Partial SRPP-like EST (AY237010) |
| MAEGK | 2 | 37, 4 | 12.7 | SRPP-like EST (AY237009) |
| MAEDED | 4 | 820, 9, 5, 1 | 14.7 | REF cDNA (X56535) |
| MAEG (1) | 1 | 64 | 19.5 | – |
| MAEG (2) | 1 | 4 | 19.6 | REF-like EST (AY430052) |
| MAEG (3) | 1 | 2 | 11.0 | – |
| MAEG (4) | 1 | 2 | 7.9 | – |
| MGEG | 1 | 11 | 11.9 | – |
| REF/SRPP protein isoform code based on N-terminus | No. of UTs | EST frequency of UTs | Molecular weight (kDa) | Available Hevea sequences in GenBank (accession no.) |
| MAEEV | 2 | 229, 7 | 22.4 | SRPP cDNA (AF051317) |
| MAEGEG | 1 | 17 | 27.0 | Partial SRPP-like EST (AY237010) |
| MAEGK | 2 | 37, 4 | 12.7 | SRPP-like EST (AY237009) |
| MAEDED | 4 | 820, 9, 5, 1 | 14.7 | REF cDNA (X56535) |
| MAEG (1) | 1 | 64 | 19.5 | – |
| MAEG (2) | 1 | 4 | 19.6 | REF-like EST (AY430052) |
| MAEG (3) | 1 | 2 | 11.0 | – |
| MAEG (4) | 1 | 2 | 7.9 | – |
| MGEG | 1 | 11 | 11.9 | – |
Nine RPMP isoforms including REF and SRPP were identified from 14 latex UTs.
| REF/SRPP protein isoform code based on N-terminus | No. of UTs | EST frequency of UTs | Molecular weight (kDa) | Available Hevea sequences in GenBank (accession no.) |
| MAEEV | 2 | 229, 7 | 22.4 | SRPP cDNA (AF051317) |
| MAEGEG | 1 | 17 | 27.0 | Partial SRPP-like EST (AY237010) |
| MAEGK | 2 | 37, 4 | 12.7 | SRPP-like EST (AY237009) |
| MAEDED | 4 | 820, 9, 5, 1 | 14.7 | REF cDNA (X56535) |
| MAEG (1) | 1 | 64 | 19.5 | – |
| MAEG (2) | 1 | 4 | 19.6 | REF-like EST (AY430052) |
| MAEG (3) | 1 | 2 | 11.0 | – |
| MAEG (4) | 1 | 2 | 7.9 | – |
| MGEG | 1 | 11 | 11.9 | – |
| REF/SRPP protein isoform code based on N-terminus | No. of UTs | EST frequency of UTs | Molecular weight (kDa) | Available Hevea sequences in GenBank (accession no.) |
| MAEEV | 2 | 229, 7 | 22.4 | SRPP cDNA (AF051317) |
| MAEGEG | 1 | 17 | 27.0 | Partial SRPP-like EST (AY237010) |
| MAEGK | 2 | 37, 4 | 12.7 | SRPP-like EST (AY237009) |
| MAEDED | 4 | 820, 9, 5, 1 | 14.7 | REF cDNA (X56535) |
| MAEG (1) | 1 | 64 | 19.5 | – |
| MAEG (2) | 1 | 4 | 19.6 | REF-like EST (AY430052) |
| MAEG (3) | 1 | 2 | 11.0 | – |
| MAEG (4) | 1 | 2 | 7.9 | – |
| MGEG | 1 | 11 | 11.9 | – |
Nine RPMP isoforms including REF and SRPP were identified from 14 latex UTs.
