-
PDF
- Split View
-
Views
-
Cite
Cite
Arenn F Carlos, Hiroaki Sekiya, Shunsuke Koga, Rodolfo G Gatto, Monica Castanedes Casey, Nha Trang Thu Pham, Irene Sintini, Mary M Machulda, Clifford R Jack, Val J Lowe, Jennifer L Whitwell, Leonard Petrucelli, R Ross Reichard, Ronald C Petersen, Dennis W Dickson, Keith A Josephs, Clinicopathologic features of a novel star-shaped transactive response DNA-binding protein 43 (TDP-43) pathology in the oldest old, Journal of Neuropathology & Experimental Neurology, Volume 83, Issue 1, January 2024, Pages 36–52, https://doi.org/10.1093/jnen/nlad105
Close - Share Icon Share
Abstract
Transactive response DNA-binding protein 43 (TDP-43) pathology is categorized as type A-E in frontotemporal lobar degeneration and as type α-β in Alzheimer disease (AD) based on inclusion type. We screened amygdala slides of 131 cases with varying ages at death, clinical/neuroimaging findings, and AD neuropathologic changes for TDP-43 pathology using anti-phospho-TDP-43 antibodies. Seven cases (5%) only showed atypical TDP-43 inclusions that could not be typed. Immunohistochemistry and immunofluorescence assessed the atypical star-shaped TDP-43 pathology including its distribution, species, cellular localization, and colocalization with tau. All 7 had died at an extremely old age (median: 100 years [IQR: 94–101]) from nonneurological causes and none had dementia (4 cognitively unimpaired, 3 with amnestic mild cognitive impairment). Neuroimaging showed mild medial temporal involvement. Pathologically, the star-shaped TDP-43-positive inclusions were found in medial (subpial) amygdala and, occasionally, in basolateral regions. Hippocampus only showed TDP-43-positive neurites in the fimbria and subiculum while the frontal lobe was free of TDP-43 inclusions. The star-shaped inclusions were better detected with antibodies against N-terminal than C-terminal TDP-43. Double-labeling studies confirmed deposition of TDP-43 within astrocytes and colocalization with tau. We have identified a novel TDP-43 pathology with star-shaped morphology associated with superaging, with a homogeneous clinicopathologic picture, possibly representing a novel, true aging-related TDP-43 pathology.
INTRODUCTION
Transactive response DNA-binding protein 43 (TDP-43) is an intranuclear protein involved in transcription regulation, alternative splicing, and messenger-RNA stabilization, thus participating in processes vital to cellular functioning and survival (1, 2). In neurodegenerative diseases, pathologic deposition of TDP-43 was first discovered in frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS), whereby TDP-43 was found to undergo hyperphosphorylation, ubiquitination, and cleavage to generate C-terminal fragments which then formed aggregates within the cytoplasm (3, 4). Subsequent studies uncovered the presence of TDP-43 pathological inclusions in cognitively unimpaired older adults (5) and in other neurodegenerative diseases, including Alzheimer disease (AD) (6), primary age-related tauopathy (PART) (7), Lewy body disease (LBD) (8, 9) and FTLD with tau (10–12), among many others.
TDP-43 pathology has a nearly universal presence in the aging brain, although its effect is heterogeneous. In people younger than 65 years of age, TDP-43 pathology is often found in isolation and is considered the driving force behind the neurodegeneration and clinical picture, as can be observed in FTLD with TDP-43 (FTLD-TDP) and ALS (13, 14). On the other hand, in people older than 65 years of age, TDP-43 is often found coexisting with other pathologies, particularly AD neuropathologic changes (ADNC) and LBD (13, 14). In TDP-43 proteinopathies, the morphological features and spatial distribution of TDP-43 inclusions are heterogenous, with involvement of different cell types (neuronal and/or glial) (15–17), cellular anatomic parts (cytoplasmic, intranuclear, and/or dendritic) (4, 18–20), and brain regions (cortical, subcortical, and/or brainstem) (21–23). Additionally, the TDP-43 species forming the pathological aggregates is not always consistent, with phosphorylated TDP-43 (pTDP-43), C-terminal TDP-43 (cTDP-43), or N-terminal (nTDP-43)/full-length TDP-43 found in varying patterns, ratios, and lesion types (24–26). Furthermore, TDP-43 can colocalize with other proteins, including tau in AD neurofibrillary tangles (27), intracellular amyloid- (A) in AD (28), and -synuclein in glial cytoplasmic inclusions in multiple system atrophy (29).
TDP-43 pathological aggregates in FTLD and AD are 2 of the most recognized and reported types of inclusions in the literature. The inclusions first described in FTLD-TDP included neuronal cytoplasmic inclusions (NCI), dystrophic neurites (DN), neuronal intranuclear inclusions (NII), and glial cytoplasmic inclusions (GCI) (30, 31). Dissimilarities in their prevalence, morphology, distribution, and relationship with clinical and genetic signatures led to the identification of 5 distinct FTLD-TDP subtypes, from type A to E (18, 32). Because of their early discovery and high frequency, these types of inclusions can often be regarded as the “typical” TDP-43 inclusions. Two major types of TDP-43 inclusions are recognized in AD: type- shows oval-shaped or ring-like NCIs, short DNs, and lentiform (cat eye-shaped) NIIs and is often described as reminiscent of those found in FTLD-TDP type A, type- is characterized by tau neurofibrillary tangle-associated TDP-43 (TAT) (27).
The current world population is an increasingly aging one. This can have huge implications in the field of neurodegenerative diseases, with age being the greatest risk factor in certain pathologies like AD and TDP-43 proteinopathies (33, 34). However, an upside is the simultaneous increase in opportunity to examine the brains of the oldest-old “superager” population. This could provide better insight into the true, isolated effects of aging. In as much as TDP-43 pathology is known to have increasing frequency with old age (34–37), it is often accompanied by other pathologies that are well accepted as contributing to the neurodegenerative process, hence the clinical presentation specific to TDP-43 is hard to assess.
Following a continuous review of cases with ADNC to determine the frequency of concomitant TDP-43 pathology, we identified 7 cases which showed TDP-43-immunoreactive inclusions that did not follow any of the described patterns in AD or aging. These cases displayed star-shaped TDP-43-immunoreactive inclusions within the amygdala. A review of their clinical features later revealed that all were “superagers” with relatively preserved cognitive functions, and all showed a relatively homogenous overall clinicopathological picture.
In the present study, we aimed to present the “atypical” star-shaped TDP-43 inclusions and describe their clinico-imaging correlates and associated copathologies. We also aimed to better characterize the spatial distribution, nature (TDP-43 species), and cellular localization of these star-shaped TDP-43 inclusions and determine whether they are codeposited with other proteins, such as tau. We hypothesized that their distribution would be restricted to the medial temporal lobe given the relatively preserved cognitive functions prior to death, that the inclusions would localize within astrocytes given their distinctive morphological features, and that there would be some degree of overlap with tau inclusions.
MATERIALS AND METHODS
Design, setting, and participants
This cross-sectional clinicopathologic study was conducted at Mayo Clinic, Rochester MN. One hundred and thirty-one cases were prospectively recruited and followed in 1 of 3 NIH-funded enterprises: the Alzheimer’s Disease Research Center (ADRC) (PI: R.C.P.), the Mayo Clinic Study of Aging (MCSA) (PI: R.C.P.), and the Neurodegenerative Research Group (NRG) (PI: J.L.W. and K.A.J.). Participants had died between January 2015 and May 2022. All had undergone genetic, neurologic, neuropsychologic, and neuropathologic evaluations. At autopsy, all showed varying degrees of ADNC and were additionally screened for TDP-43 pathology (using anti-phospho-TDP-43 antibodies) in the amygdala, which is the first region affected by TDP-43 pathology in AD (22, 38). Cases satisfying the criteria for FTLD (39), with predominant focal frontal and/or temporal degeneration observed either macroscopically or microscopically with neuronal loss, vacuolation, and gliosis were excluded. These included cases with a primary diagnosis of FTLD-TDP or FTLD-tau (progressive supranuclear palsy, corticobasal degeneration, Pick disease, globular glial tauopathy). Participants were not excluded based on the presence of concomitant LBD or vascular pathology. Sixty-eight (52%) of the 131 initial participants showed intraparenchymal typical TDP-43-immunoreactive inclusions such as NCIs, DNs, NIIs, and TATs and hence were immediately classified as TDP-43-positive (27). However, 7 of the 131 (5%) showed atypical TDP-43 inclusions that were distinct from FTLD-TDP types A-E or AD with TDP-43 type- and type- inclusions and therefore could not be easily and unequivocally classified as TDP-43-positive, let alone be typed. These 7 atypical TDP-43 participants are the focus of this study. While all 7 had data on genetic, neurologic, neuropsychologic, and neuropathologic findings, only 5 had completed a 3-T volumetric brain MRI scan and only 3 had completed an 18F-fluorodeoxyglucose (FDG)-PET scan. A case with similar cognitive status, age at death, and pathological features except for the presence of TDP-43 pathology was used as a control for the neuropathologic studies.
This study was approved by the Mayo Clinic Institutional Review Board. All participants or their proxies had signed written informed consent forms and had consented to brain donation.
