-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshinori Handa, Yasuhiro Tsutani, Takahiro Mimae, Yoshihiro Miyata, Hiroyuki Ito, Yoshihisa Shimada, Haruhiko Nakayama, Norihiko Ikeda, Morihito Okada, A multicenter propensity score-matched analysis of lymphadenectomy in N1-positve lung cancer, Japanese Journal of Clinical Oncology, Volume 53, Issue 12, December 2023, Pages 1183–1190, https://doi.org/10.1093/jjco/hyad110
Close - Share Icon Share
Abstract
Selective mediastinal lymph node dissection based on lobe-specific metastases is widely recognized in daily practice. However, the significance of mediastinal lymph node dissection for N1-positive tumors has not been elucidated.
We retrospectively reviewed 359 patients with N1-positive lung cancer who underwent lobectomy with systematic mediastinal lymph node dissection (systematic lymph node dissection) (n = 150) and lobe-specific mediastinal lymph node dissection (lobe-specific lymph node dissection) (n = 209). The operative and postoperative results and their propensity score-matched pairs were compared. The factors affecting survival were assessed using competing risk and multivariable analyses.
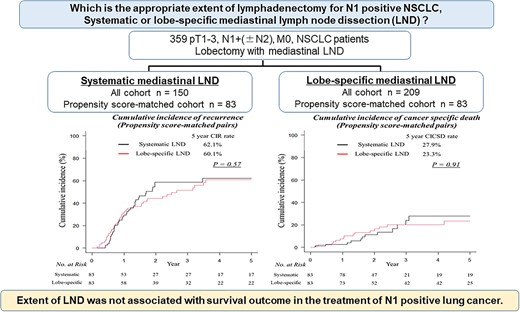
The cumulative incidence of recurrence and the cumulative incidence of cancer-specific death were not significantly different between systematic and lobe-specific lymph node dissection in entire cohort. In the propensity score-matched cohort (83 pairs), systematic lymph node dissection tended to detect N2 lymph node metastasis more frequently (55.4 vs. 41%, P = 0.087). Eleven patients (13.2%) in the systematic lymph node dissection group had a metastatic N2 lymph node ‘in the systematic lymph node dissection field’ that lobe-specific lymph node dissection did not dissect. The oncological outcomes between patients undergoing systematic lymph node dissection (5-year cumulative incidence of recurrence, 62.1%; 5-year cumulative incidence of cancer-specific death, 27.9%) and lobe-specific lymph node dissection (5-year cumulative incidence of recurrence, 60.1%; 5-year cumulative incidence of cancer-specific death, 23.3%) were similar. The propensity score-adjusted multivariable analysis for cumulative incidence of recurrence revealed that the prognosis associated with systematic lymph node dissection was comparable with the prognosis with lobe-specific lymph node dissection (hazard ratio, 1.17; 95% confidence interval, 0.82–1.67; P = 0.37).
The extent of lymph node dissection can affect accurate pathological staging; however, it was not associated with survival outcome in the treatment of N1-positive lung cancer.
Introduction
The standard surgical treatment for non-small cell lung cancer (NSCLC) has long been lobectomy with complete systematic mediastinal and hilar lymphadenectomy, known as radical systematic lymph node dissection (LND) (1,2). However, the significance of LND is controversial. There have been two contrasting opinions concerning its significance: on one hand, it has been advocated that LND is important for survival and staging (3); on the other hand, lymphadenectomy has been considered useful only for staging with no influence on prognosis (4). The appropriate extent of mediastinal LND remains also unfixed. Recently, the concept of selective mediastinal LND, namely, lobe-specific mediastinal LND, has been established. Several studies suggest that lobe-specific mediastinal LND holds comparable oncologic outcomes with systematic LND (5–7) and insist that it is inevitable that operative time, blood loss and the frequency of recurrent laryngeal nerve injury, chylothorax and bronchopleural fistula will increase because of the extent of LND. Surgeons should omit systematic LND if not needed. Taken together, lobe-specific mediastinal LND has been widely recognized in daily practice.
In particular, the significance and appropriate extent of mediastinal LND in patients with more aggressive tumors that might cause nodal metastasis has not been established. Naturally, a larger number and more widespread lymph node metastases are expected in the mediastinal field in N1-positive NSCLC than N0 NSCLC. In N1 NSCLC, more extensive mediastinal LND may provide more favorable outcomes than selective LND because of elevated tumor malignancy and a tendency to spread more extensively. Surgeons do not have a clear idea of how much the extent of lymphadenectomy affects oncological outcomes in N1 NSCLC treatment.