As shown in Table 4, the predicted molecular weights of the nine variant protein isoforms range from 7.9 to 27 kDa. Three of the nine isoforms, coded as MAEDED, MAEEV, and MAEGK, show redundancy of the genetic code as more than one UT was found in each case. A multiple alignment of the nine isoforms is shown in Fig. 3A. The four MAEG isoforms show variations in internal protein segments, suggesting alternative transcript splicing. Even so, each of them originated from independent UTs, indicating that they represent four different genes. In total, the nine protein isoforms consist of 1212 ESTs or 12% of the latex EST collection. As shown in Table 4, the REF (X56535) and SRPP (AF051317) cDNAs (or isoforms MAEDED and MAEEV, respectively) belong to UTs with the highest frequencies (820 and 229). Therefore, it is not surprising that these cDNAs were the first to be cloned (Goyvaerts et al., 1991; Oh et al., 1999). Since the analysis revealed nine protein variants derived from as many as 14 genes, they were named RPMPs or rubber particle membrane proteins. A dendrogram (Fig. 3B) indicated that the RPMPs may be categorized into three protein families. Previously, a cDNA (AF541942) encoding a 26.2 kDa guayule rubber particle protein (GHS) was proposed to be a homologue of Hevea SRPP (Kim et al., 2004). Interestingly, a 27 kDa RPMP, MAEGEG, identified in this study (Table 4) is much closer in molecular weight and does not belong to the same protein family as SRPP (Fig. 3A). Thus, a comparison was made between the GHS and MAEGEG protein sequences. The protein sequence similarity was 39.0% between GHS and MAEGEG, and 32.1% between GHS and SRPP. Therefore, it is deduced that the 27 kDa RPMP isoform (MAEGEG) is a more likely homologue of guayule GHS based on size and protein sequence similarity.

Multiple alignment (A) and dendrogram (B) of nine full-length RPMP isoforms. Protein sequence alignment was presented using BOXSHADE version 3.2 at a threshold fraction of 0.3 (no reference sequence) where black boxes indicate identical and grey boxes indicate similar residues.
Rubber particle proteins were isolated from latex for western blot analysis to detect RPMP isoforms. As shown in Fig. 4, REF and SRPP antibodies detected major proteins of molecular weights 14 kDa and 22 kDa in the latex sample. As a result of separate secondary labelling systems of the antibodies, both of these proteins were differentiated by colour. The molecular weight of SRPP has been predicted as 22.4 kDa by cDNA translation (Oh et al., 1999). This is consistent with the mass spectrometry measurements by Yeang et al. (1998) who noted that the protein tended to migrate anomalously in SDS–polyacrylamide gels. As shown in Fig. 4, the SRPP-specific antibody also detected less abundant proteins with intermediate molecular weights. The most prominent one of these may be correlated with the RPMP of molecular weight 19.5 kDa or the most abundant of the four MAEG isoforms (Table 4) since it belongs to the third highest frequency group (64) after REF and SRPP.
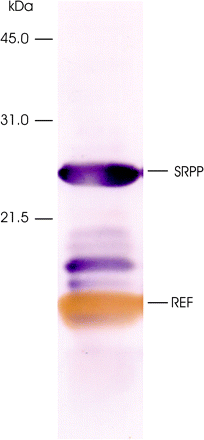
REF (brown band) and SRPP proteins (purple bands) on a western immunoblot of latex total proteins detected using antibodies against the respective proteins.
Comparative sequence analysis
A comparative analysis of RPMP homologues in other plants was performed by searching the TIGR Plant Transcript Assemblies Database (http://www.tigr.org/tdb/e2k1/plantta/). Thirty-seven matches were obtained when cDNA sequences of REF (X56535) and SRPP (AF051317) were used to perform a TBLASTX search of transcript assemblies (TAs) of ESTs of 185 species. These TA matches consist of 30 species from 10 families of the database (Table 5). The highest number of TA matches was to poplar tree species of the Salicaceae family (10) followed by species from the Asteraceae (seven) and Rosaceae (five) families. The sequence identities of these TAs fell into four annotation groups as shown in Table 5. The identity of the largest group was an Arabidopsis genomic sequence of unknown function comprising nearly 60% of the TAs. About 35% of the TAs showed matches to sequences annotated as stress-related proteins as found in two of the groups. The ‘rubber synthesis protein-related cluster’ was represented only by two lettuce species of the Asteraceae family and showed a 70.9% identity match to a rubber particle protein from guayule (Parthenium argentatum), previously reported to be an SRPP homologue (Kim et al., 2004). None of the Euphorbiaceae species in the TIGR Plant Transcript Assemblies Database was found at all in this group.
