-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandre Duvignaud, Rhett J Stoney, Kristina M Angelo, Lin H Chen, Paolo Cattaneo, Leonardo Motta, Federico G Gobbi, Emmanuel Bottieau, Daniel L Bourque, Corneliu P Popescu, Hedvig Glans, Hilmir Asgeirsson, Ines Oliveira-Souto, Stephen D Vaughan, Bhawana Amatya, Francesca F Norman, Jesse Waggoner, Marta Díaz-Menéndez, Michael Beadsworth, Silvia Odolini, Daniel Camprubí-Ferrer, Loic Epelboin, Bradley A Connor, Gilles Eperon, Eli Schwartz, Michael Libman, Denis Malvy, Davidson H Hamer, Ralph Huits, for the GeoSentinel Network, Epidemiology of travel-associated dengue from 2007 to 2022: A GeoSentinel analysis, Journal of Travel Medicine, Volume 31, Issue 7, October 2024, taae089, https://doi.org/10.1093/jtm/taae089
Close - Share Icon Share
Abstract
Dengue is a leading cause of febrile illness among international travellers. We aimed to describe the epidemiology and clinical characteristics of imported dengue in returning travellers evaluated at GeoSentinel sites from 2007 to 2022.
We retrieved GeoSentinel records of dengue among travellers residing in non-endemic countries. We considered dengue confirmed when diagnosed by a positive dengue virus (DENV)–specific reverse-transcriptase polymerase chain reaction, positive NS-1 antigen and/or anti-DENV IgG seroconversion, and probable when diagnosed by single anti-DENV IgM or high-titre anti-DENV IgG detection. Severe dengue was defined as evidence of clinically significant plasma leakage or bleeding, organ failure, or shock, according to the 2009 World Health Organization guidance. Complicated dengue was defined as either severe dengue or dengue with presence of any warning sign. Analyses were descriptive.
This analysis included 5958 travellers with confirmed (n = 4859; 81.6%) or probable (n = 1099; 18.4%) dengue. The median age was 33 years (range: <1–91); 3007 (50.5%) travellers were female. The median travel duration was 21 days (interquartile range [IQR]: 15–32). The median time between illness onset and GeoSentinel site visit was 7 days (IQR: 4–15). The most frequent reasons for travel were tourism (67.3%), visiting friends or relatives (12.2%) and business (11.0%). The most frequent regions of acquisition were South East Asia (50.4%), South Central Asia (14.9%), the Caribbean (10.9%) and South America (9.2%). Ninety-five (1.6%) travellers had complicated dengue, of whom 27 (0.5%) had severe dengue and one died. Of 2710 travellers with data available, 724 (26.7%) were hospitalized. The largest number of cases (n = 835) was reported in 2019.
A broad range of international travellers should be aware of the risk of acquiring dengue and receive appropriate pre-travel counselling regarding preventive measures. Prospective cohort studies are needed to further elucidate dengue risk by destination and over time, as well as severe outcomes and prolonged morbidity (long dengue) due to travel-related dengue.
Introduction
Dengue is endemic in most tropical and subtropical countries, many of which are popular travel destinations.1 Globally, reported dengue cases among ill international travellers have increased over the past two decades.2–5 This is part of a dynamic of global increase in the burden of dengue that is expected to continue in the decades to come under the combined influence of climate change, urbanization and international connectivity.6,7 For example, climate change has contributed to an increase in populations at risk of dengue through extension of the distribution of the mosquito vectors Aedes aegypti and A. albopictus to new areas.7–9 Rising temperatures are also expected to enhance the vectorial capacity of A. albopictus in temperate zones where it is already established and to favour the circulation of dengue in higher-altitude areas that were previously spared.10,11 Nevertheless, human mobility, including international travel, is a major driver in the spread of dengue virus (DENV) and its role should not be underestimated.12 DENV-infected travellers may introduce the virus into new geographic locations and, if suitable vectors are present and local conditions are conducive to transmission, promote circulation of dengue in previously non-endemic areas.8,13,14 Travellers with dengue may provide valuable insights into ongoing outbreaks and epidemiology of dengue in endemic areas.15–18 Several studies have investigated dengue in international travellers.14,17–25 However, most studies are limited to travellers from a single region18,21,22 or have limited sample size.5,17,18,22–25 Documented risk factors for dengue acquisition in international travellers include travel destination, season of travel, duration of travel and activities during travel.2,15,19,20,26 Dengue accounts for up to 20% of acute undifferentiated febrile illnesses in returning travellers.27 In those returning with fever from Southeast Asia,27,28 the Americas,27,29 or the Western Pacific,27 dengue is a more frequent cause of travel-related febrile illness than malaria. Dengue was the most frequently diagnosed febrile illness in travellers at the UK’s Rare & Imported Pathogens Laboratory (RIPL) for the period 2015–2020.30 Dengue is also among the top diagnoses in travellers who receive unplanned healthcare abroad.31 It may also result in hospitalization among travellers returning to non-endemic countries; ~40% of international travellers diagnosed with dengue after returning to the USA have been hospitalized.14 A prospective health economics analysis found that travellers with laboratory-confirmed dengue also incurred important direct and indirect costs because of dengue-related illness.25,32
The objective of this analysis was to describe epidemiological and clinical characteristics among returned international travellers diagnosed with dengue at GeoSentinel sites over a 15-year period.
