-
PDF
- Split View
-
Views
-
Cite
Cite
Guillaume Faure, Tiphaine Goulenok, Sylvie Lariven, Antoine Dossier, Marie-Cécile Henry-Feugeas, Nicolas Argy, Thomas Papo, Eosinophilic meningomyelitis caused by Toxocara spp. in a migrant coming from La Reunion, Journal of Travel Medicine, Volume 28, Issue 6, August 2021, taab075, https://doi.org/10.1093/jtm/taab075
Close - Share Icon Share
Case Report
On March 2018, a 23-year-old man first presented in our hospital, in Paris, with headache, back pain, progressive urinary incontinence and gait instability. The patient was raised on a farm in La Reunion, a French island in the Indian Ocean. He had travelled once to Madagascar a few years ago and moved to Paris (France) in February 2018. His medical history was unremarkable except for epilepsy during childhood, for which he received no treatment. Neurologic symptoms gradually worsened over the previous 4 months. No fever, eye, digestive or respiratory symptoms were reported.
Clinical examination showed lower limb weakness, diffuse sensory loss and dysesthesia with a sensory level at T4 and bilateral Babinski reflex.
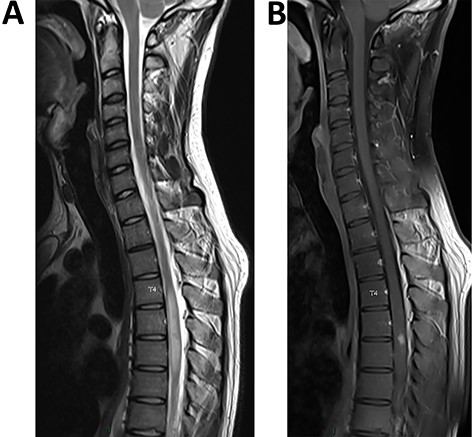
(A) (sagittal T2 sequence) Single long intramedullary lesion: spinal MRI showed swelling and diffuse T2 hyperintensity of the spinal cord from T4 to T7 levels. (B) (sagittal post-contrast T1 sequence) Nodular enhancement in the posterior region at the T5/T6 level
Blood cell count showed eosinophilia (1100 cell/mm3). MRI of the spinal cord disclosed extensive enhancing lesion of the spinal cord from T4 to T7 levels and nodular enhancement in the posterior region at the T5/T6 level (Figure 1a and b). Brain MRI was normal. CSF analysis showed 100 cells/mm3 and 82% eosinophils; protein level was 86 mg/dl (range 15–60) and glucose level was normal at 52 mg/dl (range 40–70). Blood glucose level was 77 mg/dl and C-reactive protein was 48 mg/l (range ≤5). CSF bacterial cultures and a large viral PCR panel were negative. Other causes of myelitis, as autoimmune or inflammatory diseases, were ruled out [antinuclear and anti-neutrophil cytoplasmic antibodies (ANCA), aquaporin 4 and myelin oligodendrocyte glycoprotein (MOG) antibodies in serum and CSF, intrathecal synthesis in CSF, anti-neuronal antibodies in serum and CSF were negative, 18F-fluorodesoxyglucose positron emission tomography was normal].
Parasitologic investigations such as stool examination and parasitic serologic assays for schistosomiasis, cysticercosis, strongyloidiasis, fascioliasis, toxoplasmosis, trichinellosis, echinococcosis, angiostrongyliasis and gnathostomiasis were negative. Enzyme-linked immunosorbent assay (ELISA) testing for anti-Toxocara antibodies was positive in serum [IgG anti-Toxocara canis 46 Antibody Unit (AU) (cutoff value of 9 AU)]. Western blot (WB) testing was positive in serum with three bands [p24–p35 (kDA), cutoff value of 2 bands] and in CSF with five bands highlighting a ratio (number of relevant WB bands) higher in CSF than in blood, suggesting intrathecal synthesis.
Eosinophilic meningomyelitis secondary to a toxocariasis was diagnosed.
A treatment with albendazole for 14 days (400 mg BID) and steroids (prednisone 1 mg/kg) was initiated. Clinical examination and MRI were repeated at 3, 6 and 12 months, and CSF had normalized at 12 months. At the last visit, 18 months after initial presentation, the patient could walk and stand normally with no pain but complained from some degree of sphincter dysfunction. The blood eosinophil count had normalized. Specific WB was negative in CSF. The spinal cord MRI showed partial regression of the signs of myelitis (Figure 2a and b).
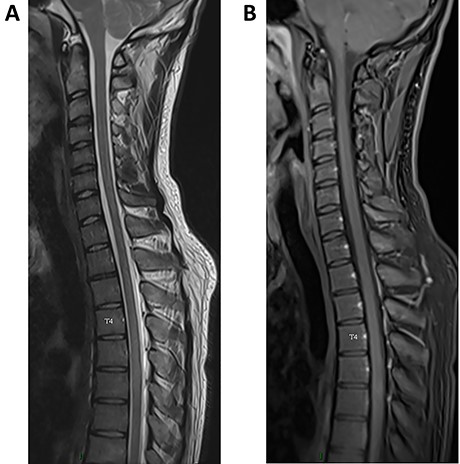
(A) (sagittal T2 sequence) Regression of the hyperintensity of the spinal cord. (B) (post-contrast T1sequence) Regression of the nodular enhancement
Toxocariasis is a ubiquitous helminthiasis infection caused by the larval stages of the roundworms Toxocara spp., mostly found in dogs and cats. In humans, transmission occurs through surface or food soiled with animal feces and can also be acquired by ingestion of undercooked meat from infected rabbit and birds. After ingestion of the eggs, the larvae reach the small bowel where they penetrate through the intestinal wall and migrate to organs such as liver, muscles, lungs, eyes and brain.1 Toxocariasis may be subclinical or responsible for lung or abdominal—visceral larva migrans—or eye—ocular larva migrans—manifestations. Neurotoxocariasis is exceedingly rare and manifests as meningitis, myelitis, encephalitis, cerebral vasculitis, brain abscess or optic neuritis.2 Diagnosis of spinal toxocariasis is based on eosinophilia, high titers of anti-Toxocara antibodies in blood or CSF and a typical spinal lesion.3 Added value of CSF is worthwhile because some cases show negative antibody levels in blood but positivity in CSF.4 Baseline seroprevalence can be very high in tropical areas, whether from cross reactions or prior infections.5 Prevalence of CSF antibodies is unknown.
ELISA detect IgG isotype antibodies directed against Toxocara excretory secretory antigens TES-Ag (ELISA-TES IgG). Sensitivity and specificity of TES-ELISA are reported to be 91 and 86%, respectively, making it an accurate diagnostic tool.6 WB showing antibody–antigen reaction bands of low molecular weight (24–35 kDa) is more specific than ELISA and is used for confirmation.6,7
Of note, eosinophilic meningomyelitis may also be caused by other helminthiases such as schistosomiasis, angiostrongylosis, gnathostomiasis, baylisascariasis or cysticercosis. The treatment usually includes albendazole or diethylcarbamazine associated with corticosteroids. Clinical, biological and MRI response to treatment stands as a confirmatory clue for the diagnosis. Outcome is usually good under anti-helmintic drugs. Mortality is very low but neurological sequelae are not uncommon.
Author contribution
G.F. and T.G. conceived the project. S.L., A.D. and N.A. collected the data. M.C.H.-F. selected and described MRI imaging. G.F., T.G. and T.P. analysed the data and wrote the manuscript. All authors have approved the final manuscript.
Funding
The authors have declared no sources of funding.
Conflict of interest: The authors have declared no conflicts of interest.