| Family | Transcript assemblies | No. of species | % Identity |
| Protein At3g05500-related cluster | |||
| Salicaceae (Willow family) | CN524303, TA178_3691, AJ779375, TA2009_3694, TA177_3691, TA1702_47664, TA8622_3694, TA2019_3695, TA7401_3695 | 5 | 69.6–73.5 |
| Asteraceae (Daisy family) | TA947_4236, TA365_75943, TA113_333970, TA4900_4232 | 4 | 66.5–68.7 |
| Rosaceae (Rose family) | TA3835_3750, DT043735, TA3834_3750, CO899615 | 2 | 72.7–74.3 |
| Malvaceae (Mallow family) | TA313_29729, TA346_3635 | 2 | 72.3 |
| Euphorbiaceae (Spurge family) | TA987_3993, DV137844 | 1 | 75.1–75.7 |
| Rutaceae (Rue family) | TA205_37656 | 1 | 72.3 |
| Stress-related protein related cluster | |||
| Euphorbiaceae (Spurge family) | TA452_3983, BP955337 | 2 | 66.1–68.1 |
| Salicaceae (Willow family) | CV243552 | 1 | 76.9 |
| Asteraceae (Daisy family) | BU011407 | 1 | 72.1 |
| Rosaceae (Rose family) | TA7857_3750 | 1 | 72.4 |
| Rutaceae (Rue family) | CN187632 | 1 | 81.3 |
| Ranunculaceae (Buttercup family) | TA5039_338618 | 1 | 71.0 |
| Rhizophoraceae (Red Mangrove family) | TA208_39984 | 1 | 70.3 |
| Fabaceae (Pea family) | TA940_47247 | 1 | 84.6 |
| Hypothetical protein P0001A07.13-related cluster, stress-related | |||
| Solanaceae (Nightshade family) | TA908_4081, TA217_62890, TA103_4072, TA9088_4113 | 4 | 60.1–62.3 |
| Rubber synthesis protein-related cluster | |||
| Asteraceae (Daisy family) | TA2822_75943, TA5585_4236 | 2 | 70.9 |
| Family | Transcript assemblies | No. of species | % Identity |
| Protein At3g05500-related cluster | |||
| Salicaceae (Willow family) | CN524303, TA178_3691, AJ779375, TA2009_3694, TA177_3691, TA1702_47664, TA8622_3694, TA2019_3695, TA7401_3695 | 5 | 69.6–73.5 |
| Asteraceae (Daisy family) | TA947_4236, TA365_75943, TA113_333970, TA4900_4232 | 4 | 66.5–68.7 |
| Rosaceae (Rose family) | TA3835_3750, DT043735, TA3834_3750, CO899615 | 2 | 72.7–74.3 |
| Malvaceae (Mallow family) | TA313_29729, TA346_3635 | 2 | 72.3 |
| Euphorbiaceae (Spurge family) | TA987_3993, DV137844 | 1 | 75.1–75.7 |
| Rutaceae (Rue family) | TA205_37656 | 1 | 72.3 |
| Stress-related protein related cluster | |||
| Euphorbiaceae (Spurge family) | TA452_3983, BP955337 | 2 | 66.1–68.1 |
| Salicaceae (Willow family) | CV243552 | 1 | 76.9 |
| Asteraceae (Daisy family) | BU011407 | 1 | 72.1 |
| Rosaceae (Rose family) | TA7857_3750 | 1 | 72.4 |
| Rutaceae (Rue family) | CN187632 | 1 | 81.3 |
| Ranunculaceae (Buttercup family) | TA5039_338618 | 1 | 71.0 |
| Rhizophoraceae (Red Mangrove family) | TA208_39984 | 1 | 70.3 |
| Fabaceae (Pea family) | TA940_47247 | 1 | 84.6 |
| Hypothetical protein P0001A07.13-related cluster, stress-related | |||
| Solanaceae (Nightshade family) | TA908_4081, TA217_62890, TA103_4072, TA9088_4113 | 4 | 60.1–62.3 |
| Rubber synthesis protein-related cluster | |||
| Asteraceae (Daisy family) | TA2822_75943, TA5585_4236 | 2 | 70.9 |
Homologues were identified based on TBLASTX matches with transcript assemblies (TAs) in the TIGR Plant Transcript Assemblies Database, and were grouped into four annotation clusters. All matches are at E-value <1.1E-23.