Methods
Data source
GeoSentinel is a global sentinel surveillance and research network that monitors infectious diseases and other adverse health events that may affect international travellers and migrants.33 It is a collaboration between the US Centers for Disease Control and Prevention (CDC) and the International Society of Travel Medicine. The Network currently consists of 71 specialized travel and tropical medicine sites in 29 countries. Data collected include demographic information (e.g. age, sex), travel characteristics (e.g. reason for travel, travel duration), exposure details (e.g. country and region of exposure), clinical details (e.g. symptoms, illness onset date, hospitalization) and diagnostic methods. Recording of symptoms began in October 2015; analysis of symptom data was therefore restricted to patient records included from that time onwards.
Inclusion criteria
All records with an international travel-related diagnosis of dengue from 1 January 2007 to 31 May 2022 were included. Records were excluded if the traveller lived in a dengue-endemic country, as we were unable to determine if dengue was acquired during travel or in the country of residence. Travellers were also excluded if multiple acute infectious diagnoses were recorded, and we could not determine where dengue was acquired. This occurred prior to 15 November 2018, when data capture methods did not allow separate entry of exposure variables in instances where multiple infectious disease diagnoses were entered.
Definitions
Dengue cases in GeoSentinel
Dengue fever was defined as a compatible clinical illness (e.g. fever, rash, headaches, myalgia, arthralgia) with appropriate exposure history, no evidence of complications and with laboratory testing as defined below. Complicated dengue was defined as dengue with either: (i) criteria for severe dengue, namely, clinically significant plasma leakage or bleeding, organ failure, or shock [i.e. adapted from criteria for severe dengue per 2009 World Health Organization (WHO) guidelines] or (ii) warning signs only (i.e. per 2009 WHO guidelines: abdominal pain, persistent vomiting, fluid accumulation, mucosal bleeding, lethargy, liver enlargement, increasing haematocrit with decreasing platelets).34 Cases of severe dengue were identified among complicated dengue cases based on supplemental data collection, as described elsewhere.35 Confirmed dengue was defined as having at least one of the following: detection of DENV nucleic acid by reverse-transcriptase polymerase chain reaction (RT-PCR), detection of NS-1 antigen or seroconversion (defined as a 4-fold rise in anti-DENV immunoglobulin (Ig)G titres). A probable case of dengue was defined as a compatible clinical illness and appropriate exposure history with either a single positive anti-DENV IgM or high positive anti-DENV IgG. Accurate entry of diagnostic methods for dengue records was verified by a medical epidemiologist (K.A.).
Analysis
All analyses were descriptive. Data were managed with Microsoft Access (Redmond, WA, USA) and analysed using SAS Version 9.4 (Cary, NC, USA) and R v4·1·1 (Vienna, Austria). The denominator for each frequency calculation was the number of patients with available data.
Ethics statement
GeoSentinel’s data collection protocol has been reviewed by a human subjects advisor at CDC’s National Center for Emerging and Zoonotic Infectious Diseases and is classified as public health surveillance and not human subjects research. Additional ethics clearances were obtained by sites as required by their respective institutions and local regulations.
Results
This analysis included 5958 travellers with confirmed (n = 4859; 81.6%) or probable (n = 1099; 18.4%) dengue. The median age was 33 years (range <1–91); 3007 (50.5%) of 5950 travellers with information available were female (Table 1).
Epidemiological, demographic and travel characteristics in international travellers with dengue, GeoSentinel Network, 1 January 2007–31 May 2022
| Characteristic . | N . | n (%) . |
|---|---|---|
| Dengue cases | 5958 | |
| • Uncomplicated | 5863 (98.4) | |
| • Complicated | 95 (1.6) | |
| Female | 5950 | 3007 (50.5) |
| Age, years Median 33 (range 0–91) | 5931 | |
| <18 | 243 (4.1) | |
| 18–24 | 986 (16.6) | |
| 25–49 | 3518 (59.3) | |
| ≥50 | 1184 (20.0) | |
| Days from illness onset to GeoSentinel visit Median 7 (IQR: 4–15) | 3524 | |
| Duration of travel, daysa Median 21 (IQR: 15–32) | 4035 | |
| Clinical setting | 5950 | |
| • Seen after travel | 5276 (88.7) | |
| • Seen during travel | 674 (11.3) | |
| Region of exposureb | 5851 | |
| • South East Asia | 2946 (50.4) | |
| • South Central Asia | 872 (14.9) | |
| • Caribbean | 635 (10.9) | |
| • South America | 538 (9.2) | |
| • Sub-Saharan Africa | 389 (6.7) | |
| • Central America | 337 (5.8) | |
| • Oceania (except Australia and New Zealand) | 91 (1.6) | |
| • North East Asia | 19 (0.3) | |
| • Otherc | 24 (0.4) | |
| Reason for travel | 5958 | |
| • Tourism | 4009 (67.3) | |
| • Visiting friends or relatives | 725 (12.2) | |