This study compared oncological outcomes between lobectomy with systematic mediastinal LND and lobectomy with lobe-specific mediastinal LND in patients with N1-positive NSCLC identified in a multicenter database and adjusted for preoperative factors. We aim to evaluate the appropriate extent of LND in lung cancer treatment.
Patients and methods
Study population
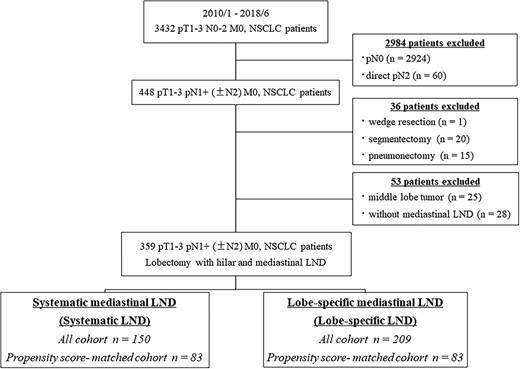
Overall, 3432 patients with surgical resection for pathological T1-3 N0-2 M0 NSCLC between January 2010 and June 2018 were identified at Hiroshima University Hospital, Kanagawa Cancer Center and Tokyo Medical University (Fig. 1). Of these, 448 N1 patients in which a surgical resection other than lobectomy was performed (n = 36), with a middle lobe tumor (n = 25) and without mediastinal LND (n = 28), were excluded. In total, 359 patients with T1-3 N1+ (±N2) M0 NSCLC were evaluated. All patients were preoperatively assessed using high-resolution computed tomography (HRCT) and 18F-fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT). Tumors were staged according to the TNM Classification of Malignant Tumors, eighth edition (8). In the preoperative evaluation, lymph node metastasis was defined as positive when swollen mediastinal or hilar lymph nodes measuring >1 cm on the short axis were evident on HRCT and FDG, accumulating a maximum standard uptake value (SUVmax) of >1.5 in these lymph nodes based on FDG-PET. Endobronchial ultrasonography or mediastinoscopy was not routinely performed.
The institutional review boards of the participating institutions approved this retrospective review of a prospective database and waived the requirement for informed consent from individual patients. (IRB number; E1216, Approve date; 13 June 2018).
Pathologic examination
Pathologic invasive size was defined as the maximum dimension of the invasive tumor component, excluding the previously described lepidic growth component (9). Lymphatic and vascular invasion were assessed using D2-40 immunohistochemistry, which stains the lymphatic ducts, and Verhoeff–van Gieson staining, which stains the elastic fiber of the vessels. Elastic tissue fibers were subjected to Verhoeff–van Gieson staining to evaluate pleural invasion. Pleural invasion was positive if cancer had invaded the elastic layer, including the invasion of the visceral pleura or neighboring organs. Lymphatic and vascular invasion were positive when spreading or penetration was detected as an extension of a malignant neoplasm. Pathologists from each institution performed histologic examinations.
Extent of LND
Hilar (N1) and mediastinal (N2) LND were performed in selected patients. These patients were divided into two groups based on the extent of mediastinal nodal evaluation. We categorized N2 lymph nodes into two regional groups: superior mediastinal (stations 2R and 4R on the right side and stations 4L, 5 and 6 on the left side) and inferior mediastinal (stations 7, 8 and 9). The patients with an evaluation of only the mediastinal region closest to the lobe containing the malignancy (superior mediastinum for upper lobe cancers and inferior mediastinum for lower lobe cancers) were placed in the lobe-specific LND group. The patients who underwent lymph node evaluation of the superior and inferior mediastinal stations were placed in the systematic LND group. We dissected 4L, 5 and 6 lymph nodes as ‘lobe-specific’ LND in left upper NSCLC. (We did not make difference between the upper segment tumor and the lingular segment tumor.)
We defined the N2 lymph node that lobe-specific LND could dissect as the N2 in the ‘lobe-specific LND field.’ Conversely, we defined the N2 lymph node that could not be dissected by lobe-specific LND but could be dissected by systematic LND as the N2 in ‘the systematic LND field.’ To provide more context, in right upper lobectomy, stations 2R and 4R are in the ‘lobe-specific LND field’ that lobe-specific LND (and, of course, systematic LND) could dissect. Meanwhile, stations 7, 8 and 9 are in the ‘systematic LND field,’ which only systematic LND could dissect.
Follow-up evaluation
All patients were followed from the day of surgery and followed up with a physical examination and chest radiography every three months and chest and abdominal CT every 6 months for the first 2 years from the day after lung resection. Then, a physical examination and chest radiography were repeated every 6 months, and chest CT proceeded annually. Tumor recurrence was classified into three subgroups: (1) locoregional (recurrent tumor within the same side lobes and ipsilateral hilum or mediastinal lymph node metastasis), (2) distant and (3) locoregional and distant.
