-
PDF
- Split View
-
Views
-
Cite
Cite
Sylvia Lemos Hinrichsen, Mariana Vieira Neves, Matheus Vinicius de Araújo Lucena, Tatiana de Aguiar Santos Vilella, Gnathostoma spp. infection in a traveller to the Amazon region, Journal of Travel Medicine, Volume 30, Issue 8, December 2023, taad142, https://doi.org/10.1093/jtm/taad142
Close - Share Icon Share
A 53-year-old female, residing in the city of São Paulo, presented with a complaint of migratory swelling ~3 days after consuming raw fish, such as Peacock Bass (Tucunaré), following a leisure trip. She stayed at an ecotourism resort located in the Amazon region, alongside the banks of the Roosevelt River, for about a week. The trip took place in October 2020, during a period of lower COVID-19 cases, after the liberation of air travel to destinations considered of lower risk for pandemic-related illnesses.
The patient reported that her clinical condition began with a primary skin lesion in the epigastric region. Her family members, who were also with her during the trip, developed similar lesions on their feet and ankles. They attempted self-treatment with topical copaiba oil, a single dose of albendazole and also ivermectina, besides cefuroxime without therapeutic success.
Following empirical therapy, the lesions on the family members regressed, but the patient’s lesion persisted, accompanied by worsening epigastric discomfort. This prompted her to seek out a gastroenterologist. During the physical examination, a topic manipulation by the physician revealed a lesion with migratory characteristics, affecting both the left and right breasts (Figure 1), as well as the back (Figure 2).
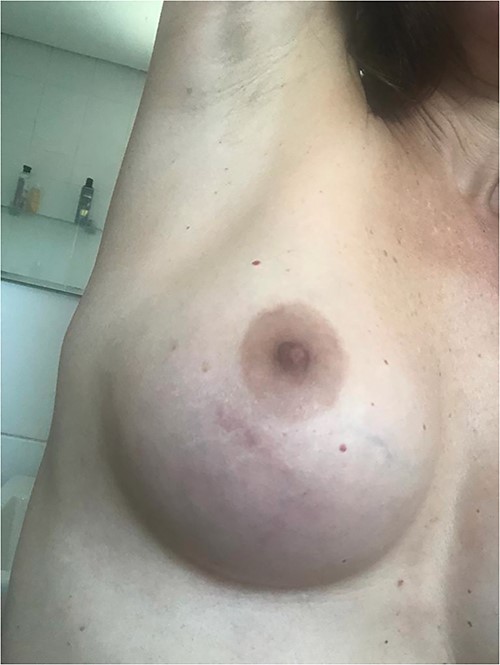
Erythematous-oedematous lesion with serpiginous linear path of migratory nature located on the right breast.
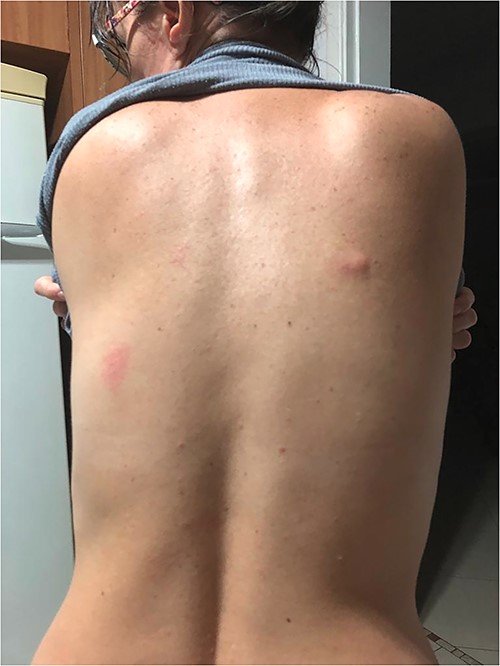
Erythematous-oedematous lesions with linear path located on the back.
Due to the new presentation of the skin lesions, the patient underwent imaging tests, which were inconclusive. After consulting an infectious disease specialist in São Paulo, she was referred to a specialized centre to undergo a biopsy of the lesions. However, she did not proceed with the biopsy as she opted to seek a second opinion from another specialist through a teleconsultation in the field of infectious diseases, which took place 1 month after the onset of signs and symptoms.
During this teleconsultation, considering the clinical presentation, persistent eosinophilia and the history of travel to the Amazon region with consumption of raw fish, Peacock Bass (Tucunaré), by both the patient and all other family members during their stay, suspicion has arisen regarding Gnathostoma spp. infection.