| • Business | 656 (11.0) | |
| • Missionary/Volunteer/Researcher/Aid work | 336 (5.6) | |
| • Education/Study abroad | 101 (1.7) | |
| • Migration | 99 (1.7) | |
| • Otherd | 32 (0.5) | |
| Pre-travel visit | 5802 | |
| • Yes | 2087 (36.0) | |
| • No | 2370 (40.8) | |
| • Don’t know/Unknown/Not applicable | 1345 (23.2) |
| Characteristic . | N . | n (%) . |
|---|---|---|
| Dengue cases | 5958 | |
| • Uncomplicated | 5863 (98.4) | |
| • Complicated | 95 (1.6) | |
| Female | 5950 | 3007 (50.5) |
| Age, years Median 33 (range 0–91) | 5931 | |
| <18 | 243 (4.1) | |
| 18–24 | 986 (16.6) | |
| 25–49 | 3518 (59.3) | |
| ≥50 | 1184 (20.0) | |
| Days from illness onset to GeoSentinel visit Median 7 (IQR: 4–15) | 3524 | |
| Duration of travel, daysa Median 21 (IQR: 15–32) | 4035 | |
| Clinical setting | 5950 | |
| • Seen after travel | 5276 (88.7) | |
| • Seen during travel | 674 (11.3) | |
| Region of exposureb | 5851 | |
| • South East Asia | 2946 (50.4) | |
| • South Central Asia | 872 (14.9) | |
| • Caribbean | 635 (10.9) | |
| • South America | 538 (9.2) | |
| • Sub-Saharan Africa | 389 (6.7) | |
| • Central America | 337 (5.8) | |
| • Oceania (except Australia and New Zealand) | 91 (1.6) | |
| • North East Asia | 19 (0.3) | |
| • Otherc | 24 (0.4) | |
| Reason for travel | 5958 | |
| • Tourism | 4009 (67.3) | |
| • Visiting friends or relatives | 725 (12.2) | |
| • Business | 656 (11.0) | |
| • Missionary/Volunteer/Researcher/Aid work | 336 (5.6) | |
| • Education/Study abroad | 101 (1.7) | |
| • Migration | 99 (1.7) | |
| • Otherd | 32 (0.5) | |
| Pre-travel visit | 5802 | |
| • Yes | 2087 (36.0) | |
| • No | 2370 (40.8) | |
| • Don’t know/Unknown/Not applicable | 1345 (23.2) |
IQR, interquartile range.
Those who went to one destination only.
Region of exposure was not ascertainable for 44 and missing for 63.
Western Europe (n = 8); Middle East (n = 5); North Africa (n = 5); North America (n = 3); Australia/New Zealand (n = 2); French territories of the Scattered Islands in the Indian Ocean (n = 1).
Military (n = 14); not ascertainable (n = 12); planned medical care (n = 6).
Epidemiological, demographic and travel characteristics in international travellers with dengue, GeoSentinel Network, 1 January 2007–31 May 2022
| Characteristic . | N . | n (%) . |
|---|---|---|
| Dengue cases | 5958 | |
| • Uncomplicated | 5863 (98.4) | |
| • Complicated | 95 (1.6) | |
| Female | 5950 | 3007 (50.5) |
| Age, years Median 33 (range 0–91) | 5931 | |
| <18 | 243 (4.1) | |
| 18–24 | 986 (16.6) | |
| 25–49 | 3518 (59.3) | |
| ≥50 | 1184 (20.0) | |
| Days from illness onset to GeoSentinel visit Median 7 (IQR: 4–15) | 3524 | |
| Duration of travel, daysa Median 21 (IQR: 15–32) | 4035 | |
| Clinical setting | 5950 | |
| • Seen after travel | 5276 (88.7) | |
| • Seen during travel | 674 (11.3) | |
| Region of exposureb | 5851 | |
| • South East Asia | 2946 (50.4) | |
| • South Central Asia | 872 (14.9) | |
| • Caribbean | 635 (10.9) | |
| • South America | 538 (9.2) | |
| • Sub-Saharan Africa | 389 (6.7) | |
| • Central America | 337 (5.8) | |
| • Oceania (except Australia and New Zealand) | 91 (1.6) | |
| • North East Asia | 19 (0.3) | |
| • Otherc | 24 (0.4) | |
| Reason for travel | 5958 | |
| • Tourism | 4009 (67.3) | |
| • Visiting friends or relatives | 725 (12.2) | |
| • Business | 656 (11.0) | |
| • Missionary/Volunteer/Researcher/Aid work | 336 (5.6) | |
| • Education/Study abroad | 101 (1.7) | |
| • Migration | 99 (1.7) | |
| • Otherd | 32 (0.5) | |
| Pre-travel visit | 5802 | |
| • Yes | 2087 (36.0) | |
| • No | 2370 (40.8) | |
| • Don’t know/Unknown/Not applicable | 1345 (23.2) |
| Characteristic . | N . | n (%) . |
|---|---|---|
| Dengue cases | 5958 | |
| • Uncomplicated | 5863 (98.4) | |
| • Complicated | 95 (1.6) | |
| Female | 5950 | 3007 (50.5) |
| Age, years Median 33 (range 0–91) | 5931 | |
| <18 | 243 (4.1) | |
| 18–24 | 986 (16.6) | |
| 25–49 | 3518 (59.3) | |
| ≥50 | 1184 (20.0) | |
| Days from illness onset to GeoSentinel visit Median 7 (IQR: 4–15) | 3524 | |
| Duration of travel, daysa Median 21 (IQR: 15–32) | 4035 | |
| Clinical setting | 5950 | |
| • Seen after travel | 5276 (88.7) | |
| • Seen during travel | 674 (11.3) | |
| Region of exposureb | 5851 | |
| • South East Asia | 2946 (50.4) | |
| • South Central Asia | 872 (14.9) | |
| • Caribbean | 635 (10.9) | |
| • South America | 538 (9.2) | |
| • Sub-Saharan Africa | 389 (6.7) | |
| • Central America | 337 (5.8) | |
| • Oceania (except Australia and New Zealand) | 91 (1.6) | |
| • North East Asia | 19 (0.3) | |
| • Otherc | 24 (0.4) | |
| Reason for travel | 5958 | |
| • Tourism | 4009 (67.3) | |
| • Visiting friends or relatives | 725 (12.2) | |
| • Business | 656 (11.0) | |
| • Missionary/Volunteer/Researcher/Aid work | 336 (5.6) | |
| • Education/Study abroad | 101 (1.7) | |
| • Migration | 99 (1.7) | |
| • Otherd | 32 (0.5) | |
| Pre-travel visit | 5802 | |
| • Yes | 2087 (36.0) | |
| • No | 2370 (40.8) | |
| • Don’t know/Unknown/Not applicable | 1345 (23.2) |
IQR, interquartile range.