Statistical analysis
The summarized data are presented as mean or median and interquartile range. The differences in the various variables between the systematic and lobe-specific LND groups were evaluated using Fisher’s exact test for categoric variables and the Mann–Whitney U test for continuous variables. Time-to-event end points were analyzed using competing risk analysis. The risk of recurrence [defined as the cumulative incidence of recurrence (CIR)] and cancer-specific death [defined as the cumulative incidence of cancer-specific death (CICSD)] were estimated using a cumulative incidence function, which accounted for death without recurrence as a competing event. In CIR, patients were censored if they were alive and without recurrence at the time of the last follow-up. In CICSD, patients were censored if they were alive or dead caused by something other than cancer at the last follow-up. The differences in CIR and CICSD between groups were assessed using the methods of Gray (in univariate nonparametric analyses) and Fine and Gray (in analyses adjusted for the propensity score). We performed our survival analysis mainly using CIR and CICSD to focus more clearly on the oncologic outcomes of both groups.
Using an optimized performance matching algorithm, we conducted a propensity score matching analysis to compare the oncological outcomes of systematic and lobe-specific LND (1:1 matched for each paired group). The propensity score distribution was also compared using a t-test for continuous variables and a χ2 test for categorical variables. In each analysis, we generated a propensity score for matched groups using logistic regression based on the patients’ potential confounding baseline characteristics, including age, sex, whole tumor size, solid component size, SUVmax, clinical T, clinical N, histology, pathological whole tumor size and pathological invasive size. Propensity score-adjusted multivariable analyses for CIR and CICSD were used to identify the prognostic impact of the extent of LND in N1-positive NSCLC. P values < 0.05 were considered significant. The propensity score was used as a covariate. All statistical analyses were performed using EZR version 1.51 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
All cohort analysis
Table 1 summarizes the characteristics of the 359 patients. Of the 359 patients, 150 underwent lobectomy with systematic mediastinal LND and 209 underwent lobectomy with lobe-specific mediastinal LND. Systematic LND was performed more often in patients with higher SUVmax tumors of clinical factors and tumors with lymphatic invasion. These results were believed to reflect the pattern that systematic LND was performed more often for patients with radiologically and pathologically more invasive tumors.
Patient and tumor characteristics of the systematic and lobe-specific LND groups in all cohort
| Variables . | Systematic LND n = 150 . | Lobe-specific LND n = 209 . | P value . | |
|---|---|---|---|---|
| Clinical variables | Age, years | 66 (60–72) | 70 (64–76) | <0.0001 |
| Sex, male/female | 111 (74.0%)/39 (26.0%) | 146 (69.9%)/63 (30.1%) | 0.41 | |
| Whole tumor size, cm | 3.1 (2.4–4.3) | 3.1 (2.3–4.0) | 0.45 | |
| Solid component size, cm | 3.0 (2.3–4.1) | 2.8 (2.2–3.8) | 0.17 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.24 | |
| SUVmax | 10.3 (6.2–15.4) | 8.8 (4.7–13.9) | 0.042 | |
| cT, T1/T2/T3/T4 | 67(44.7%)/54(36.0%)/21(14.0%)/8(5.3%) | 106(50.7%)/71(34.0%)/22(10.5%)/10(4.8%) | 0.64 | |