Genetic assessment
Peripheral blood samples were obtained antemortem from all 7 participants and were used to extract genomic DNA for apolipoprotein E (APOE) genotyping performed through analysis of single-nucleotide polymorphisms (SNP), rs429358 (4-coding SNP) and rs7412 (2-coding SNP), using TaqMan genotyping assay.
Clinical and neuropsychological evaluations
All 7 participants were seen for a baseline and at least 1 follow-up comprehensive evaluation. The neurological evaluation included administration of the Mini Mental State Examination (MMSE) (40) to screen for overall cognitive impairment and the Clinical Dementia Rating scale—Sum of Boxes (CDR-SOB) (41) to estimate functional independence in the activities of daily living. Parkinsonism and gait dysfunction were also estimated using the Unified Parkinson’s Disease Rating Scale (UPDRS) (42). The neuropsychological evaluation consisted of the Auditory Verbal Learning Test (AVLT) (43) to test for verbal episodic memory, the Boston Naming Test for confrontational naming, Trail Making Test-A (44) for visuomotor speed, Trail Making Test-B (44) for cognitive flexibility, Weschler Adult Intelligence Scale-Revised Block Design Test (WAIS-BD) (45) for visuospatial and constructional function, and the Neuropsychiatric Inventory (46) for mood and behavioral changes.
All neurological and neuropsychological findings, as well as results from auxiliary laboratory or imaging studies, were reviewed during consensus meetings to establish a diagnosis of cognitive state (ie, cognitively unimpaired [CU], mild cognitive impairment [MCI], or dementia) (47, 48). Information on other medical conditions and circumstances occurring prior to or at the time of death was retrieved from clinical records.
Plots showing the longitudinal changes in clinical scores from baseline to last follow-up closest to death were generated using the ggplot2 package of R statistical software version 4.2.
Neuroimaging acquisition and processing
The 3-T volumetric MRI protocol included a magnetization-prepared rapid gradient echo (MPRAGE) sequence. Voxel-based morphometry (VBM) of MRI data was performed using Statistical Parametric Mapping (SPM) 12 (Wellcome Trust Centre for Neuroimaging, London, UK) to evaluate group-level patterns of gray matter volume loss across the brain. Multiple regression was used to compare gray matter volumes between the star-shaped TDP-43 group (n = 5) and healthy controls (n = 12), with age at MRI scan as a covariate. The 12 healthy controls had been recruited by the MCSA and had undergone the same neuroimaging protocols as the main study participants. The controls were 40% female (n = 5), had a median education of 15 years (IQR: 12–16), and were matched by age at scan (control and atypical TDP-43 group median age at scan = 88 years, IQR: 86–91 and IQR: 86–95, respectively). Clinically, all controls had received a diagnosis of “cognitively unimpaired” prior to death and their last MMSE showed a median score of 28 (IQR: 27–29). Pathologically, they showed no TDP-43 pathology, absent-to-low ADNC, no LBD, and mostly absent-to-moderate cerebrovascular pathology. The primary threshold for significance was set at p < .001, uncorrected. Because only a few voxels survived the primary threshold, no further correction for multiple comparisons was performed. Neuroimaging figures were generated with BrainNet Viewer (49).
The 18F-FDG-PET scans were acquired using a PET/CT scanner (GE Healthcare, Milwaukee, WI). Individual-level patterns of hypometabolism were assessed using 3-dimensional stereotactic surface projections with CortexID Suite (GE Healthcare), whereby activity at each voxel is normalized to the pons and Z-scored to an age-segmented normative database.
Neuropathologic evaluations
All cases underwent systematic postmortem examination of the brain. Except for 1 case, 1 hemibrain (typically the left) was processed to yield formalin-fixed, paraffin-embedded tissue blocks which were sectioned into 5- to 8-m-thick slices used for microscopic studies. Recommendations from the National Institute on Aging-Alzheimer’s Association guidelines (50) were followed for tissue sampling and neuropathologic assessments. All tissue samples were routinely stained with hematoxylin and eosin (H&E) and a modified Bielschowsky stain. Immunohistochemistry using antibodies to phospho-tau (AT8, 1:1000: Endogen, Woburn, MA) was performed to determine a Braak neurofibrillary tangle (NFT) stage (51) and a Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuritic plaque score (supplemented by modified Bielschowsky stain) (52), as well as to detect argyrophilic grains disease (AGD) (53) (supplemented by modified Bielschowsky stain) and aging-related tau astrogliopathy (ARTAG) (54) (supplemented by 4R tau immunohistochemistry). Immunohistochemistry for amyloid- (A) using clone 6F/3D (1:10, Novocastra Vector Labs, Burlingame, CA) was performed to establish a Thal A phase (55). An overall severity of ADNC was estimated based on the “ABC” scoring system (50). Sections immunostained for -synuclein (clone LB509, 1:200, Zymed, San Francisco, CA) were used to assess for LBD (56). Hippocampal sclerosis was considered present when moderate to severe neuronal loss and gliosis were seen in the subiculum and/or CA1 region of the hippocampus with H&E staining. Slides stained with H&E were also used to assess for cerebral amyloid angiopathy (CAA) (supplemented with A histochemistry), arteriolosclerosis, microinfarcts and lacunar or large infarcts.
TDP-43 pathology evaluations
Screening for TDP-43 pathology was performed on amygdala sections immunostained with antibodies against TDP-43 with phosphorylated epitopes (pS409/410, 1:1500, mouse monoclonal, Cosmo Bio Co., Tokyo, Japan) using a DAKO Autostainer (Universal Staining System, Carpinteria, CA). The segmentation of amygdala in its subnuclei and regions was performed based on previous publication (57). Cases that showed predominant NCIs, DNs, and occasional NIIs in intraparenchymal regions of the amygdala were categorized as type-, while cases with a preponderance of TATs (whether “apple-bite” or “flame-shaped”) were categorized as type-. As already mentioned, 7 cases showed features suggestive of true TDP-43 inclusions and did not resemble artifacts or “dirt,” nonetheless, they were atypical and consequently could not be typed. To determine the extent of distribution of the star-shaped TDP-43 pathology and whether these cases would show “typical” inclusions beyond the amygdala, we also stained coronal sections of the temporal lobe cut at the level of the lateral geniculate nucleus, comprising of the posterior hippocampal proper and entorhinal cortex, and coronal sections of the frontal lobe containing the superior and middle frontal gyri. Moreover, to assess the nature of the TDP-43 species that constitutes these atypical inclusions, we performed supplementary TDP-43 immunostaining of amygdala sections using antibodies against the N-terminal TDP-43 fragments found only in full-length TDP-43 (MC2079, 1:2500, rabbit polyclonal, from Dr. Leonard Petrucelli, Mayo Clinic, FL) and antibodies against a peptide sequence in the 25-kDa C-terminal fragment (MC2085, 1:2500, rabbit polyclonal, from Dr. Leonard Petrucelli, Mayo Clinic, FL) (58). Initial immunohistochemistry analyses showed that antibodies against pTDP-43 and nTDP-43 were most efficient at detecting the star-like inclusions. For this reason, we have used both antibodies to conduct double-labeling studies. Since the morphology of the inclusions was reminiscent of pathologic protein inclusions depositing in astrocytes, we performed double-labeling immunohistochemistry staining on amygdala sections using the combination of pTDP-43 antibody (Rb3655, 1:1000, rabbit polyclonal, from Dr. Leonard Petrucelli, Mayo Clinic, FL) or nTDP-43 antibody (MC2079, 1:2500, rabbit polyclonal) and anti-glial fibrillary acidic protein ([GFAP], a marker of astrocytes) (clone GA-5, 1:5000, mouse monoclonal, BioGenex) to confirm the astrocytic cellular localization. Furthermore, since TDP-43 is known to colocalize with tau in both neurofibrillary tangles and neuritic plaques in AD, we also screened for potential colocalization of TDP-43 and tau proteins within the star-shaped TDP-43 inclusions using double-labeling immunohistochemistry for pTDP-43 (Rb3655, 1:1000, rabbit polyclonal) or nTDP-43 antibody (MC2079, 1:2500, rabbit polyclonal) and phospho-tau (CP13, 1:1000, mouse monoclonal, from Dr. Peter Davies, Feinstein Institute, North Shore Hospital, Manhasset, NY).
We successively performed double-labeling immunofluorescence studies to further investigate a potential colocalization using antibodies again against pTPD-43 (Rb3655, 1:500, rabbit polyclonal) or nTDP-43 (MC2079, 1:2500, rabbit polyclonal) and antibodies against GFAP (clone GA-5, 1:5000, mouse monoclonal) or phospho-tau (CP13, 1:1000, mouse monoclonal). Sections were successively incubated with secondary antibodies Alexa Fluor 568 (1:500, Thermo Fisher Scientific, Waltham, MA) and Alexa Fluor 488 (1:500, Thermo Fisher Scientific), diluted with Antibody Diluent with Background Reducing Components (DAKO), treated with 1% Sudan Black, and finally mounted with Vectashield mounting media containing DAPI (Vector Laboratories). For all double-labeling studies, we used an age-matched case that is known to lack TDP-43 pathologic inclusions as a negative control.