Those who went to one destination only.
Region of exposure was not ascertainable for 44 and missing for 63.
Western Europe (n = 8); Middle East (n = 5); North Africa (n = 5); North America (n = 3); Australia/New Zealand (n = 2); French territories of the Scattered Islands in the Indian Ocean (n = 1).
Military (n = 14); not ascertainable (n = 12); planned medical care (n = 6).
The median travel duration was 21 days (interquartile range [IQR]: 15–32), and the median time between illness onset and GeoSentinel site visit was 7 days (IQR: 4–15). The most frequent reasons for travel were tourism (n = 4009; 67.3%), visiting friends and relatives (VFR) (n = 725; 12.2%) and business (n = 656; 11.0%). Of 5802 travellers with information available, 2370 (40.8%) had a pre-travel consultation (Table 1).
The most frequent regions of dengue exposure were Southeast Asia (n = 2946; 50.4%), South-Central Asia (n = 872; 14.9%), the Caribbean (n = 635; 10.9%) and South America (n = 538; 9.2%). Among 5586 travellers with information on destination of exposure, most were exposed in Thailand (n = 1231; 22.0%), Indonesia (n = 635; 11.4%) or India (n = 506; 9.1%). All destinations of exposure are shown in Appendix 1. Some travellers acquired dengue in destinations considered non-endemic (e.g. USA, France, Spain, Portugal).
Ninety-five (1.6%) travellers had complicated dengue, of whom 27 (0.5%) had severe dengue and 1 died. The clinical characteristics of those severe dengue cases (n = 27) have been described previously.35 Their epidemiological characteristics are further described in Appendix 2. Among 2710 travellers with available data regarding the highest level of care received, 1968 (72.6%) were outpatients, 724 (26.7%) were inpatients and 18 (0.7%) had been admitted to an intensive care unit.
Symptom data were available for 3051 (51.2%) travellers (from October 2015 onwards). Fever (n = 2657, 87%), headache (n = 1135, 37%), myalgia (n = 1105, 36%), fatigue (n = 1053, 35%) and rash (n = 885, 29%) were the five more frequently reported symptoms (Table 2). Abdominal pain was present in one-third of complicated cases.
Symptoms among travellers with uncomplicated or complicated dengue, reported to the GeoSentinel Network, January 1, 2015- May 31, 2022*
| Symptom . | Uncomplicated dengue (%) (N=2,999 travellers) . | Complicated dengue (%) (N=52 travellers) . |
|---|---|---|
| Fever/sweat/chills | 2,608 (87.0) | 49 (94.2) |
| Headache | 1,117 (37.2) | 18 (34.6) |
| Myalgia | 1,084 (36.1) | 21 (40.4) |
| Fatigue | 1,034 (34.5) | 19 (36.5) |
| Rash | 866 (28.9) | 19 (36.5) |
| Arthralgia | 637 (21.2) | 12 (23.1) |
| Acute diarrhoea | 421 (14.0) | 10 (19.2) |
| Nausea | 399 (13.3) | 12 (23.1) |
| Abdominal pain/discomfort | 282 (9.4) | 17 (32.7) |
| Vomiting | 239 (8.0) | 8 (15.4) |
| Cough | 167 (5.6) | 4 (7.7) |
| Symptom . | Uncomplicated dengue (%) (N=2,999 travellers) . | Complicated dengue (%) (N=52 travellers) . |
|---|---|---|
| Fever/sweat/chills | 2,608 (87.0) | 49 (94.2) |
| Headache | 1,117 (37.2) | 18 (34.6) |
| Myalgia | 1,084 (36.1) | 21 (40.4) |
| Fatigue | 1,034 (34.5) | 19 (36.5) |
| Rash | 866 (28.9) | 19 (36.5) |
| Arthralgia | 637 (21.2) | 12 (23.1) |
| Acute diarrhoea | 421 (14.0) | 10 (19.2) |
| Nausea | 399 (13.3) | 12 (23.1) |
| Abdominal pain/discomfort | 282 (9.4) | 17 (32.7) |
| Vomiting | 239 (8.0) | 8 (15.4) |
| Cough | 167 (5.6) | 4 (7.7) |
*Only symptoms with frequencies >5% are shown.