| cN, N0/N1/N2 | 64 (42.7%)/57 (38.0%)/29 (19.3%) | 124 (59.3%)/65 (31.1%)/20(9.6%) | 0.0025 | |
| Side, right/left | 91 (60.7%)/59 (39.3%) | 107 (51.2%)/102 (48.8%) | 0.085 | |
| Lobe, upper/lower | 86 (57.3%)/64 (42.7%) | 114 (54.5%)/95 (45.5%) | 0.67 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 100 (66.7%) 31 (20.7%) 19 (12.6%) | 127 (60.8%) 49 (23.4%) 33 (15.8%) | 0.50 |
| Pathologic tumor size, cm | 3.0 (2.5–4.2) | 3.0 (2.3–4.0) | 0.34 | |
| Pathologic invasive size, cm | 3.0 (2.1–4.0) | 2.6 (1.9–3.5) | 0.11 | |
| Lymphatic invasion | 107 (71.3%) | 118 (56.5%) | 0.0041 | |
| Vascular invasion | 121 (80.7%) | 158 (75.6%) | 0.30 | |
| Pleural invasion | 71 (47.3%) | 85 (40.7%) | 0.24 | |
| Number of dissected LNs | 21 (16–29) | 17 (13–25) | <0.0001 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | <0.0001 | |
| Pathologic N status N1 N2 | 76 (50.7%) 74 (49.3%) | 139 (66.5%) 70 (33.5%) | 0.0032 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c67 (44.7%) c25 (16.7%) | 70 (33.5%) 0 (0%) | 0.037 <0.0001 | |
| Adjuvant chemotherapy | 76 (50.7%) | 101 (48.3%) | 0.67 | |
| Recurrence | 77 (51.3%) | 96 (45.9%) | 0.34 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 16 (20.8%) 37 (48.1%) 24 (31.1%) | 35 (36.5%) 38 (39.6%) 23 (23.9%) | 0.079 | |
| Lymph node relapse | 24 (16.0%) | 41 (19.6%) | 0.41 | |
| Variables . | Systematic LND n = 150 . | Lobe-specific LND n = 209 . | P value . | |
|---|---|---|---|---|
| Clinical variables | Age, years | 66 (60–72) | 70 (64–76) | <0.0001 |
| Sex, male/female | 111 (74.0%)/39 (26.0%) | 146 (69.9%)/63 (30.1%) | 0.41 | |
| Whole tumor size, cm | 3.1 (2.4–4.3) | 3.1 (2.3–4.0) | 0.45 | |
| Solid component size, cm | 3.0 (2.3–4.1) | 2.8 (2.2–3.8) | 0.17 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.24 | |
| SUVmax | 10.3 (6.2–15.4) | 8.8 (4.7–13.9) | 0.042 | |
| cT, T1/T2/T3/T4 | 67(44.7%)/54(36.0%)/21(14.0%)/8(5.3%) | 106(50.7%)/71(34.0%)/22(10.5%)/10(4.8%) | 0.64 | |
| cN, N0/N1/N2 | 64 (42.7%)/57 (38.0%)/29 (19.3%) | 124 (59.3%)/65 (31.1%)/20(9.6%) | 0.0025 | |
| Side, right/left | 91 (60.7%)/59 (39.3%) | 107 (51.2%)/102 (48.8%) | 0.085 | |
| Lobe, upper/lower | 86 (57.3%)/64 (42.7%) | 114 (54.5%)/95 (45.5%) | 0.67 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 100 (66.7%) 31 (20.7%) 19 (12.6%) | 127 (60.8%) 49 (23.4%) 33 (15.8%) | 0.50 |
| Pathologic tumor size, cm | 3.0 (2.5–4.2) | 3.0 (2.3–4.0) | 0.34 | |
| Pathologic invasive size, cm | 3.0 (2.1–4.0) | 2.6 (1.9–3.5) | 0.11 | |
| Lymphatic invasion | 107 (71.3%) | 118 (56.5%) | 0.0041 | |
| Vascular invasion | 121 (80.7%) | 158 (75.6%) | 0.30 | |
| Pleural invasion | 71 (47.3%) | 85 (40.7%) | 0.24 | |
| Number of dissected LNs | 21 (16–29) | 17 (13–25) | <0.0001 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | <0.0001 | |
| Pathologic N status N1 N2 | 76 (50.7%) 74 (49.3%) | 139 (66.5%) 70 (33.5%) | 0.0032 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c67 (44.7%) c25 (16.7%) | 70 (33.5%) 0 (0%) | 0.037 <0.0001 | |
| Adjuvant chemotherapy | 76 (50.7%) | 101 (48.3%) | 0.67 | |
| Recurrence | 77 (51.3%) | 96 (45.9%) | 0.34 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 16 (20.8%) 37 (48.1%) 24 (31.1%) | 35 (36.5%) 38 (39.6%) 23 (23.9%) | 0.079 | |
| Lymph node relapse | 24 (16.0%) | 41 (19.6%) | 0.41 | |
Values are median (interquartile range) or n (%). LND, lymph node dissection; C/T ratio, consolidation tumor ratio; SUV, standard uptake value; LNs, lymph nodes.