The parasite’s life cycle is metaxenic, developing in intermediate hosts (crustaceans and freshwater and saltwater fish), and having domestic and wild mammals as definitive hosts, with the possible involvement of paratenic hosts including humans, reptiles and birds.1,2
Therefore, given the possibility of gnathostomiasis, treatment with oral albendazole was initiated at a dose of 400 mg/day for 21 days. After 10 days, a regression of the lesions was observed, with only the persistence of erythematous macules remaining, which began to regress from the 15th day. Following the completion of the prescribed treatment, a complete resolution of the condition was achieved.
Gnathostomiasis is a zoonotic disease that remains poorly studied in many countries around the world, despite its significant importance. It is particularly relevant among travellers in endemic areas where people consume raw or undercooked freshwater fish and eels, which serve as intermediate hosts for the parasite. An example of such a fish is the Tucunaré (Cichla ocellaris), found in countries such as Thailand, Japan, Peru, Ecuador, various countries in Central and South America, including Brazil and even in Europe.1–3.
Between 1987 and 1999, four cases of gnathostomiasis were described in Peru, linked to the ingestion of raw fish, and in Brazil, the first reported case occurred in 2009 in a patient from Peru. However, the first autochthonous case occurred in 2012 in a patient from the northern region of the country, and subsequently, two other cases from the Brazilian Amazon region were reported.3–6
This parasitic infection is caused by third-stage larvae of helminths belonging to the Gnathostoma genus. It is prevalent mainly in tropical and subtropical regions but remains clinically less understood outside endemic areas, leading to challenges in diagnosis. In the presented case, a patient returned to São Paulo, Brazil, from the Amazon region and experienced symptoms without an etiological diagnosis for a month after consuming infected Tucunaré fish, likely causing illness in both her and her family.
Diagnosis of Gnathostoma spp. infection is based on clinical-epidemiological findings, typically presenting as a single subcutaneous nodule along with peripheral eosinophilia. Histological examination often reveals dense eosinophilic infiltrates in the dermis and hypodermis, reflecting larval migration. However, larval identification through histopathology varies in frequency, ranging from 23 to 34%.7
In the described case, the diagnosis was established based on the patient’s compatible clinical-epidemiological presentation, ruling out larva migrans caused by Ancylostoma braziliensis due to the absence of epidermal involvement. Serological tests (Enzyme-Linked Immunosorbent Assay and western blot) were not conducted due to their low sensitivity, specificity and potential cross-reactivity.3–6
For treatment, albendazole is the preferred drug, administered at 400–800 mg/day for 21 days, achieving cure rates of 93.9 and 94.1%. Ivermectin as a single dose (200 μg/kg) is also effective but less so than albendazole, and it may lead to relapses. It is important to note that multiple treatment courses might be necessary in some cases, and antiparasitic use can cause larval migration to the skin surface, resulting in new lesions, as observed with the patient following short-term albendazole treatment and lesion manipulation.1,8
Corticosteroids, specifically prednisolone at 60 mg/day for 7 days, can be cautiously administered alone in severe cases with visceral, ocular or central nervous system involvement. However, their use should be monitored closely due to the potential risk of larval migration and death.1
Hence, conducting focused clinical investigations into diseases that are less prevalent in certain regions, known mainly locally where they are endemic, is essential to enable early recognition of etiological diagnoses, especially among travellers, as demonstrated in this case. Accurate disease aetiology determination is crucial for effective treatment, which was delayed in the present case for a month after initial symptoms appeared.
Authors’ contributions
Sylvia Lemos Hinrichsen—Contributions: study concept and design, data collection; analysis, interpretation, and manuscript preparation.
Mariana Vieira Neves and Matheus Vinicius de Araújo Lucena—Contributions: study concept and design; data collection.
Tatiana de Aguiar Santos Vilella—Contributions: critical revision and intellectual content addition.
Conflict of interest: None declared.
Author Biographies
Sylvia Lemos Hinrichsen—PhD in Tropical Medicine. Email: [email protected]
Mariana Vieira Neves—Graduate student in Medicina. Email: [email protected]
Matheus Vinicius de Araújo Lucena—Graduate student in Medicina. Email: [email protected]
Tatiana de Aguiar Santos Vilella—M.Sc in Tropical Medicine. Email: [email protected]