Symptoms among travellers with uncomplicated or complicated dengue, reported to the GeoSentinel Network, January 1, 2015- May 31, 2022*
| Symptom . | Uncomplicated dengue (%) (N=2,999 travellers) . | Complicated dengue (%) (N=52 travellers) . |
|---|---|---|
| Fever/sweat/chills | 2,608 (87.0) | 49 (94.2) |
| Headache | 1,117 (37.2) | 18 (34.6) |
| Myalgia | 1,084 (36.1) | 21 (40.4) |
| Fatigue | 1,034 (34.5) | 19 (36.5) |
| Rash | 866 (28.9) | 19 (36.5) |
| Arthralgia | 637 (21.2) | 12 (23.1) |
| Acute diarrhoea | 421 (14.0) | 10 (19.2) |
| Nausea | 399 (13.3) | 12 (23.1) |
| Abdominal pain/discomfort | 282 (9.4) | 17 (32.7) |
| Vomiting | 239 (8.0) | 8 (15.4) |
| Cough | 167 (5.6) | 4 (7.7) |
| Symptom . | Uncomplicated dengue (%) (N=2,999 travellers) . | Complicated dengue (%) (N=52 travellers) . |
|---|---|---|
| Fever/sweat/chills | 2,608 (87.0) | 49 (94.2) |
| Headache | 1,117 (37.2) | 18 (34.6) |
| Myalgia | 1,084 (36.1) | 21 (40.4) |
| Fatigue | 1,034 (34.5) | 19 (36.5) |
| Rash | 866 (28.9) | 19 (36.5) |
| Arthralgia | 637 (21.2) | 12 (23.1) |
| Acute diarrhoea | 421 (14.0) | 10 (19.2) |
| Nausea | 399 (13.3) | 12 (23.1) |
| Abdominal pain/discomfort | 282 (9.4) | 17 (32.7) |
| Vomiting | 239 (8.0) | 8 (15.4) |
| Cough | 167 (5.6) | 4 (7.7) |
*Only symptoms with frequencies >5% are shown.
The annual numbers of dengue cases varied by year; the number of sites reporting dengue cases also varied (Figure 1). In 2020 (n = 225) and in 2021 (n = 77), the number of reported dengue cases dropped dramatically following reductions in global travel volume during the COVID-19 pandemic.
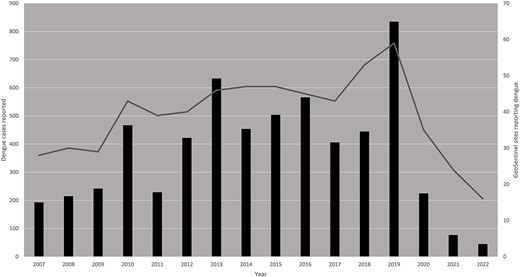
International travel-related dengue cases reported by year, GeoSentinel Network, January 1, 2007-May 31, 2022 (N=5,958). The bars (left axis) represent the number of dengue cases reported by GeoSentinel sites, by year. The curve (right axis) represents the number of sites reporting at least one dengue case in that particular year.
Discussion
This is the largest analysis of international travel-related dengue to date. Travellers with dengue reported globally were characterized by their epidemiology and clinical characteristics using data collected by the GeoSentinel Network.
Peak reporting years in this analysis (2010, 2013, 2015, 2016 and 2019) corresponded to years with the highest number of dengue cases reported globally to WHO.36 Of note, 2016 and 2019 also corresponded to years with the highest number of travel-associated dengue cases imported into the USA.14 Globally, the number of symptomatic dengue cases and the number of dengue-related deaths are estimated to have increased between 1990 and 2019 from 31 to 57 million and from 28 000 to 36 000, respectively.1 Reports of dengue cases (including outbreaks) reflected among international travellers are driven by multiple factors, including population growth, urbanization and air travel.7 Numbers of dengue cases reported through the network declined in 2020 and 2021, corresponding to the start of the COVID-19 pandemic. While reductions in international travel volume may partly explain this drop, other factors likely contributed. A recent modelling study highlighted the strong association between the decline of dengue incidence in highly endemic areas of Southeast Asia and Latin America and the public health and social measures aimed at tackling SARS-CoV-2 transmission.37 In 2022, a large number of dengue cases reported to the GeoSentinel Network were imported to Europe from Cuba, reflecting an ongoing local outbreak.18 In this analysis, over two-thirds of travellers with dengue travelled for tourism, but all travel types were represented. This indicates that all types of travellers to endemic areas should be concerned about acquiring dengue and take appropriate mitigation measures to minimize the risk of acquiring the virus. Dengue was reported from almost all continents; two-thirds of infections were acquired in Asia, with more than half imported from South East Asia. This is consistent with previous studies on dengue in international travellers,3–5,15,30 and likely a reflection of the burden of disease present in these areas and also the number of international travellers to these destinations. Other regions saw changes in the number of cases reported over time, but it is unclear if this is a true reflection of travel-related dengue epidemiology or if it is attributable to reporting patterns from GeoSentinel sites.
Severe dengue in travellers is rare.38 The proportion of travellers with severe dengue was 0.5% in our sample. A recent Spanish retrospective study on imported arboviral diseases reported six (1.3%) patients with severe dengue among 456 with confirmed dengue monoinfection.39 A similar study conducted in Israel reported four (1.6%) severe dengue among 245 dengue cases.40 Recently published US data reported 1–2% of dengue cases to be severe.14 Although dengue is a benign and self-limiting disease in most travellers, it can nevertheless lead to significant healthcare consumption and costs, as illustrated by a recently published cost analysis ancillary to the GeoSentinel’s CHIDEZIMA cohort study.25,32,41 Dengue is also a frequent cause of healthcare seeking during travel.31 While the risk of prolonged morbidity following dengue fever (long dengue), including chronic fatigue, and its contribution to the global burden of the disease remains underexplored,42 some investigations are underway to estimate its prevalence.41 Further studies are still needed to elucidate the specifics of travel-related dengue clinical care as well as its long-term effects and contribution to the travel-related dengue burden.