aN2 in lobe-specific LND field is defined as N2 lymph node that could be dissected by lobe-specific LND
bN2 in systematic LND field is defined as N2 lymph node that could not be dissected by lobe-specific LND but could be dissected by systematic LND
c18 patients had both N2 lymph node in lobe-specific LND and systematic LND fields
Patient and tumor characteristics of the systematic and lobe-specific LND groups in all cohort
| Variables . | Systematic LND n = 150 . | Lobe-specific LND n = 209 . | P value . | |
|---|---|---|---|---|
| Clinical variables | Age, years | 66 (60–72) | 70 (64–76) | <0.0001 |
| Sex, male/female | 111 (74.0%)/39 (26.0%) | 146 (69.9%)/63 (30.1%) | 0.41 | |
| Whole tumor size, cm | 3.1 (2.4–4.3) | 3.1 (2.3–4.0) | 0.45 | |
| Solid component size, cm | 3.0 (2.3–4.1) | 2.8 (2.2–3.8) | 0.17 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.24 | |
| SUVmax | 10.3 (6.2–15.4) | 8.8 (4.7–13.9) | 0.042 | |
| cT, T1/T2/T3/T4 | 67(44.7%)/54(36.0%)/21(14.0%)/8(5.3%) | 106(50.7%)/71(34.0%)/22(10.5%)/10(4.8%) | 0.64 | |
| cN, N0/N1/N2 | 64 (42.7%)/57 (38.0%)/29 (19.3%) | 124 (59.3%)/65 (31.1%)/20(9.6%) | 0.0025 | |
| Side, right/left | 91 (60.7%)/59 (39.3%) | 107 (51.2%)/102 (48.8%) | 0.085 | |
| Lobe, upper/lower | 86 (57.3%)/64 (42.7%) | 114 (54.5%)/95 (45.5%) | 0.67 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 100 (66.7%) 31 (20.7%) 19 (12.6%) | 127 (60.8%) 49 (23.4%) 33 (15.8%) | 0.50 |
| Pathologic tumor size, cm | 3.0 (2.5–4.2) | 3.0 (2.3–4.0) | 0.34 | |
| Pathologic invasive size, cm | 3.0 (2.1–4.0) | 2.6 (1.9–3.5) | 0.11 | |
| Lymphatic invasion | 107 (71.3%) | 118 (56.5%) | 0.0041 | |
| Vascular invasion | 121 (80.7%) | 158 (75.6%) | 0.30 | |
| Pleural invasion | 71 (47.3%) | 85 (40.7%) | 0.24 | |
| Number of dissected LNs | 21 (16–29) | 17 (13–25) | <0.0001 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | <0.0001 | |
| Pathologic N status N1 N2 | 76 (50.7%) 74 (49.3%) | 139 (66.5%) 70 (33.5%) | 0.0032 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c67 (44.7%) c25 (16.7%) | 70 (33.5%) 0 (0%) | 0.037 <0.0001 | |
| Adjuvant chemotherapy | 76 (50.7%) | 101 (48.3%) | 0.67 | |
| Recurrence | 77 (51.3%) | 96 (45.9%) | 0.34 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 16 (20.8%) 37 (48.1%) 24 (31.1%) | 35 (36.5%) 38 (39.6%) 23 (23.9%) | 0.079 | |
| Lymph node relapse | 24 (16.0%) | 41 (19.6%) | 0.41 | |
| Variables . | Systematic LND n = 150 . | Lobe-specific LND n = 209 . | P value . | |
|---|---|---|---|---|
| Clinical variables | Age, years | 66 (60–72) | 70 (64–76) | <0.0001 |
| Sex, male/female | 111 (74.0%)/39 (26.0%) | 146 (69.9%)/63 (30.1%) | 0.41 | |
| Whole tumor size, cm | 3.1 (2.4–4.3) | 3.1 (2.3–4.0) | 0.45 | |
| Solid component size, cm | 3.0 (2.3–4.1) | 2.8 (2.2–3.8) | 0.17 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.24 | |
| SUVmax | 10.3 (6.2–15.4) | 8.8 (4.7–13.9) | 0.042 | |
| cT, T1/T2/T3/T4 | 67(44.7%)/54(36.0%)/21(14.0%)/8(5.3%) | 106(50.7%)/71(34.0%)/22(10.5%)/10(4.8%) | 0.64 | |
| cN, N0/N1/N2 | 64 (42.7%)/57 (38.0%)/29 (19.3%) | 124 (59.3%)/65 (31.1%)/20(9.6%) | 0.0025 | |
| Side, right/left | 91 (60.7%)/59 (39.3%) | 107 (51.2%)/102 (48.8%) | 0.085 | |
| Lobe, upper/lower | 86 (57.3%)/64 (42.7%) | 114 (54.5%)/95 (45.5%) | 0.67 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 100 (66.7%) 31 (20.7%) 19 (12.6%) | 127 (60.8%) 49 (23.4%) 33 (15.8%) | 0.50 |
| Pathologic tumor size, cm | 3.0 (2.5–4.2) | 3.0 (2.3–4.0) | 0.34 | |
| Pathologic invasive size, cm | 3.0 (2.1–4.0) | 2.6 (1.9–3.5) | 0.11 | |
| Lymphatic invasion | 107 (71.3%) | 118 (56.5%) | 0.0041 | |
| Vascular invasion | 121 (80.7%) | 158 (75.6%) | 0.30 | |
| Pleural invasion | 71 (47.3%) | 85 (40.7%) | 0.24 | |
| Number of dissected LNs | 21 (16–29) | 17 (13–25) | <0.0001 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | <0.0001 | |
| Pathologic N status N1 N2 | 76 (50.7%) 74 (49.3%) | 139 (66.5%) 70 (33.5%) | 0.0032 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c67 (44.7%) c25 (16.7%) | 70 (33.5%) 0 (0%) | 0.037 <0.0001 | |
| Adjuvant chemotherapy | 76 (50.7%) | 101 (48.3%) | 0.67 | |
| Recurrence | 77 (51.3%) | 96 (45.9%) | 0.34 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 16 (20.8%) 37 (48.1%) 24 (31.1%) | 35 (36.5%) 38 (39.6%) 23 (23.9%) | 0.079 | |
| Lymph node relapse | 24 (16.0%) | 41 (19.6%) | 0.41 | |
Values are median (interquartile range) or n (%). LND, lymph node dissection; C/T ratio, consolidation tumor ratio; SUV, standard uptake value; LNs, lymph nodes.