RESULTS
Patient characteristics
Of the 7 cases with star-shaped TDP-43 inclusions, 3 were male and 4 were female. Information on demographic and clinical features is found in Table 1. All 7 were “superagers,” with a median age at death of 99.8 years (IQR: 93.5–101.4). Only 1 (14%) was a carrier of a single copy of the APOE 4 allele. All had died from consequences of heart failure, pneumonia, cancer, or severe fall-induced injuries, after having a prolonged agonal period. All suffered from varying degrees of cardiovascular disease, including coronary artery disease, valvular heart disease, arrhythmias, and hypertension. Three also suffered from recurrent falls. None of the 7 had been diagnosed with dementia in life: 4 had preserved normal cognition until death and 3 developed cognitive impairment with mild loss of episodic memory in their 90s. The matched control died at age 99.2 years and was also cognitively normal prior to death.
Demographic and clinical characteristics of star-shaped TDP-43 cases and control case.
| Characteristics . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Sex . | M . | F . | M . | M . | F . | F . | F . | M . |
| Handedness | Right | Right | Right | Left | Right | Right | Right | Right |
| Education, years | 20 | 15 | 18 | 13 | 20 | 17 | 13 | 8 |
| APOE ε4 carrier | No | No | No | No | Yes | No | No | No |
| Age at death, years | 93.5 | 101.4 | 91.9 | 99.8 | 94.0 | 101.2 | 103.7 | 99.2 |
| Last cognitive diagnosis | CU | Multi-MCI with amnestic | MCI-amnestic | CU | Multi-MCI with amnestic | CU | CU | CU |
| Immediate cause of death | Decompensated heart failure and pneumonia | Metastatic breast carcinoma | Decompensated heart failure | Fall with GI bleed | Pneumonia | Fall with multiple fractures | Decompensated heart failure | Decompensated heart failure |
| Past medical history | Hypertensive cardiomyopathy, CAD, AF, HTN, renal cell carcinoma | HTN, AR, colon cancer | CAD with MI, HTN, AF, hyperlipidemia, OSA, hypothyroidism, gait impairment with recurrent falls | Hypertensive cardiomyopathy, CAD, MI, AS, Sick sinus syndrome, recurrent pneumonia, diverticular disease, recurrent falls | Hypertensive cardiomyopathy, Amyloid cardiomyopathy, AS, myelodysplastic syndrome | HTN, AS, Macular degeneration | HTN, CAD, AF, PMR, Hypothyroidism, Hyperparathyroidism Diverticular disease, Infarcts | HTN, CAD, AF, TR, transthyretin cardiac amyloidosis |
| Characteristics . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Sex . | M . | F . | M . | M . | F . | F . | F . | M . |
| Handedness | Right | Right | Right | Left | Right | Right | Right | Right |
| Education, years | 20 | 15 | 18 | 13 | 20 | 17 | 13 | 8 |
| APOE ε4 carrier | No | No | No | No | Yes | No | No | No |
| Age at death, years | 93.5 | 101.4 | 91.9 | 99.8 | 94.0 | 101.2 | 103.7 | 99.2 |
| Last cognitive diagnosis | CU | Multi-MCI with amnestic | MCI-amnestic | CU | Multi-MCI with amnestic | CU | CU | CU |
| Immediate cause of death | Decompensated heart failure and pneumonia | Metastatic breast carcinoma | Decompensated heart failure | Fall with GI bleed | Pneumonia | Fall with multiple fractures | Decompensated heart failure | Decompensated heart failure |
| Past medical history | Hypertensive cardiomyopathy, CAD, AF, HTN, renal cell carcinoma | HTN, AR, colon cancer | CAD with MI, HTN, AF, hyperlipidemia, OSA, hypothyroidism, gait impairment with recurrent falls | Hypertensive cardiomyopathy, CAD, MI, AS, Sick sinus syndrome, recurrent pneumonia, diverticular disease, recurrent falls | Hypertensive cardiomyopathy, Amyloid cardiomyopathy, AS, myelodysplastic syndrome | HTN, AS, Macular degeneration | HTN, CAD, AF, PMR, Hypothyroidism, Hyperparathyroidism Diverticular disease, Infarcts | HTN, CAD, AF, TR, transthyretin cardiac amyloidosis |
AF, atrial fibrillation; APOE, apolipoprotein E; AS, aortic stenosis; CAD, coronary artery disease; CHF, chronic heart failure; CU, cognitively unimpaired; GI, gastrointestinal; HTN, hypertension; MCI, mild cognitive impairment; MI, myocardial infarction; OSA, obstructive sleep apnea; PMR, polymyalgia rheumatica; TR, tricuspid regurgitation.
Demographic and clinical characteristics of star-shaped TDP-43 cases and control case.
| Characteristics . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Sex . | M . | F . | M . | M . | F . | F . | F . | M . |
| Handedness | Right | Right | Right | Left | Right | Right | Right | Right |
| Education, years | 20 | 15 | 18 | 13 | 20 | 17 | 13 | 8 |
| APOE ε4 carrier | No | No | No | No | Yes | No | No | No |
| Age at death, years | 93.5 | 101.4 | 91.9 | 99.8 | 94.0 | 101.2 | 103.7 | 99.2 |
| Last cognitive diagnosis | CU | Multi-MCI with amnestic | MCI-amnestic | CU | Multi-MCI with amnestic | CU | CU | CU |
| Immediate cause of death | Decompensated heart failure and pneumonia | Metastatic breast carcinoma | Decompensated heart failure | Fall with GI bleed | Pneumonia | Fall with multiple fractures | Decompensated heart failure | Decompensated heart failure |
| Past medical history | Hypertensive cardiomyopathy, CAD, AF, HTN, renal cell carcinoma | HTN, AR, colon cancer | CAD with MI, HTN, AF, hyperlipidemia, OSA, hypothyroidism, gait impairment with recurrent falls | Hypertensive cardiomyopathy, CAD, MI, AS, Sick sinus syndrome, recurrent pneumonia, diverticular disease, recurrent falls | Hypertensive cardiomyopathy, Amyloid cardiomyopathy, AS, myelodysplastic syndrome | HTN, AS, Macular degeneration | HTN, CAD, AF, PMR, Hypothyroidism, Hyperparathyroidism Diverticular disease, Infarcts | HTN, CAD, AF, TR, transthyretin cardiac amyloidosis |
| Characteristics . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Sex . | M . | F . | M . | M . | F . | F . | F . | M . |
| Handedness | Right | Right | Right | Left | Right | Right | Right | Right |
| Education, years | 20 | 15 | 18 | 13 | 20 | 17 | 13 | 8 |
| APOE ε4 carrier | No | No | No | No | Yes | No | No | No |
| Age at death, years | 93.5 | 101.4 | 91.9 | 99.8 | 94.0 | 101.2 | 103.7 | 99.2 |
| Last cognitive diagnosis | CU | Multi-MCI with amnestic | MCI-amnestic | CU | Multi-MCI with amnestic | CU | CU | CU |
| Immediate cause of death | Decompensated heart failure and pneumonia | Metastatic breast carcinoma | Decompensated heart failure | Fall with GI bleed | Pneumonia | Fall with multiple fractures | Decompensated heart failure | Decompensated heart failure |
| Past medical history | Hypertensive cardiomyopathy, CAD, AF, HTN, renal cell carcinoma | HTN, AR, colon cancer | CAD with MI, HTN, AF, hyperlipidemia, OSA, hypothyroidism, gait impairment with recurrent falls | Hypertensive cardiomyopathy, CAD, MI, AS, Sick sinus syndrome, recurrent pneumonia, diverticular disease, recurrent falls | Hypertensive cardiomyopathy, Amyloid cardiomyopathy, AS, myelodysplastic syndrome | HTN, AS, Macular degeneration | HTN, CAD, AF, PMR, Hypothyroidism, Hyperparathyroidism Diverticular disease, Infarcts | HTN, CAD, AF, TR, transthyretin cardiac amyloidosis |
AF, atrial fibrillation; APOE, apolipoprotein E; AS, aortic stenosis; CAD, coronary artery disease; CHF, chronic heart failure; CU, cognitively unimpaired; GI, gastrointestinal; HTN, hypertension; MCI, mild cognitive impairment; MI, myocardial infarction; OSA, obstructive sleep apnea; PMR, polymyalgia rheumatica; TR, tricuspid regurgitation.
Clinical and neuropsychological scores
Scores from specific clinical and neuropsychological assessments were plotted based on the time from when the scores were obtained at first and last visit to when the patients died (Fig. 1). Consistent with the relatively preserved cognitive functions, the mean MMSE scores of the cohort were consistently above the cut-off of 26/30 for cognitive impairment. Mean CDR-SOB was also 1.0, indicative of well-maintained functional independence. Among the neuropsychological tests, the AVLT delayed recall scores were the most affected, suggesting impaired verbal episodic memory. There was also evidence for impairment of the executive function domain, as reflected by the increasing times to complete the TMT-B test which is dependent on the integrity of cognitive flexibility. All other domains (language, attention, visuospatial, behavior/frontal, and motor) showed little changes over the years, and mean performance scores were predominantly in the normal or clinically acceptable ranges.