All cases of dengue included in this analysis were imported to non-endemic countries. From a public health perspective, an important consequence of dengue importation into non-endemic countries by viremic travellers is the risk of secondary autochthonous transmission.8,14,43,44 This risk is not negligible in temperate areas where A. albopictus is already present. Because DENV infection is asymptomatic in around 60% of cases,43,45 the potential for secondary autochthonous transmission by viremic returning travellers is probably underestimated. In recent years, autochthonous dengue cases or outbreaks occurred sporadically in several southern European countries,46–50 in Japan43 and in the USA.14,44,51–53 In mainland France, where both the caseload and the geographic extension of autochthonous DENV transmission have increased, 65 cases of dengue occurred in 2022 that could be traced back to nine transmission events,47 and a cluster also occurred in the Paris region in 2023.49,50 The increasingly frequent occurrence of imported dengue cases in non-endemic areas represents a diagnostic challenge for frontline healthcare professionals who are unfamiliar with the disease.44,52–54 The increasing frequency of autochthonous cases in non-endemic areas may also present a diagnostic challenge to experienced specialists (e.g. infectious disease or travel medicine specialists) because the index of suspicion is typically based on a patient having been present in an endemic area. Importantly, the risk of autochthonous transmission will likely increase in the future due to the change in climatic conditions that will favour the establishment of more efficient dengue vector populations, such as A. aegypti,8,10–12, or enhance the transmission capacity of A. albopictus.10 Clinician education aimed at increasing the index of suspicion for both imported cases and the possibility of autochthonous transmission of dengue should be considered. Regarding the latter, besides vectorial transmission, the possible occurrence of dengue cases transmitted from infected blood and organ donors represents an additional risk that call for appropriate mitigation strategies.
Preventing DENV importation to non-endemic areas is challenging not only from an individual health and economic perspective but also in terms of public health.1,25,55 In travellers, personal protective measures such as the use of long clothing and mosquito repellents limit the risk of DENV infection and constitute the basis of prevention. However, the effectiveness of such strategies is limited by a low awareness of travellers to dengue-endemic countries regarding the risk of contracting the disease as well as low adherence to appropriate protective measures.56 Receipt of pre-travel advice by and counselling towards at-risk travellers remain suboptimal, demonstrated in this analysis where just over 40% of travellers saw a healthcare professional before travel. Current preventive measures could be supplemented by tetravalent dengue vaccines. In fact, a recent assessment of travel-related risks based on monthly incidence rates has ranked dengue among high-priority vaccines considered for international travel.57 Yet, vaccination-based strategies in travellers are currently compromised by three important barriers: (i) the lack of availability of short schemes adapted to immediate pre-travel immunization; (ii) the suboptimal safety and/or efficacy profile of existing dengue vaccines in seronegative subjects, which is the situation of the vast majority of travellers residing in non-endemic countries and (iii) the lack of data for those aged over 60 years.58–62 Moreover, vaccinating at-risk travellers may not be sufficient to prevent DENV introduction in non-endemic countries, depending on vaccine affordability, uptake and capacity to suppress viremia in people exposed to various DENV serotypes.
Limitations
Our analysis has several limitations. Regarding case detection, we only captured data from travellers who sought care at a GeoSentinel site, so this analysis is not representative of all travellers with dengue. Moreover, GeoSentinel does not collect data on travellers who are not ill. For all these reasons, this large convenience sample cannot be used to calculate risk or incidence. Before 2015, we did not capture dengue diagnostic methods in the GeoSentinel database, and cases were classified as laboratory-confirmed or probable by the clinicians. Therefore, the accuracy of the diagnosis prior to 2015 could not be verified. Also, the probable case definition included a high-positive IgG titre, but this may not necessarily indicate a recent DENV infection and may represent a recent infection with or vaccination against a related flavivirus; ‘high’ was also not quantified in the definition and was at the discretion of the treating clinician. Concerning the description of the disease course, the time to presentation to network sites may be prolonged since sites may serve as travel and tropical medicine referral centers. In addition, the duration of symptoms is not captured. Lastly, symptoms data were subject to the discretion of the reporting clinician and may have included symptoms and signs that were present during the evaluation and warning signs that had resolved at the time of evaluation. Recall or reporting biases can therefore not be excluded. Data on differentiating primary versus secondary dengue were also not captured.
Conclusion
A broad range of international travellers are at increasing risk of acquiring dengue and should be aware of that risk. Counselling regarding appropriate personal protective measures remains the cornerstone of dengue prevention. Dengue vaccine could represent a promising prevention tool, but questions remain regarding its application to the prevention of travel-related dengue. Prospective cohort studies are needed to further elucidate dengue risk by destination, by serotype/genotype and over time, as well as serious outcomes and prolonged morbidity (long-dengue) due to travel-related dengue.
Funding
This work was supported by the US Centers for Disease Control and Prevention through a Cooperative Agreement with the International Society of Travel Medicine (ISTM) [Federal Award Number: 1 U01CK000632-01-00], as well as by the Public Health Agency of Canada through a grant to ISTM and by the GeoSentinel Foundation.