aN2 in lobe-specific LND field is defined as N2 lymph node that could be dissected by lobe-specific LND
bN2 in systematic LND field is defined as N2 lymph node that could not be dissected by lobe-specific LND but could be dissected by systematic LND
c18 patients had both N2 lymph node in lobe-specific LND and systematic LND fields
The median follow-up period was 37.9 months for all enrolled patients. The CIR of patients undergoing lobectomy with systematic mediastinal LND tended to be worse than those with lobe-specific mediastinal LND (62.4 vs. 56.6%; P = 0.054) (Fig. 2A), reflecting a clinicopathological difference between the two groups. There was no significant difference in CICSD between patients who underwent lobectomy with systematic mediastinal LND and lobectomy with lobe-specific mediastinal LND (5-year CICSD rates, 27.9 vs. 24.6%; P = 0.85) (Fig. 2B).
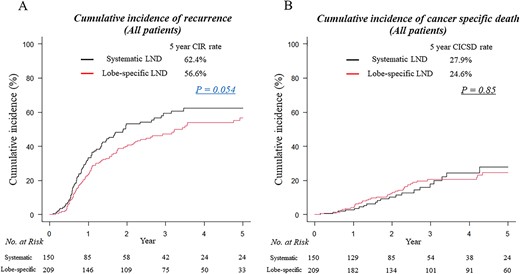
Cumulative incidence of recurrence (CIR) (A) and cumulative incidence of cancer-specific death (CICSD) curves (B) for patients who underwent lobectomy with systematic lymph node dissection (LND) (black lines) and lobectomy with lobe-specific mediastinal LND (red lines). No., number.
Propensity score-matched analysis
When propensity score matching was used, the systematic LND group and lobe-specific LND group were well matched (83 patients each) with no significant differences in clinicopathological factors (Table 2).
Patient and tumor characteristics of systematic and lobe-specific LND groups in propensity score-matched cohort
| Variables . | Systematic LND n = 83 . | Lobe-specific LND n = 83 . | P value . | SD . | |
|---|---|---|---|---|---|
| Clinical variables | Age, years | 67 (62–73) | 67 (60–73) | 0.76 | 0.073 |
| Sex, male/female | 60 (72.3%)/23 (27.7%) | 60 (72.3%)/23 (27.7%) | 1.00 | 0 | |
| Whole tumor size, cm | 3.0 (2.4–4.0) | 3.0 (2.3–3.8) | 0.73 | 0.037 | |
| Solid component size, cm | 2.8 (2.2–4.0) | 2.8 (2.1–3.8) | 0.79 | 0.0073 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.40 | 0.065 | |
| SUVmax | 8.4 (5.9–13.5) | 8.9 (4.9–13.8) | 0.86 | 0.016 | |
| cT, T1/T2/T3 | 40 (48.2%)/29 (35.0%)/14 (16.8%) | 38 (45.8%)/31 (37.4%)/14 (16.8%) | 0.94 | 0.057 | |
| cN, N0/N1/N2 | 41 (49.4%)/31 (37.4%)/11 (13.2%) | 47 (56.6%)/25 (30.1%)/11 (13.3%) | 0.59 | 0.17 | |
| Side, right/left | 52 (62.7%)/31 (37.3%) | 45 (54.2%)/38 (45.8%) | 0.35 | 0.18 | |
| Lobe, upper/lower | 48 (57.8%)/35 (42.2%) | 49 (59.0%)/34 (41.0%) | 1.00 | 0.027 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 58 (69.9%) 15 (18.1%) 10 (12.0%) | 60 (72.3%) 13 (15.7%) 10 (12.0%) | 0.92 | 0.070 |
| Pathologic tumor size, cm | 3.0 (2.5–4.0) | 3.0 (2.2–4.0) | 0.59 | 0.031 | |
| Pathologic invasive size, cm | 3.0 (2.0–4.0) | 2.7 (2.0–3.5) | 0.73 | 0.024 | |
| Lymphatic invasion | 63 (75.9%) | 57 (68.7%) | 0.39 | 0.17 | |
| Vascular invasion | 66 (79.5%) | 64 (77.1%) | 0.85 | 0.064 | |