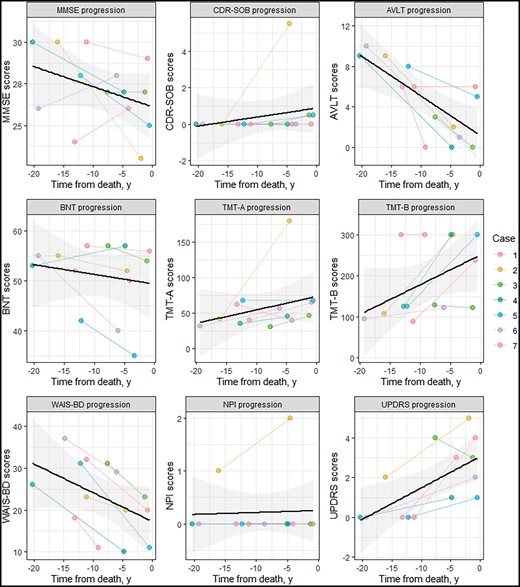
Longitudinal changes in clinical and neuropsychologic scores. The spaghetti plots show the progression of clinical or neuropsychological scores across the time from baseline to death. For each patient and each test, 2 values are shown representing scores at baseline and at the last follow-up visit closest to death. The black line represents the mean score for the cohort and the shaded gray area represents confidence intervals. Noteworthy changes are seen with the drop in AVLT delayed scores, suggesting impaired episodic memory, and the lengthening of time to complete the TMT-B test, indicating worsening cognitive flexibility. Abbreviations: AVLT, auditory verbal learning test; BNT, Boston naming test; CDR-SOB, Clinical Dementia Rating Scale—Sum of Boxes; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory; TMT-A, Trail Making Test-A; TMT-B, Trail Making Test-B; UPDRS, Unified Parkinson’s Disease Rating Scale; WAIS-BD, Weschler Adult Intelligence Scale-Revised Block Design Test.
Neuroimaging findings
Brain MRI scans from 5 cases individually revealed moderate generalized cerebral and cerebellar atrophy as well as marked atrophy in temporal lobes with leukoaraiosis. Effect maps showed predominant differences in gray matter volumes in the medial and lateral temporal areas, as well as the precuneus and posterior cingulate, between the star-shaped TDP-43 group (n = 5) and the cognitively and neuropathologically normal, age-matched control group (n = 12) (Fig. 2A). However, only a few regions in the lateral temporo-occipital and anterior temporal regions (darker yellow to orange regions in Fig. 2A) had significantly smaller volumes than controls at p < .001, potentially due to our limited number of cases. Regarding the FDG-PET scans, 2 of the 3 cases showed no clear pattern of abnormal hypometabolism congruent with any neurodegenerative disease. Interestingly, 1 case (case 5) who had undergone longitudinal FDG-PET scans (n = 3) taken within 2 years of each other showed focal progressive hypometabolism of the left medial temporal lobe (Fig. 2B–D).
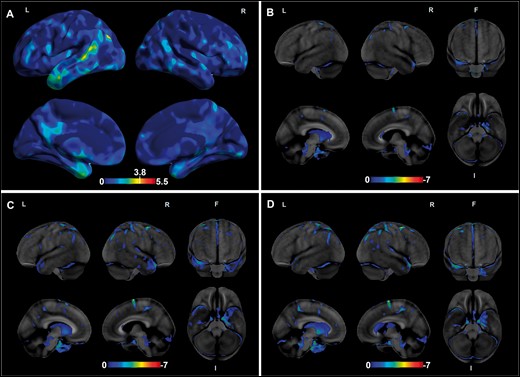
Volume loss and hypometabolism on neuroimaging. The effect map shows differences in gray matter volumes between the star-shaped TDP-43 group (n = 5) and the healthy control group (n = 12) (A). In the top lateral views, involvement of the anterior temporal pole and the posterosuperior temporal region is seen. In the bottom medial views, involvement of the medial temporal pole, precuneus and posterior cingulate are evident. The color bar represents height threshold T-values ranging from 0 (blue) to 5.5 (red). Regions that showed significantly less gray matter volumes in the star-shaped TDP-43 group at p < .001 are those with T > 3.78 (dark yellow to red). (B–D) Results from longitudinal FDG-PET scans (taken 2 years apart) from 1 case (case 4) showing progressive left medial temporal lobe hypometabolism. Abbreviations: FDG-PET, 18F-fluorodeoxyglucose-positron emission tomography; F, front; I, inferior; L, left; R, right.
Neuropathologic findings
Overall neuropathologic findings
The median weight of 1 fixed hemibrain was 634 g (IQR: 553–656). Detailed information on neuropathologic scores from the 7 atypical TDP-43 cases and the age-matched control is found in Table 2. The median scores for ADNC were 2 for Thal A phase, III for Braak NFT stage, and 1 or sparse for CERAD neuritic plaque score. Individually, none of the 7 cases had severe AD pathology. Indeed, 1 case showed low ADNC, 3 cases intermediate ADNC, and 3 cases were classified as having PART, with the presence of NFTs in the medial temporal lobe in the absence of A plaques. Other frequent copathologies present included ARTAG, which was found in all 7 cases, with tau-immunoreactive astrocytic lesions found in the same regions as the star-shaped TDP-43 inclusions, and AGD, with tau-immunoreactive grains found in the hippocampus and/or entorhinal cortex in 6 of the 7 cases. Lewy body pathology was present in 4 cases, with a brainstem-predominant pattern in 3 and a transitional pattern of -synuclein pathology in 1 case. Hippocampal sclerosis was absent to mild in severity in 6 cases and moderate in severity in 1 case. Concerning vascular pathology, 3 cases had CAA, while arteriolosclerosis was found in all. Five cases also showed infarcts and/or microinfarcts at autopsy.
Neuropathologic findings in atypical star-shaped TDP-43 cases and in a control case.
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Brain weight, fixed, g . | 656 (left) . | 503 (left) . | 648 (left) . | 691 (left) . | 634 (mean) . | 553 (left) . | 560 (left) . | 637 (left) . |
| TDP-43 Pathology | ||||||||
| Amygdala (star-shaped)* | Subpial | Subpial | Subpial | Subpial > basolateral | Subpial > basolateral | Subpial | Subpial | None |
| Hippocampus (neurites)† | Absent | Absent | Absent | Fimbria > Subiculum | Subiculum > Fimbria | Subiculum > Fimbria | Entorhinal > Fimbria > Subiculum | None |
| Superior/middle frontal | Absent | Absent | Absent | Absent | Absent | Absent | Absent | None |
| N-terminal (full-length specie) TDP-43 | Star-shaped with short processes, thin superficial neurites | Several well-formed star-shaped inclusions, thin neurites | Granular star-shaped, few thin neurites | Absent | Several well-formed star-shaped inclusions, thin superficial neurites | Granular star-shaped inclusions, Few thin superficial neurites | Only thin superficial neurites | None |
| C-terminal TDP-43 | Absent | Granular star-shaped inclusions | Only 1 NCI | Absent | Star-shaped with short processes, few thin superficial neurites, deeper comma-shaped neurites | Only few thin superficial neurites, few NCI | Granular star-shaped, few thin superficial neurites | None |
| Other pathologies | ||||||||
| Hippocampal sclerosis | Mild | Mild | Absent | Mild | Mild | Moderate | Mild | Absent |
| Thal A phase | 2 | 4 | 0 | 3 | 0 | 3 | 0 | 0 |
| Braak NFT stage | III | III | I-II | IV | I | IV | I | II |
| CERAD | Sparse | Moderate | Normal | Moderate | Normal | Moderate | Normal | Normal |
| ADNC | Low (A1, B2, C1) | Intermediate (A3, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | PART (A0, B1, C0) |
| AGD | Absent | Present | Present | Present | Present | Present | Present | Absent |
| ARTAG | Present | Present | Present | Present | Present | Present | Present | Absent |
| LBD status | Present | Present | Absent | Absent | Present | Present | Absent | Absent |
| LBD stage | Brainstem-predominant | Brainstem-predominant | N/A | N/A | Brainstem-predominant | Transitional | N/A | N/A |
| CAA | Absent | Absent | Absent | Severe | Moderate | Mild | Absent | Moderate |
| Arteriolosclerosis | Mild | Mild | Moderate | Severe | Moderate | Moderate | Severe | Mild |
| Infarcts | Present | Absent | Absent | Present | Absent | Present | Present | Absent |
| Microinfarcts | Absent | Absent | Absent | Absent | Present | Absent | Present | Absent |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Brain weight, fixed, g . | 656 (left) . | 503 (left) . | 648 (left) . | 691 (left) . | 634 (mean) . | 553 (left) . | 560 (left) . | 637 (left) . |
| TDP-43 Pathology | ||||||||
| Amygdala (star-shaped)* | Subpial | Subpial | Subpial | Subpial > basolateral | Subpial > basolateral | Subpial | Subpial | None |
| Hippocampus (neurites)† | Absent | Absent | Absent | Fimbria > Subiculum | Subiculum > Fimbria | Subiculum > Fimbria | Entorhinal > Fimbria > Subiculum | None |
| Superior/middle frontal | Absent | Absent | Absent | Absent | Absent | Absent | Absent | None |
| N-terminal (full-length specie) TDP-43 | Star-shaped with short processes, thin superficial neurites | Several well-formed star-shaped inclusions, thin neurites | Granular star-shaped, few thin neurites | Absent | Several well-formed star-shaped inclusions, thin superficial neurites | Granular star-shaped inclusions, Few thin superficial neurites | Only thin superficial neurites | None |
| C-terminal TDP-43 | Absent | Granular star-shaped inclusions | Only 1 NCI | Absent | Star-shaped with short processes, few thin superficial neurites, deeper comma-shaped neurites | Only few thin superficial neurites, few NCI | Granular star-shaped, few thin superficial neurites | None |
| Other pathologies | ||||||||
| Hippocampal sclerosis | Mild | Mild | Absent | Mild | Mild | Moderate | Mild | Absent |
| Thal A phase | 2 | 4 | 0 | 3 | 0 | 3 | 0 | 0 |
| Braak NFT stage | III | III | I-II | IV | I | IV | I | II |
| CERAD | Sparse | Moderate | Normal | Moderate | Normal | Moderate | Normal | Normal |
| ADNC | Low (A1, B2, C1) | Intermediate (A3, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | PART (A0, B1, C0) |
| AGD | Absent | Present | Present | Present | Present | Present | Present | Absent |
| ARTAG | Present | Present | Present | Present | Present | Present | Present | Absent |
| LBD status | Present | Present | Absent | Absent | Present | Present | Absent | Absent |
| LBD stage | Brainstem-predominant | Brainstem-predominant | N/A | N/A | Brainstem-predominant | Transitional | N/A | N/A |
| CAA | Absent | Absent | Absent | Severe | Moderate | Mild | Absent | Moderate |
| Arteriolosclerosis | Mild | Mild | Moderate | Severe | Moderate | Moderate | Severe | Mild |
| Infarcts | Present | Absent | Absent | Present | Absent | Present | Present | Absent |
| Microinfarcts | Absent | Absent | Absent | Absent | Present | Absent | Present | Absent |
ADNC, Alzheimer disease neuropathologic score; AGD, argyrophilic grains disease; ARTAG, aging-related tau astrogliopathy; CAA, cerebral amyloid angiopathy; CERAD, consortium to establish a registry for Alzheimer’s disease; LBD, Lewy body disease; NFT, neurofibrillary tangle; TDP-43, transactive response DNA-binding protein 43.