| Family | Transcript assemblies | No. of species | % Identity |
| Protein At3g05500-related cluster | |||
| Salicaceae (Willow family) | CN524303, TA178_3691, AJ779375, TA2009_3694, TA177_3691, TA1702_47664, TA8622_3694, TA2019_3695, TA7401_3695 | 5 | 69.6–73.5 |
| Asteraceae (Daisy family) | TA947_4236, TA365_75943, TA113_333970, TA4900_4232 | 4 | 66.5–68.7 |
| Rosaceae (Rose family) | TA3835_3750, DT043735, TA3834_3750, CO899615 | 2 | 72.7–74.3 |
| Malvaceae (Mallow family) | TA313_29729, TA346_3635 | 2 | 72.3 |
| Euphorbiaceae (Spurge family) | TA987_3993, DV137844 | 1 | 75.1–75.7 |
| Rutaceae (Rue family) | TA205_37656 | 1 | 72.3 |
| Stress-related protein related cluster | |||
| Euphorbiaceae (Spurge family) | TA452_3983, BP955337 | 2 | 66.1–68.1 |
| Salicaceae (Willow family) | CV243552 | 1 | 76.9 |
| Asteraceae (Daisy family) | BU011407 | 1 | 72.1 |
| Rosaceae (Rose family) | TA7857_3750 | 1 | 72.4 |
| Rutaceae (Rue family) | CN187632 | 1 | 81.3 |
| Ranunculaceae (Buttercup family) | TA5039_338618 | 1 | 71.0 |
| Rhizophoraceae (Red Mangrove family) | TA208_39984 | 1 | 70.3 |
| Fabaceae (Pea family) | TA940_47247 | 1 | 84.6 |
| Hypothetical protein P0001A07.13-related cluster, stress-related | |||
| Solanaceae (Nightshade family) | TA908_4081, TA217_62890, TA103_4072, TA9088_4113 | 4 | 60.1–62.3 |
| Rubber synthesis protein-related cluster | |||
| Asteraceae (Daisy family) | TA2822_75943, TA5585_4236 | 2 | 70.9 |
| Family | Transcript assemblies | No. of species | % Identity |
| Protein At3g05500-related cluster | |||
| Salicaceae (Willow family) | CN524303, TA178_3691, AJ779375, TA2009_3694, TA177_3691, TA1702_47664, TA8622_3694, TA2019_3695, TA7401_3695 | 5 | 69.6–73.5 |
| Asteraceae (Daisy family) | TA947_4236, TA365_75943, TA113_333970, TA4900_4232 | 4 | 66.5–68.7 |
| Rosaceae (Rose family) | TA3835_3750, DT043735, TA3834_3750, CO899615 | 2 | 72.7–74.3 |
| Malvaceae (Mallow family) | TA313_29729, TA346_3635 | 2 | 72.3 |
| Euphorbiaceae (Spurge family) | TA987_3993, DV137844 | 1 | 75.1–75.7 |
| Rutaceae (Rue family) | TA205_37656 | 1 | 72.3 |
| Stress-related protein related cluster | |||
| Euphorbiaceae (Spurge family) | TA452_3983, BP955337 | 2 | 66.1–68.1 |
| Salicaceae (Willow family) | CV243552 | 1 | 76.9 |
| Asteraceae (Daisy family) | BU011407 | 1 | 72.1 |
| Rosaceae (Rose family) | TA7857_3750 | 1 | 72.4 |
| Rutaceae (Rue family) | CN187632 | 1 | 81.3 |
| Ranunculaceae (Buttercup family) | TA5039_338618 | 1 | 71.0 |
| Rhizophoraceae (Red Mangrove family) | TA208_39984 | 1 | 70.3 |
| Fabaceae (Pea family) | TA940_47247 | 1 | 84.6 |
| Hypothetical protein P0001A07.13-related cluster, stress-related | |||
| Solanaceae (Nightshade family) | TA908_4081, TA217_62890, TA103_4072, TA9088_4113 | 4 | 60.1–62.3 |
| Rubber synthesis protein-related cluster | |||
| Asteraceae (Daisy family) | TA2822_75943, TA5585_4236 | 2 | 70.9 |
Homologues were identified based on TBLASTX matches with transcript assemblies (TAs) in the TIGR Plant Transcript Assemblies Database, and were grouped into four annotation clusters. All matches are at E-value <1.1E-23.
Discussion
Hevea latex ESTs are derived from the cytoplasmic content of a single cell type, the laticifer. As such, latex transcriptome analysis is unique because, in itself, it constitutes a study of single-cell genomics. The rubber tree is estimated to have a genome DNA content of about 2 pg (1C) based on flow cytometry (Bennet and Leitch, 1997). The 3441 UTs identified in the present work represent a very small proportion of expressed genes, and the high proportion of ESTs of unknown function, especially the abundant ones, will be a challenge for functional characterization in future. However, the larger scale of EST analysis in the present work compared with the earlier analysis of 1176 latex ESTs by Ko et al. (2003) provides a new estimate of gene families as reflected by the number of UTs. While Ko et al. (2003) reported that 51.9% of 1176 latex ESTs analysed code for seven gene families, 53.5% of total ESTs in this study generated 360 UTs. Clearly, greater depth of EST generation leads to discovery of more genes expressed in the laticifer. EST redundancy rate analysis indicated that it is still feasible to discover new UTs by sequencing more clones from the latex cDNA library. It is proposed that only when the EST redundancy shows a drastic escalation should the level of complexity of the latex transcriptome be re-evaluated.