| Pleural invasion | 39 (47.0%) | 43 (51.8%) | 0.64 | 0.10 | |
| Number of dissected LNs | 20 (16–26) | 17 (13–25) | 0.026 | 0.28 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | 0.014 | 0.33 | |
| Pathologic N status N1 N2 | 37 (44.6%) 46 (55.4%) | 49 (59.0%) 34 (41.0%) | 0.087 | 0.32 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c43 (51.8%) c11 (13.2%) | 34 (41.0%) 0 (0%) | 0.21 0.0007 | 0.21 0.55 | |
| Adjuvant chemotherapy | 44 (53.0%) | 48 (57.8%) | 0.64 | 0.10 | |
| Recurrence | 40 (48.2%) | 45 (54.2%) | 0.60 | 0.13 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 9 (22.5%) 20 (50.0%) 11 (27.5%) | 14 (31.1%) 18 (40.0%) 13 (28.9%) | 0.59 | 0.18 | |
| Lymph node relapse | 12 (14.5%) | 17 (20.5%) | 0.41 | 0.18 | |
| Variables . | Systematic LND n = 83 . | Lobe-specific LND n = 83 . | P value . | SD . | |
|---|---|---|---|---|---|
| Clinical variables | Age, years | 67 (62–73) | 67 (60–73) | 0.76 | 0.073 |
| Sex, male/female | 60 (72.3%)/23 (27.7%) | 60 (72.3%)/23 (27.7%) | 1.00 | 0 | |
| Whole tumor size, cm | 3.0 (2.4–4.0) | 3.0 (2.3–3.8) | 0.73 | 0.037 | |
| Solid component size, cm | 2.8 (2.2–4.0) | 2.8 (2.1–3.8) | 0.79 | 0.0073 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.40 | 0.065 | |
| SUVmax | 8.4 (5.9–13.5) | 8.9 (4.9–13.8) | 0.86 | 0.016 | |
| cT, T1/T2/T3 | 40 (48.2%)/29 (35.0%)/14 (16.8%) | 38 (45.8%)/31 (37.4%)/14 (16.8%) | 0.94 | 0.057 | |
| cN, N0/N1/N2 | 41 (49.4%)/31 (37.4%)/11 (13.2%) | 47 (56.6%)/25 (30.1%)/11 (13.3%) | 0.59 | 0.17 | |
| Side, right/left | 52 (62.7%)/31 (37.3%) | 45 (54.2%)/38 (45.8%) | 0.35 | 0.18 | |
| Lobe, upper/lower | 48 (57.8%)/35 (42.2%) | 49 (59.0%)/34 (41.0%) | 1.00 | 0.027 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 58 (69.9%) 15 (18.1%) 10 (12.0%) | 60 (72.3%) 13 (15.7%) 10 (12.0%) | 0.92 | 0.070 |
| Pathologic tumor size, cm | 3.0 (2.5–4.0) | 3.0 (2.2–4.0) | 0.59 | 0.031 | |
| Pathologic invasive size, cm | 3.0 (2.0–4.0) | 2.7 (2.0–3.5) | 0.73 | 0.024 | |
| Lymphatic invasion | 63 (75.9%) | 57 (68.7%) | 0.39 | 0.17 | |
| Vascular invasion | 66 (79.5%) | 64 (77.1%) | 0.85 | 0.064 | |
| Pleural invasion | 39 (47.0%) | 43 (51.8%) | 0.64 | 0.10 | |
| Number of dissected LNs | 20 (16–26) | 17 (13–25) | 0.026 | 0.28 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | 0.014 | 0.33 | |
| Pathologic N status N1 N2 | 37 (44.6%) 46 (55.4%) | 49 (59.0%) 34 (41.0%) | 0.087 | 0.32 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c43 (51.8%) c11 (13.2%) | 34 (41.0%) 0 (0%) | 0.21 0.0007 | 0.21 0.55 | |
| Adjuvant chemotherapy | 44 (53.0%) | 48 (57.8%) | 0.64 | 0.10 | |
| Recurrence | 40 (48.2%) | 45 (54.2%) | 0.60 | 0.13 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 9 (22.5%) 20 (50.0%) 11 (27.5%) | 14 (31.1%) 18 (40.0%) 13 (28.9%) | 0.59 | 0.18 | |
| Lymph node relapse | 12 (14.5%) | 17 (20.5%) | 0.41 | 0.18 | |
Values are median (interquartile range) or n (%). SD, standardized difference.