*Distribution refers to that of star-shaped TDP-43 inclusions.
†Distribution refers to that of thin superficial neurites.
Neuropathologic findings in atypical star-shaped TDP-43 cases and in a control case.
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Brain weight, fixed, g . | 656 (left) . | 503 (left) . | 648 (left) . | 691 (left) . | 634 (mean) . | 553 (left) . | 560 (left) . | 637 (left) . |
| TDP-43 Pathology | ||||||||
| Amygdala (star-shaped)* | Subpial | Subpial | Subpial | Subpial > basolateral | Subpial > basolateral | Subpial | Subpial | None |
| Hippocampus (neurites)† | Absent | Absent | Absent | Fimbria > Subiculum | Subiculum > Fimbria | Subiculum > Fimbria | Entorhinal > Fimbria > Subiculum | None |
| Superior/middle frontal | Absent | Absent | Absent | Absent | Absent | Absent | Absent | None |
| N-terminal (full-length specie) TDP-43 | Star-shaped with short processes, thin superficial neurites | Several well-formed star-shaped inclusions, thin neurites | Granular star-shaped, few thin neurites | Absent | Several well-formed star-shaped inclusions, thin superficial neurites | Granular star-shaped inclusions, Few thin superficial neurites | Only thin superficial neurites | None |
| C-terminal TDP-43 | Absent | Granular star-shaped inclusions | Only 1 NCI | Absent | Star-shaped with short processes, few thin superficial neurites, deeper comma-shaped neurites | Only few thin superficial neurites, few NCI | Granular star-shaped, few thin superficial neurites | None |
| Other pathologies | ||||||||
| Hippocampal sclerosis | Mild | Mild | Absent | Mild | Mild | Moderate | Mild | Absent |
| Thal A phase | 2 | 4 | 0 | 3 | 0 | 3 | 0 | 0 |
| Braak NFT stage | III | III | I-II | IV | I | IV | I | II |
| CERAD | Sparse | Moderate | Normal | Moderate | Normal | Moderate | Normal | Normal |
| ADNC | Low (A1, B2, C1) | Intermediate (A3, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | PART (A0, B1, C0) |
| AGD | Absent | Present | Present | Present | Present | Present | Present | Absent |
| ARTAG | Present | Present | Present | Present | Present | Present | Present | Absent |
| LBD status | Present | Present | Absent | Absent | Present | Present | Absent | Absent |
| LBD stage | Brainstem-predominant | Brainstem-predominant | N/A | N/A | Brainstem-predominant | Transitional | N/A | N/A |
| CAA | Absent | Absent | Absent | Severe | Moderate | Mild | Absent | Moderate |
| Arteriolosclerosis | Mild | Mild | Moderate | Severe | Moderate | Moderate | Severe | Mild |
| Infarcts | Present | Absent | Absent | Present | Absent | Present | Present | Absent |
| Microinfarcts | Absent | Absent | Absent | Absent | Present | Absent | Present | Absent |
| Characteristic . | Case 1 . | Case 2 . | Case 3 . | Case 4 . | Case 5 . | Case 6 . | Case 7 . | Control . |
|---|---|---|---|---|---|---|---|---|
| Brain weight, fixed, g . | 656 (left) . | 503 (left) . | 648 (left) . | 691 (left) . | 634 (mean) . | 553 (left) . | 560 (left) . | 637 (left) . |
| TDP-43 Pathology | ||||||||
| Amygdala (star-shaped)* | Subpial | Subpial | Subpial | Subpial > basolateral | Subpial > basolateral | Subpial | Subpial | None |
| Hippocampus (neurites)† | Absent | Absent | Absent | Fimbria > Subiculum | Subiculum > Fimbria | Subiculum > Fimbria | Entorhinal > Fimbria > Subiculum | None |
| Superior/middle frontal | Absent | Absent | Absent | Absent | Absent | Absent | Absent | None |
| N-terminal (full-length specie) TDP-43 | Star-shaped with short processes, thin superficial neurites | Several well-formed star-shaped inclusions, thin neurites | Granular star-shaped, few thin neurites | Absent | Several well-formed star-shaped inclusions, thin superficial neurites | Granular star-shaped inclusions, Few thin superficial neurites | Only thin superficial neurites | None |
| C-terminal TDP-43 | Absent | Granular star-shaped inclusions | Only 1 NCI | Absent | Star-shaped with short processes, few thin superficial neurites, deeper comma-shaped neurites | Only few thin superficial neurites, few NCI | Granular star-shaped, few thin superficial neurites | None |
| Other pathologies | ||||||||
| Hippocampal sclerosis | Mild | Mild | Absent | Mild | Mild | Moderate | Mild | Absent |
| Thal A phase | 2 | 4 | 0 | 3 | 0 | 3 | 0 | 0 |
| Braak NFT stage | III | III | I-II | IV | I | IV | I | II |
| CERAD | Sparse | Moderate | Normal | Moderate | Normal | Moderate | Normal | Normal |
| ADNC | Low (A1, B2, C1) | Intermediate (A3, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | Intermediate (A2, B2, C2) | PART (A0, B1, C0) | PART (A0, B1, C0) |
| AGD | Absent | Present | Present | Present | Present | Present | Present | Absent |
| ARTAG | Present | Present | Present | Present | Present | Present | Present | Absent |
| LBD status | Present | Present | Absent | Absent | Present | Present | Absent | Absent |
| LBD stage | Brainstem-predominant | Brainstem-predominant | N/A | N/A | Brainstem-predominant | Transitional | N/A | N/A |
| CAA | Absent | Absent | Absent | Severe | Moderate | Mild | Absent | Moderate |
| Arteriolosclerosis | Mild | Mild | Moderate | Severe | Moderate | Moderate | Severe | Mild |
| Infarcts | Present | Absent | Absent | Present | Absent | Present | Present | Absent |
| Microinfarcts | Absent | Absent | Absent | Absent | Present | Absent | Present | Absent |
ADNC, Alzheimer disease neuropathologic score; AGD, argyrophilic grains disease; ARTAG, aging-related tau astrogliopathy; CAA, cerebral amyloid angiopathy; CERAD, consortium to establish a registry for Alzheimer’s disease; LBD, Lewy body disease; NFT, neurofibrillary tangle; TDP-43, transactive response DNA-binding protein 43.
*Distribution refers to that of star-shaped TDP-43 inclusions.
†Distribution refers to that of thin superficial neurites.