Both broad and narrow approaches were adopted for functional classification of UTs. While GO produced broad-based functional categories, manual curation of gene identities provided insights specific to the latex transcriptome especially in rubber biosynthesis. Bioinformatic analysis of latex ESTs revealed that genes for rubber biosynthesis pathway proteins rank as the most abundant category of transcripts. Both EST and QRT-PCR analyses also showed that REF and SRPP dominate the latex expression profile. The high prevalence of rubber biosynthesis-related genes is consistent with the role of the laticifer as the main site of cis-polyisoprene biosynthesis, but wide variations in gene expression suggest different degrees of transcriptional control for each pathway step. Besides being a source of natural rubber, a role commonly ascribed to laticifer function is defence. Support for this has been shown by detection of high levels of defence-related proteins such as chalcone synthase, chitinase, phenylalanine ammonia-lyase, β-1,3-glucanase, and lysozymes (Kush et al., 1990; Martin, 1991; Chye and Cheung, 1995), and hevein, a latex anti-fungal protein (Broekaert et al., 1990; Van Parjis et al., 1991). Further support is provided by the finding that expression of these genes is higher in latex than in other plant parts such as leaves, stems, and roots. Unlike hevein, ESTs for these proteins were not highly expressed, probably due to the depth of EST generation in this study. Based on structural studies, cis-polyisoprene is also proposed to be a scavenger of free radicals and, thus, controls cellular oxidative damage (Tangpakdee and Tanaka, 1998; Sakdapipanich et al., 1999). A high abundance of ESTs with defence and stress responses to biotic and abiotic factors has been detailed in the present analysis of latex ESTs. Complementing this biochemical battery is the invasive laticiferous network, not present only in the axis of the embryo (Parkin, 1900), which together confer a highly efficient infrastructure for eliciting systemic defence responses.
Plants which conduct both pathways of IDP synthesis utilize the plastidic MEP pathway for synthesis of chloroplast-located isoprenoids and the cytosolic mevalonate pathway for synthesis of sterols (Arigoni et al., 1997; Lichtenthaler, 1999; Eisenreich et al., 2001). A specialized plastid of Hevea latex, the Frey–Wyssling particle, is notably rich in carotenes (Gomez and Moir, 1979) and is thus likely to contain MEP pathway metabolism. Additionally, Frey–Wyssling particles have been suggested to perform important metabolic functions including rubber biosynthesis (Dickenson, 1969). This concurs with reports on the biosynthesis capacity of non-rubber particles of latex bottom fraction which contains predominantly Frey–Wyssling particles and lutoids (Tangpakdee et al., 1997; Wititsuwannakul et al., 2003). IDP generated by Frey–Wyssling particles may be transported out into the cytosol as a substrate for further IDP condensation in the mevalonate pathway. This could explain the highest expression of RPMP genes as shown by EST frequency analysis and QRT-PCR data in order to cater for substrates supplied by two independent routes. Constitutive production of these rubber particle proteins may be required to maintain the large rubber fraction in the laticifer which constitutes 30–50% by weight of latex (Kekwick, 1989). Nonetheless, there is no experimental evidence for dual IDP sources for Hevea rubber biosynthesis although evidence of metabolite crossover between both IDP-generating pathways has been shown in Arabidopsis (Kasahara et al., 2002) and tobacco (Hemmerlin et al., 2003). In future, biosynthesis assays comparing the in vitro capacity of washed rubber particles and Frey–Wyssling particles to incorporate substrates of MVA and MEP pathways will be useful for investigating the role of both organelles in rubber biosynthesis.