Acknowledgements
We thank the following members of the GeoSentinel network who contributed cases included in this study: Carsten Schade Larsen, MD (Department of Infectious Disease-Aarhus University Hospital, Aarhus, Denmark); Christian Wejse, MD, PhD (Department of Infectious Disease-Aarhus University Hospital, Aarhus, Denmark); Abraham Goorhuis, MD, PhD (Center for Tropical & Travel Medicine—University of Amsterdam, Amsterdam, The Netherlands); Henry Wu, MD, DTM (Travel Well—Emory University, Dowman Drive, Atlanta, GA, USA); Marc Shaw, MD (WORLDWISE Health Saint Marks Road, Remuera, Auckland, New Zealand); Annemarie Hern, MD (WORLDWISE Health Saint Marks Road, Remuera, Auckland, New Zealand); Noreen A. Hynes, MD, MPH (John Hopkins Univ. School of Medicine—Geographic Medicine Center—Division of Infectious Diseases, Baltimore, MD, USA); Watcharapong Piyaphanee, MD (Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand); Udomsak Silachamroon, MD (Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand); Israel Molina, MD (Antiga Escola de Infermeria, Hospital Universitari Vall d'Hebron, Barcelona, Spain); Fernando Salvador, MD (Antiga Escola de Infermeria, Hospital Universitari Vall d'Hebron, Barcelona, Spain); Frank Mockenhaupt, MD (Charité—Universitaets medizin Berlin, Germany); Gundel Harms Zwingenberger, MD (Charité—Universitaets medizin Berlin, Germany); Elizabeth Bartnett (Boston University Chobanian & Avedisian School of Medicine, Boston, MA, USA); Francesco Castelli, MD (Clinica di Malattie Infettive e Tropicali—University of Brescia, Italy); Alberto Matteel, MD (Clinica di Malattie Infettive e Tropicali—University of Brescia, Italy); Christina Coyle, MD (Tropical Medicine Clinic—Jacobi Medical Center, The Bronx, NY, USA); Paul Kelly, MD (Division of Infectious Diseases—Bronx-Lebanon Hospital Center, The Bronx, NY, USA); Cosmina Zeana, MD (Division of Infectious Diseases—Bronx-Lebanon Hospital Center, The Bronx, NY, USA); Susan Kuhn, MD (University of Calgary, University Drive Northwest, Calgary, AB, Canada); Marc Mendelson, MD (Division of Infectious Diseases & HIV Medicine—Groote Schuur Hospital, Cape Town, South Africa); Salim Parker, MD (Division of Infectious Diseases & HIV Medicine—Groote Schuur Hospital, Cape Town, South Africa); Félix Djossou, MD (Centre Hospitalier Andrée Rosemon, Cayenne, French Guiana); Cecilia Perret, MD, and Thomas Weitzel, MD (Centro de Enfermedades—Tropicales y Salud del Viajero—Pontificia Universidad Católica de Chile, Santiago, Chile); Francois Chappuis, MD (Division of Tropical and Humanitarian Medicine—Geneva University Hospitals, Geneva, Switzerland); Matteo Bassetti, MD (University of Genova, Italy—Ospedale Policlinico San Martino, Genova, Italy); Sabine Jordan, MD, and Christof Vinnemeier, MD (University Medical Centre Hamburg Eppendorf, Department of Medicine, Division of Infectious Diseases and Tropical Medicine, Bernhard-Nocht-Kilini, Hamburg, Germany); Jasper Chan, MD (Hong Kong University, Hong Kong, China); Kelvin Chiu, MD (Hong Kong University, Hong Kong, China); Rosa de Miguel Buckley, MD (Hospital Universitario La Paz-Carlos III, Madrid, Spain); Tamar Lachish, MD (The Center of Geographical Medicine & Tropical Diseases, Sheba Medical Center, Ramat Gan, Israel); Christina Greenaway, MD (Jewish General Hospital, Division of Infectious Diseases, Montreal, Canada); Albie De Frey, MD (National Institute for Communicable Diseases, Sandringham, Johannesburg, Republic of South Africa); Lucille Blumberg, MD (National Institute for Communicable Diseases, Sandringham, Johannesburg, Republic of South Africa); Prativa Pandey, MD (The CIWEC Clinic Travel Medicine Center, Lainchaur, Kathmandu, Nepal); Mauro Saio, MD (The Nairobi Hospital, Nairobi, Kenya); Hugo Siu, MD (Clinica Anglo Americana, Lima, Peru); Jose Antonio Perez Molina, MD (University Hospital Ramón y Cajal. Madrid, Spain); Emilie Javelle, MD (IHU Méditerranée Infection, Marseille, France); Karin Leder, MD (Victorian Infectious Diseases Service—Royal Melbourne Hospital, Parkville, Australia); Sapha Bakarati, MD (Clinique Santé-voyage de la Fondation du CHUM, Montreal, Canada); Cedric Yansouni, MD (Clinique Santé-voyage de la Fondation du CHUM, Montreal, Canada); Arpita Chakravarti, MD (Centre Hospitalier de l'Université de Montréal, Canada); Camilla Rothe, MD (LMU University Hospital Munich, Div. of Infectious Diseases and Tropical Medicine, Munich, Germany); Mirjam Schunk, MD (LMU University Hospital Munich, Div. of Infectious Diseases and Tropical Medicine, Munich, Germany); Marina Rogova, NP (The New York Center for Travel and Tropical Medicine, New York, NY, USA); John Cahill, MD, and Benjamin Wyler, MD (Mount Sinai Doctors: Infectious Disease, New York, NY, USA); Carmelo Licitra, MD (Orlando Health Travel Medicine, Orlando, FL, USA); Anne McCarthy, MD (The Ottawa Hospital Civic Campus, Ottawa, Canada); Eric Caumes, MD (Service des Maladies Infectieuses et Tropicales—Hôpital Pitié-Salpêtrière, Sorbonne Université, Paris, France); Paul