Atypical TDP-43 inclusions
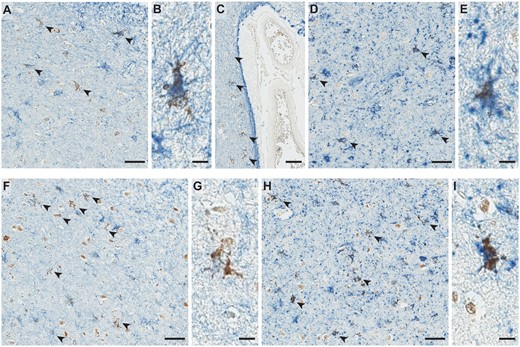
The star-shaped TDP-43 inclusions were consistently found in the medial subpial regions, particularly in the cortical nucleus, around perivascular regions, and in basolateral white matter and subependymal regions (Fig. 3A). Representative images are shown from the superficial (Fig. 4A), white matter (Fig. 4B), and subependymal regions (Fig. 4C). Distinct morphologic variants of the star-shaped inclusions were found within the amygdala. Some of the most mature-looking lesions had TDP-43 deposition in the cell body surrounding the nucleus, with long branching projections likely representing protein aggregates within cellular processes (Fig. 4D, E). Others demonstrated less involvement of the perinuclear region and appeared to have a more lace-like pattern of involvement of more distal processes (Fig. 4F). However, in cases that had the lowest burden, some of the star-shaped inclusions showed a more granular involvement of perinuclear regions and shorter, stubby processes, with the appearance of a less mature preinclusion (Fig. 4G, H). Occasionally, some inclusions had a crown-like (Fig. 4I) or thorn-like (Fig. 4J) appearance. Rare slender comma-shaped inclusions reminiscent of tau-immunoreactive coiled bodies were also seen (Fig. 4K). Lastly, thread-like neurites and dot-like neuropil were also common in very superficial regions, particularly in cases with less mature star-shaped pre-inclusions (Fig. 4A, L). The control case was free of any kind of TDP-43 inclusion, whether typical or atypical and is therefore not shown.
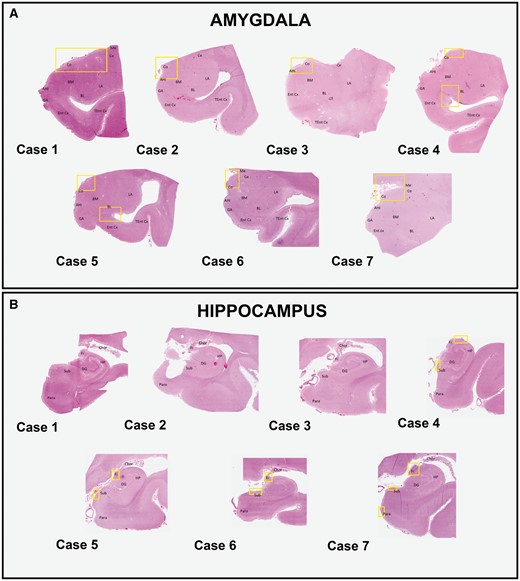
Distribution of TDP-43-immunoreactive inclusions. Whole slide imaging of digitalized sections from the amygdala (A) and hippocampus (B) are shown. To easily identify anatomical regions, sections stained with hematoxylin and eosin from each patient are selected. The amygdaloid subregions are labeled. Areas where the star-shaped TDP-43 inclusions are observed in the amygdala are delineated by the yellow boxes (A). These mainly involve the corticomedial regions in all cases and additionally the basolateral white matter and subependymal regions in case 4 and 5. Areas where the thread-like neurites are seen in the hippocampal regions are similarly delineated by the yellow boxes (B). These include the fibers of the fimbria and the subiculum in all cases, with the addition of the entorhinal cortex in case 7. Abbreviations: Ahi, amygdala hippocampal transition area; BL, basolateral nucleus; BM, basomedial nucleus; Ce, central nucleus; Chor, choroid plexus; Co, cortical nucleus; DG, dentate gyrus; Ent cx, entorhinal cortex; Fi, fimbria; GA, gyrus accumbens; HP, hippocampal proper; LA, lateral nucleus; Me, medial nucleus; Para, parahippocampal gyrus; Sub, subiculum; Tent cx, transentorhinal cortex.
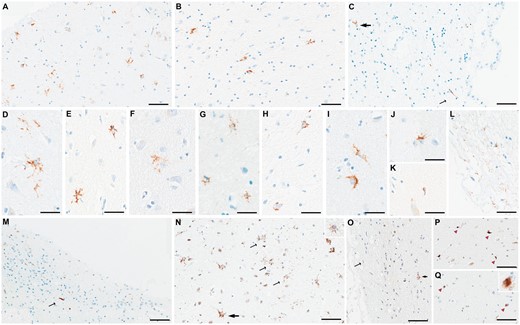
Representative figures of microscopic TDP-43 findings. Amygdala slides stained with antibodies against phospho-TDP-43 showed star-shaped TDP-43-immunoreactive inclusions in medial subpial regions (A) and basolateral white matter (B) and subependymal regions (thick arrow in C). Additionally, dot-like neuropil (A) and a long thread-like neurite (thin arrow in C) are seen. Different morphologies of star-shaped inclusions are shown: star-shaped with long, branching processes (D, E), with lace-like processes (F), with granular appearance (G, H), or with crown-like (I) or thorn-like (J) appearance. An occasional coiled body-like inclusion is shown (K). Superficial thin, thread-like neurites are commonly seen (L). In the hippocampus, a variety of neurites from thin to thick and short to long are seen in the fimbria (M), as well as the subiculum and entorhinal cortex (not shown). Using antibodies against N-terminal TDP-43 (N), star-shaped TDP-43 inclusions with long processes are also seen (thick arrow), although many of the inclusions are more granular in appearance (thin arrows and inset in N). Using antibodies against the C-terminal region (O), only a few granular star-shaped inclusions are seen (thick arrow) and some superficial neurites (thin arrow). However, some comma-shaped thick neurites (pink arrowheads in P) and neuronal cytoplasmic inclusions (pink arrowheads and inset in Q) are additionally seen. Scale bars: A-C, M-Q = 40 m, D-K and all insets = 30 m.
TDP-43 pathology beyond the amygdala
Hippocampal and frontal lobe slides immunostained with antibodies against pTDP-43 did not reveal extension of the star-shaped TDP-43 lesions to either of these regions. Nonetheless, 4 cases did harbor some other type of TDP-43-immunoreactive inclusions in the hippocampal region (Fig. 3B). These merely included fine thread-like neurites (with occasional fat neurites) found among the fibers of the fimbria (Fig. 4M) and in superficial regions of the subiculum. The severity of lesions in each region varied among the individual cases, with some showing more frequent neurites in the subiculum, while others showed more in the fimbria (Table 2). The entorhinal cortex was also involved in 1 case, which intriguingly was the region harboring the greatest number of neurites. None of the 7 cases had any “typical” or “atypical” TDP-43-immunoreactive inclusions in the superior and middle frontal gyri.
Antibodies against N-terminal regions (full-length specie)
Five cases showed star-shaped inclusions in medial (subpial) regions following the staining of amygdala slides for nTDP-43. Three cases showed frequent well-formed star-shaped inclusions with long branching processes, as well as more granular star-shaped inclusions (Fig. 4N), corresponding to the 3 cases with the highest burden of similar inclusions seen with pTDP-43 antibody. They also showed many thin superficial neurites, which increased in number more than what was observed with the pTDP-43 antibody. Two cases, however, only showed star-shaped preinclusions with shorter processes (Fig. 4N inset). One case only showed several fine subpial neurites. None showed TDP-43 inclusions in basolateral white matter or subependymal regions. Finally, 1 case did not have any type of TDP-43 pathological inclusions.
Antibodies against C-terminal regions
Using slides stained with antibodies to cTDP-43, only 3 showed a few star-shaped inclusions mostly of the granular type, with shorter processes than the ones found using the pTDP-43 antibody (Fig. 4O). Two of these cases also showed thin neurites, however, 1 of the 2 additionally showed more intraparenchymal thicker, comma-shaped neurites that were not detected with pTDP-43 antibodies (Fig. 4P). Moreover, 2 cases did not show star-shaped inclusions and instead showed few “typical” NCIs (Fig. 4Q and inset). Comparable to the results with nTDP-43 staining, no TDP-43 inclusions were detected in the basolateral regions. Two cases were free of pathologic inclusions.
Cellular localization of star-shaped inclusions
The unique star-shaped appearance of these atypical inclusions closely resembled other pathological inclusions found within astrocytes. Results of the double-labeling immunohistochemistry for pTDP-43 or nTDP-43 and GFAP (a marker of astrocyte activation) indeed disclosed evidence of localization of the TDP-43 inclusions within astrocytes (Fig. 5A–C for pTDP-43; Fig. 5F, G for nTDP-43). There is also evidence of colocalization of TDP-43 and GFAP in perivascular lesions (Fig. 5C).
Representative images of double-labeling immunohistochemistry findings. Double-labeling immunohistochemistry showed colocalization of pTDP-43 (brown: Rb3655) and GFAP (blue: GA-5) within the star-shaped inclusions in subpial (indicated by arrowheads in (A) and magnified inclusion in (B)) and perivascular regions (indicated by black arrowheads in (C)). Double labeling immunohistochemistry for pTDP-43 (brown: Rb3655) and phospho-tau (blue: CP13) also showed colocalization of the 2 proteins in the star-shaped inclusions (arrowheads in (D) and magnified inclusion in (E)). Similar colocalizations are seen using anti-nTDP-43 antibodies (brown: MC2079) and anti-GFAP (blue: GA-5) in panels (F) and (G), as well as using anti-nTDP-43 (brown: MC2069) and anti-phospho-tau (blue: CP13) in panels (H) and (I). Scale bars: A, C, D, F, H = 40 m, B, E, G, I = 10 m. Abbreviation: GFAP, glial fibrillary acidic protein.