Historically, the 14 kDa REF and 22 kDa SRPP proteins were first named in the context of their roles in rubber biosynthesis and have received the most attention because they are the most abundant rubber particle membrane proteins. In the past, additional proteins of different molecular weights have been observed in SDS–polyacrylamide gels, although less consistently, and were generally regarded as breakdown or aggregate forms (Yeang et al., 1996) or contaminating latex bottom fraction proteins (Wititsuwannakul et al., 2004). However, immunological detection of REF and SRPP isoforms in addition to the 14 kDa and 22 kDa proteins in this report further confirms the existence of multiple isoforms. The partial and inconsistent presence of isoforms in independent membrane protein preparations from equivalent latex collections (which may vary physiologically due to field environmental conditions that are beyond control) could be a result of differential expression of isoforms. Evidence for this is suggested by the broad range of abundance of RPMPs and by the reverse proportions of REF and SRPP when comparing EST and QRT-PCR expression data. In this respect, it is postulated that the nine isoforms, or subsets of them, could play specific roles in rubber formation. Enhancement of IDP incorporation based on in vitro biosynthesis assays containing isolated rubber particles has previously been demonstrated for recombinant SRPP (Oh et al., 1999) and REF proteins (KS Chow, unpublished data). In future, similar assays using recombinant proteins of the other RPMP isoforms would be a useful approach for comparing their relative effects on IDP incorporation.
The occurrence of RPMP-like sequences in other plant species indicates that they are far less unique to Hevea than previously anticipated in spite of their role in cis-polyisoprene biosynthesis. A significant association with genes of unknown function in both latex-bearing and non-bearing plants indicates a diversification of physiological function in the plant kingdom. In the Hevea rubber tree, numerous matches with stress-related proteins suggest that the RPMP sequences may have evolved to perform a dual role played in stress response and rubber biosynthesis. Unlike the Asteraceae family, sequences of Euphorbiaceae species with rubber biosynthesis function were not identified from the TIGR Plant Transcript Assemblies Database. This is probably because of the lack of rubber-producing species in the database, especially major species such as guayule (P. argentatum), Ficus elastica, Ficus carica, and Ficus benghalensis. Although ESTs are currently not available for these species, much has been researched on their rubber particle proteins and their functions in the rubber biosynthesis machinery (Kang et al., 2000a, b; Cornish, 2001). Studies have indicated that while Hevea SRPP did not have homologues in rubber particles from F. carica and F. benghalensis (Singh et al., 2003), a guayule cDNA encoding a 26.2 kDa protein (GHS) isolated by Kim et al. (2004) was proposed to correspond to a 26 kDa protein of guayule rubber particles (Cornish and Backhaus, 1990; Backhaus et al., 1991) and to be the guayule homologue for Hevea SRPP. However, the present investigation of three Hevea RPMP protein families suggests that a previously unreported 27 kDa RPMP may be a more likely homologue of the guayule rubber particle protein.
In conclusion, a genomics approach in the form of EST sequence analysis was undertaken to gain insights into rubber biosynthesis in Hevea. Initial transcriptome profiling consequently led to detailed sequence analysis of RPMPs, the most abundant category of ESTs, and further experimental investigations using QRT-PCR and rubber particle membrane protein analyses supported the EST observations. The findings continue to reinforce the fact that the entire process of Hevea cis-polyisoprene biosynthesis involves the participation of numerous proteins, varying regulatory control of their expression, and interactions between them in the rubber biosynthesis machinery.
Abbreviations
- acetyl CoA
acetyl coenzyme A
- ASR
abscisic acid, stress and ripening
- BLAST
basic local alignment search tool
- DMAPP
dimethylallyl diphosphate
- DXPS
1-deoxy-d-xylulose 5-phosphate synthase
- EST
expressed sequence tag
- FPP
farnesyl diphosphate
- GA3P
D-glyceraldehyde-3-phosphate
- GGPP
geranylgeranyl diphosphate
- GO
Gene Ontology
- GPP
geranyl diphosphate
- HMBPP
1-hydroxy-2-methyl-2-butenyl-4-diphosphate
- HMG CoA
3-hydroxy-3-methylglutaryl coenzyme A
- IDP
isopentenyl diphosphate
- MEP
2-C-methyl-d-erythritol 4-phosphate
- MVA
mevalonate
- PBS
phosphate-buffered saline
- QRT-PCR
quantitative reverse transcription-PCR
- REF
rubber elongation factor
- RIN
RNA integrity number
- RPMP
rubber particle membrane protein
- SDS–PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- SRPP
small rubber particle protein
- TA
transcript assembly
- TBS
Tris-buffered saline
- UT
unique transcript
We thank the Malaysian Rubber Board and the Malaysian Ministry of Science, Technology and Innovation for research funding, and Salimah Mohd. Lim, Halimah Alias, Nancy Liew, Janet Loh, Amos Tan, and Yin-Ho Loke for technical support.
References
Author notes
Present address: Sime Darby Technology Centre, Jalan Tandang, 46050 Petaling Jaya, Selangor, Malaysia




Comments