Henri Consigny, MD, and Oula Itani, MD (Institut Pasteur, Paris, France); Nancy Piper Jenks, MD (Hudson River Health Care, Peeksill, NY, USA); Els van Nood, MD (Erasmus MC University Hospital, Rotterdam, The Netherlands); Daniel Leung, MD (International Travel Clinic—University of Utah Hospital and Clinics, Salt Lake City, UT, USA); Terri Sofarelli, PA-C (International Travel Clinic—University of Utah Hospital and Clinics, Salt Lake City, UT, USA); Ann Settgast, MD, and Patricia F. Walker (HealthPartners, Minneapolis, MN, USA); Mugen Ujiie, MD (Travel Clinic—National Center for Global Health and Medicine (NCGM), Tokyo, Japan); Kei Yamamoto (Travel Clinic—National Center for Global Health and Medicine (NCGM), Tokyo, Japan); Katherine Plewes, MD (Tropical Medicine Expert Group BC—Vancouver General Hospital, Canada); Yazdan Mirzanejad (Tropical Medicine Expert Group BC- Vancouver General Hospital, Canada); Pierre Plourde, MD (Travel Health and Tropical Medicine Clinic, Winnipeg, Canada); Yukihiro Yoshimura, MD (Department of Infectious Diseases—Yokohama Municipal Citizen’s Hospital, Yokohama, Japan); Natsuo Tachikawa, MD (Department of Infectious Diseases—Yokohama Municipal Citizen’s Hospital, Yokohama, Japan); Patricia Schlagenhauf, PhD (University of Zürich Centre for Travel Medicine, WHO Collaborating Centre for Travellers' Health; Zurich, Switzerland); Annelies Zinkernagel, MD, PhD (University of Zürich Centre for Travel Medicine, WHO Collaborating Centre for Travellers' Health; Zurich, Switzerland).
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Author contributions
Alexandre Duvignaud (Conceptualization, Methodology, Writing—original draft, Writing—review & editing, Supervision [lead], Data curation, Formal analysis [supporting], Investigation, Validation, Visualization [equal]), Rhett J. Stoney (Conceptualization, Writing—original draft, Writing—review & editing [supporting], Data curation, Formal analysis, Visualization [lead], Investigation, Methodology, Validation [equal]), Kristina M. Angelo (Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing, Visualization [supporting], Investigation, Methodology, Validation [equal]), Lin H. Chen (Investigation, Writing—review & editing, Validation [equal]), Paolo Cattaneo (Investigation, Writing—review & editing, Validation [equal]), Leonardo Motta (Investigation, Writing—review & editing, Validation [equal]), Federico G. Gobbi (Investigation, Writing—review & editing, Validation [equal]), Emmanuel Bottieau (Investigation, Writing—review & editing, Validation [equal]), Daniel L. Bourque (Investigation, Writing—review & editing, Validation [equal]), Corneliu P. Popescu (Investigation, Writing—review & editing, Validation [equal]), Hedvig Glans (Investigation, Writing—review & editing, Validation [equal]), Hilmir Asgeirsson (Investigation, Writing—review & editing, Validation [equal]), Ines Oliveira-Souto (Investigation, Writing—review & editing, Validation [equal]), Stephen D. Vaughan (Investigation, Writing—review & editing, Validation [equal]), Bhawana Amatya (Investigation, Writing—review & editing, Validation [equal]), Francesca F. Norman (Investigation, Writing—review & editing, Validation [equal]), Jesse Waggoner (Investigation, Writing—review & editing, Validation [equal]), Marta Diaz-Menendez (Investigation, Writing—review & editing, Validation [equal]), Michael Beadsworth (Investigation, Writing—review & editing, Validation [equal]), Silvia Odolini (Investigation, Writing—review & editing, Validation [equal]), Daniel Camprubí-Ferrer (Investigation, Writing—review & editing, Validation [equal]), Loic Epelboin (Investigation, Writing—review & editing, Validation [equal]), Bradley A. Connor (Investigation, Writing—review & editing, Validation [equal]), Gilles Eperon (Investigation, Writing—review & editing, Validation [equal]), Eli Schwartz (Investigation, Writing—review & editing, Validation [equal]), Michael Libman (Conceptualization, Data curation, Formal analysis, Visualization [supporting], Investigation, Methodology, Writing—review & editing, Validation [equal]), Denis Malvy (Conceptualization, Data curation, Formal analysis, Writing—original draft, Visualization [supporting], Investigation, Methodology, Writing—review & editing, Validation [equal]), Davidson H. Hamer (Conceptualization, Investigation, Methodology, Writing—original draft, Writing—review & editing, Validation-[equal], Data curation, Formal analysis, Visualization [supporting]), Ralph Huits (Conceptualization, Methodology [lead], Data curation, Formal analysis, Visualization-Supporting, Investigation, Writing—original draft, Writing—review & editing, Validation [equal]).
Conflict of interest
None declared.
References
World Health Organization. Improving data for dengue. https://www.who.int/activities/improving-data-for-dengue (accessed July 19, 2023).
Menon S, Wilder-Smith A. New Vaccines on the Immediate Horizon for Travelers: Chikungunya and Dengue Vaccines. Curr Infect Dis Rep 2023;