Relationship between TDP-43 and tau
Since all 7 cases showed ARTAG in the amygdala and, more importantly, in the same markedly delineated regions as those affected by the star-shaped TDP-43 inclusions, we speculated whether TDP-43 might be co-depositing with tau in these regions. Results of double-labeling immunohistochemistry for pTDP-43 or nTDP-43 and phospho-tau (using CP13) showed colocalization of the 2 proteins (Fig. 5D, E for pTDP-43; Fig. 5H, I for nTDP-43).
Colocalization with double-labeling immunofluorescence
The star-like inclusions were on average scarce in quantity and difficult to detect with immunofluorescence. However, we found that MC2079 (anti-nTDP-43) identified more atypical inclusions than Rb3655 (anti-pTDP-43). Results of immunofluorescence double-labeling corroborated the findings of double-labeling immunohistochemistry, by providing more evidence that the TDP-43 inclusions are found within astrocytes (Fig. 6A) and that the TDP-43 in star-like inclusions partially colocalizes with tau (Fig. 6B). These findings were not seen in the control case (Fig. 6C, D).
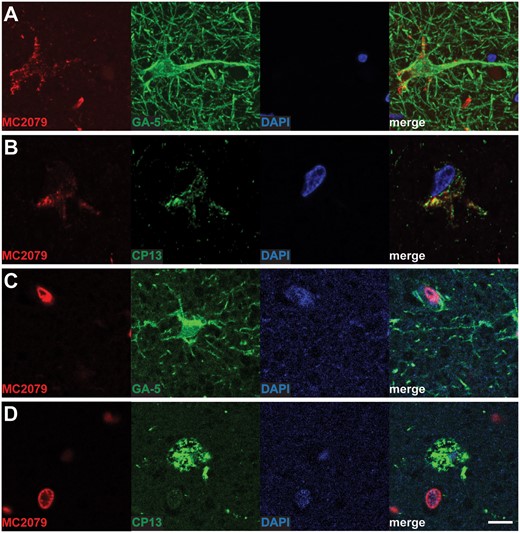
Representative images of double-labeling immunofluorescence findings. Double-labeling immunofluorescence (red: MC2079, green: GFAP) shows localization of TDP-43 within astrocytes (A). Similarly, double-staining immunofluorescence with MC2079 (red) and CP13 (green) showed colocalization of TDP-43 and tau within the atypical inclusions (B). No star-shaped TDP-43 pathologic inclusions localized in astrocytes (C) or colocalizing with tau (D) are seen with the negative control case. Scale bar = 10 m. Abbreviation: GFAP, glial fibrillary acidic protein.
DISCUSSION
In the present study, we identified a novel star-shaped morphology of TDP-43 pathologic inclusions detected within the amygdala of 7 nonagenarian and centenarian “super-agers” who had died of non-neurologic disorders and had relatively preserved, or only mildly impaired, cognitive functions. Those who had impaired cognition showed mild deterioration of episodic memory and, to a lesser extent, executive function, while all other cognitive domains remained essentially preserved given their very old age. Neuroimaging findings using volumetric MRI and FDG-PET showed relatively mild volume loss and hypometabolism predominantly involving the temporal lobes, respectively. The in vivo findings were later proven congruent with the results of postmortem brain examination, as the brunt of overall neurodegenerative pathology was observed in the temporal regions. The distribution of star-shaped TDP-43 inclusions appeared restricted to the amygdala, with the hippocampus only showing TDP-43-immunoreactive neurites in the superficial subiculum and fibers of the fimbria and the frontal regions lacking all types of TDP-43 inclusions. Furthermore, the star-shaped TDP-43 inclusions were best detected using antibodies against pTDP-43 and nTDP-43, while antibodies against cTDP-43 clearly underperformed. As we had hypothesized given their distinct morphology, we found evidence suggesting that the star-shaped TDP-43 inclusions were indeed deposited within astrocytes. Moreover, the star-shaped TDP-43 pathology was found in the same amygdala regions affected by ARTAG, and indeed further analyses showed evidence for colocalization of TDP-43 and tau. Taken together, these findings suggest that the star-shaped TDP-43 pathology could represent a true aging-related TDP-43 proteinopathy.
Based on morphology and double-labeling experiments, we found evidence of TDP-43 depositing in medial temporal lobe astrocytes among the oldest old participants. Astrocytes play a crucial role in maintaining an optimal brain environment by regulating extracellular homeostasis and concentration of neurotransmitters at synapses, controlling neurogenesis and synaptogenesis, producing trophic factors and neurotransmitter precursors, and maintaining the integrity of the blood-brain barrier (BBB) (59–61). TDP-43 is expressed in both neuronal and glial cells, however, there is typically a preferential accumulation of pathological TDP-43 in neuronal cells compared to glial cells (62, 63). Nonetheless, TDP-43 deposition in astrocytes has been reported in many non-primary TDP-43 proteinopathies, including Alexander disease (17), Cockayne syndrome (64), corticobasal degeneration (12, 65), multiple system atrophy (29), as well as in Rosenthal fibers and eosinophilic granular bodies found in pilocytic astrocytomas (66). In primary TDP-43 proteinopathies, such as FTLD-TDP and ALS, TDP-43 deposition in white matter was initially found only within oligodendrocytes and not astrocytes (16). Later in vitro and animal model studies provided increasing evidence of astrocytic contribution to the neurodegenerative process through a combination of different mechanisms, including decreased responses to oxidative stress (67), dysregulation of astroglial metabolism and metabolic support of neurons (68), and failure of astrocytes to induce a protective anti-inflammatory response to degenerating neurons (69). Another study using induced pluripotent stem cell-derived motor neurons and astrocytes found evidence of TDP-43 aggregates propagating from motor neurons into astrocytes, suggesting a neuroprotective role which allows unloading of TDP-43 pathologic aggregates from neurons and clearance by astrocytes (62). Furthermore, some authors have suggested that the accumulation of TDP-43 in astrocytes increases with age which consequently boosts astrocyte-mediated toxicity (63). In this study, the general lack of traditional neuronal TDP-43 inclusions would support the theory of pathological inclusions forming in astrocytes as a way to prevent their deposition in neurons and/or to increase protein clearance. This would suggest an underlying neuroprotective mechanism and would be in line with the observed resilient clinical presentation. However, it is also possible that the pathologic TDP-43 deposition within astrocytes could simply be an age-related phenomenon as other authors have suggested (63), with astrocytes being less able to clear accumulated protein over time as they age.
In primary TDP-43 proteinopathies like FTLD-TDP and ALS, pathologic TDP-43 undergoes phosphorylation, ubiquitination, and proteolytic cleavage of the N-terminal region to yield aberrant C-terminal segments which then aggregate to form pathologic inclusions (3, 4). It appears that phosphorylated C-terminal fragments are crucial to the pathogenesis. However, more recent studies have shown that while the C-terminal segment is more prone to phosphorylation, this process is not indispensable for inclusion formation and that toxic gain of functions of the C-terminal region are playing a key role in neurodegeneration (58). Other studies have also shown that even non-truncated or full-length TDP-43 is also involved in inclusion formation and could be the predominant TDP-43 species in certain FTLD-TDP subtypes and individual inclusion types, like pre-inclusions or perivascular inclusions (25). Similarly, in AD with TDP-43, variations in TDP-43 species content are associated with different clinical presentations, suggesting different disease mechanisms (24). In this study, we found that phosphorylated TDP-43 species, C-terminal species, and full-length TDP-43 species containing the N-terminal region are all present in the star-shaped inclusions. We found that the star-shaped TDP-43 inclusions were best detected using the pTDP-43 antibody, which binds to both phosphorylated C-terminal and phosphorylated full-length TDP-43 species (25). Intriguingly, we found that the nTDP-43 antibody which detects full-length species was also optimal in detecting the star-shaped inclusions and that the cTDP-43 antibody was the least reliable. Furthermore, aside from the different number of inclusions detected, there were also differences in terms of the morphology of inclusions observed: the pTDP-43 antibody detected more mature and varied star-shaped inclusions, the nTDP-43 antibody detected more granular inclusions, with shorter processes than mature star-shaped inclusions, and more thin superficial neurites, and finally, the cTDP-43 antibody only detected granular star-shaped inclusions. The use of 3 antibodies specific to different TDP-43 species makes it highly unlikely that we were detecting nonspecific binding. This is supported by the fact that we did not detect star-shaped inclusions in the age-matched case. The different antibodies used in our studies had also been validated and confirmed to be specific to their target TDP-43 species (58). Moreover, the cTDP-43 antibody also detected NCIs and short, thick comma-shaped neurites that were not detected with the other 2 antibodies. Our findings suggest that the star-shaped inclusions are predominantly composed of phosphorylated and unphosphorylated full-length TDP-43, which is different from NCIs and short, comma-shaped neurites that consist mainly of cleaved C-terminal fragments. This would suggest a different mechanism underlying the formation of these star-shaped inclusions. With the preponderance of full-length TDP-43, a mechanism whereby decreased protein degradation by astrocytic cells occurs is more likely. It is possible that with aging, an increasing number of normal TDP-43 proteins accumulate in astrocytes which also coincidentally have less ability to clear said proteins. The presence of minimally toxic C-terminal segments would also be in line with less neurodegeneration and cognitive resilience observed in life.
Despite our cases being nonagenarians or centenarians at death, they showed strikingly preserved cognition with none of the cases diagnosed with dementia prior to death. Although 3 cases did show mild cognitive impairment first occurring in their 90s, we cannot confirm or negate an effect of the star-shaped TDP-43 on cognition since other pathologies were found in the medial temporal lobe including ADNC (albeit mild) or other “age-related” tauopathies, including AGD and ARTAG, which could have contributed to the mild impairment of episodic memory and executive function observed. As is widely known, the typical presentation of AD is that of amnestic dementia with chief impairment in learning and recall of recently learned information (70). However, based on the degree of pathology detected at postmortem, there was an intermediate likelihood that the cognitive symptoms could be explained by the AD pathology solely in 1 case (case 4), and that AD pathology was not sufficient to explain the clinical symptoms in the remaining 2 cases (50). Although not the case in the present study, we did find impaired executive functions in association with severe limbic ADNC and a limbic predominant pattern of TDP-43 pathology in another study (13). Moreover, AGD is another pathology that is strongly associated with old age and has been linked to an amnestic type of cognitive impairment and personality or behavioral changes (53, 71, 72). While the above-mentioned neurodegenerative diseases are all probable contributors, it is also possible that other factors like multiple miscellaneous medical conditions and multimedications could have also contributed to the mild cognitive decline.
Another factor that would support the theory of age-related increase in TDP-43 accumulation in astrocytes is that the TDP-43 star-shaped inclusions were found in regions affected by ARTAG, and double-labeling studies have revealed partial colocalization of tau and TDP-43 within the same star-shaped inclusions. ARTAG is a primary tauopathy characterized by the pathologic deposition of 4-repeat tau within the perikarya and proximal processes of astrocytes in subpial, subependymal, perivascular, white, and gray matter regions of the brain, whose presence is strongly associated with increasing age (54, 73, 74). Although there is no consensus yet on the clinical correlate of ARTAG, some studies have shown associations with aphasia, parkinsonism, and dementia (54, 75, 76). High prevalence of thorn-shaped astrocytes has been described in the amygdala, yet some studies focusing on ARTAG in the medial temporal lobe have not found a correlation with dementia (74, 77). Additionally, ARTAG also shares overlapping features with chronic traumatic encephalopathy (CTE) (54), and it is interesting to note that several of our cases suffered from recurrent falls in life, with some leading to significant injuries. However, based on the review of medical records, none of our cases had any diagnosis of TBI, nor presented with any clinical features (eg, altered mental status, cognitive decline, or neuropsychiatric symptoms) or imaging features (eg, subdural hematoma, epidural hematoma, subarachnoid hemorrhage, contusion, or diffuse axonal injury) following recorded falls, possibly suggestive of TBI. While TDP-43 has been found to colocalize with tau in neurofibrillary tangles (6, 27) and neuritic plaques (78) in AD, as well as in astrocytic plaques in CBD (12, 65), colocalization with tau in thorn-shaped astrocytes or granular fuzzy astrocytes of ARTAG has not yet been reported. It remains unclear whether TDP-43 is the cause or the consequence of tau deposition in these cases.
In this study, we found that the star-shaped TDP-43 inclusions were restricted to the amygdala and are often accompanied by thread-like neurites in superficial subpial or subependymal regions. The presence of said subpial or subependymal neurites has been described before in the context of normal cognition (5, 79), and some authors have theorized their localization within astrocytic processes (79). In cases with a primary neuropathological diagnosis of multiple system atrophy, it was also suggested that subpial astrocytic inclusions could be the earliest evidence of TDP-43 pathology (29). Of note, the inclusions we present in this study are different from those published by previous authors, particularly in regard to the star-shaped appearance and overall clinicopathologic features. Moreover, in our study, the neurites found are best detected using nTDP-43 antibody, less with pTDP-43 antibody, and least with cTDP-43 antibody. They are also the only type of TDP-43-immunoreactive inclusions observed in the hippocampus. As discussed above, pre-inclusions in FTLD-TDP type B are predominantly made of nTDP-43 (25). Taken together, it is then reasonable to hypothesize that these neurites could be a precursor of the star-shaped inclusions. It is also possible that, over time, the hippocampus will likely develop similar star-shaped inclusions. Another interesting finding is the location or distribution of the star-shaped TDP-43 lesions. It appears that the pathology starts in the medial subpial regions of the amygdala, particularly the cortical nucleus. Involvement of the basolateral white matter and subependymal regions is likely a later phenomenon. Similar medial regions of hippocampal sections are affected by neurites. We are unable to determine whether other regions outside the medial temporal lobe would be involved if natural progression was allowed. Furthermore, it is curious that the cortical region of the amygdala and the fimbria and subiculum of the hippocampus are all regions adjacent to blood vessels. Given that astrocytes are involved in maintaining the integrity of the BBB by wrapping their foot processes around blood vessels (80), astrocytic TDP-43 pathology could be associated with damage to the BBB. Indeed, bilobed or multi-lobed perivascular TDP-43-immunoreactive inclusions have been previously described and, although the authors did not perform double-labeling studies to confirm the astrocytic localization, they believed the perivascular inclusions were formed by astrocytic foot processes filled with TDP-43 aggregates (81). In this study, we found evidence of star-shaped TDP-43 pathology within astrocytes in perivascular regions. It is also worth mentioning that like the bilobed/multi-lobed perivascular inclusions, our star-shaped perivascular TDP-43 inclusions were also better detected with pTDP-43 and nTDP-43 antibodies than with the cTDP-43 antibody. Yet again, one would not be completely wrong to hypothesize that the bilobed/multi-lobed perivascular TDP-43 inclusions could eventually develop into full-blown star-shaped inclusions. Nonetheless, there is evidence to suggest the involvement of the blood-brain barrier in the pathogenesis of TDP-43 proteinopathies, and future studies should investigate associations between vasculopathies and TDP-43 pathology. Of note, our cases all had severe cardiovascular disease and hypertension. Neuroimaging studies also showed leukoaraiosis in all 5 cases who had an MRI.
A strength of this study is that all cases were enrolled in National Institutes of Health (NIH) funded studies and were followed longitudinally over time to death. In addition, they also had their clinical care performed at our institution. This allowed us to gather detailed information on their medical and neurological histories. Another strength is the use of our in-house anti-nTDP-43 and anti-cTDP-43 antibodies targeting various TDP-43 epitopes that lowered the likelihood of any possible off-target binding and provided a clue to possible disease mechanisms that appear to be different from those found in FTLD-TDP or ALS. A limitation of the study is the relatively small number of cases completing advanced neuroimaging, especially FDG-PET scans. Future studies with larger sample sizes are needed to validate and advance our findings.
In conclusion, we found evidence of TDP-43 pathology depositing within astrocytes of the medial temporal lobe (chiefly in the amygdala) of “superagers” who had died at a very old age (close to 100 years) and had shown relatively preserved cognitive functions. Other common pathologies found at postmortem included ARTAG and AGD which are both associated with old age. There is evidence to suggest that the mechanism underlying the formation of star-shaped TDP-43 inclusions is different from those found in primary TDP-43 proteinopathies and that old age and vascular factors could be the major drivers of pathology. Given the relatively benign clinical presentation associated with the star-shaped TDP-43 pathology, we believe they could represent a true, aging-related TDP-43 proteinopathy.
FUNDING
This study was supported by National Institutes of Health (NIH)/National Institute on Aging grants R01 AG037491 (PI: K.A.J.), R01-NS120992 (PI: K.A.J.), P30 AG062677 (PI: R.C.P.), and U01 AG006786 (PI: R.C.P.). The NIH had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
ACKNOWLEDGMENTS
We would like to thank the patients and their families who donated their brains to help further our research on neurodegenerative diseases. We are also grateful to Ms. Nirubol Tosakulwong for neuropsychological data support and Kris Johnson, RN for histologic support.
CONFLICT OF INTEREST
The authors have no duality or conflicts of interest to declare.
DISCLOSURES
Drs. Josephs, Dickson, Jack, Lowe, Machulda, Petersen, and Whitwell receive research support from the US National Institutes of Health (NIH). Dr Josephs serves as an Associate Editor for Annals of Clinical and Translational Neurology. Drs. Josephs and Dickson serve on the editorial board of Acta Neuropathologica. Dr. Petersen reported consulting for Roche, Merck, Genentech, Biogen, and Eli Lilly and Company and receives research support from the NIH. Dr. Jack serves on an independent data monitoring board for Roche, has served as a speaker for Eisai, and consulted for Biogen, but he receives no personal compensation from any commercial entity. He receives research support from NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Lowe reported consulting for Bayer Schering Pharma, Piramal Life Sciences, Life Molecular Imaging, Eisai Inc., AVID Radiopharmaceuticals, and Merck Research and receiving research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, and the NIH (NIA, NCI).