aN2 in lobe-specific LND field is defined as N2 lymph node that could be dissected by lobe-specific LND
bN2 in systematic LND field is defined as N2 lymph node that could not be dissected by lobe-specific LND but could be dissected by systematic LND
c8 patients had both N2 lymph node in lobe-specific LND and systematic LND fields
Patient and tumor characteristics of systematic and lobe-specific LND groups in propensity score-matched cohort
| Variables . | Systematic LND n = 83 . | Lobe-specific LND n = 83 . | P value . | SD . | |
|---|---|---|---|---|---|
| Clinical variables | Age, years | 67 (62–73) | 67 (60–73) | 0.76 | 0.073 |
| Sex, male/female | 60 (72.3%)/23 (27.7%) | 60 (72.3%)/23 (27.7%) | 1.00 | 0 | |
| Whole tumor size, cm | 3.0 (2.4–4.0) | 3.0 (2.3–3.8) | 0.73 | 0.037 | |
| Solid component size, cm | 2.8 (2.2–4.0) | 2.8 (2.1–3.8) | 0.79 | 0.0073 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.40 | 0.065 | |
| SUVmax | 8.4 (5.9–13.5) | 8.9 (4.9–13.8) | 0.86 | 0.016 | |
| cT, T1/T2/T3 | 40 (48.2%)/29 (35.0%)/14 (16.8%) | 38 (45.8%)/31 (37.4%)/14 (16.8%) | 0.94 | 0.057 | |
| cN, N0/N1/N2 | 41 (49.4%)/31 (37.4%)/11 (13.2%) | 47 (56.6%)/25 (30.1%)/11 (13.3%) | 0.59 | 0.17 | |
| Side, right/left | 52 (62.7%)/31 (37.3%) | 45 (54.2%)/38 (45.8%) | 0.35 | 0.18 | |
| Lobe, upper/lower | 48 (57.8%)/35 (42.2%) | 49 (59.0%)/34 (41.0%) | 1.00 | 0.027 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 58 (69.9%) 15 (18.1%) 10 (12.0%) | 60 (72.3%) 13 (15.7%) 10 (12.0%) | 0.92 | 0.070 |
| Pathologic tumor size, cm | 3.0 (2.5–4.0) | 3.0 (2.2–4.0) | 0.59 | 0.031 | |
| Pathologic invasive size, cm | 3.0 (2.0–4.0) | 2.7 (2.0–3.5) | 0.73 | 0.024 | |
| Lymphatic invasion | 63 (75.9%) | 57 (68.7%) | 0.39 | 0.17 | |
| Vascular invasion | 66 (79.5%) | 64 (77.1%) | 0.85 | 0.064 | |
| Pleural invasion | 39 (47.0%) | 43 (51.8%) | 0.64 | 0.10 | |
| Number of dissected LNs | 20 (16–26) | 17 (13–25) | 0.026 | 0.28 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | 0.014 | 0.33 | |
| Pathologic N status N1 N2 | 37 (44.6%) 46 (55.4%) | 49 (59.0%) 34 (41.0%) | 0.087 | 0.32 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c43 (51.8%) c11 (13.2%) | 34 (41.0%) 0 (0%) | 0.21 0.0007 | 0.21 0.55 | |
| Adjuvant chemotherapy | 44 (53.0%) | 48 (57.8%) | 0.64 | 0.10 | |
| Recurrence | 40 (48.2%) | 45 (54.2%) | 0.60 | 0.13 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 9 (22.5%) 20 (50.0%) 11 (27.5%) | 14 (31.1%) 18 (40.0%) 13 (28.9%) | 0.59 | 0.18 | |
| Lymph node relapse | 12 (14.5%) | 17 (20.5%) | 0.41 | 0.18 | |
| Variables . | Systematic LND n = 83 . | Lobe-specific LND n = 83 . | P value . | SD . | |
|---|---|---|---|---|---|
| Clinical variables | Age, years | 67 (62–73) | 67 (60–73) | 0.76 | 0.073 |
| Sex, male/female | 60 (72.3%)/23 (27.7%) | 60 (72.3%)/23 (27.7%) | 1.00 | 0 | |
| Whole tumor size, cm | 3.0 (2.4–4.0) | 3.0 (2.3–3.8) | 0.73 | 0.037 | |
| Solid component size, cm | 2.8 (2.2–4.0) | 2.8 (2.1–3.8) | 0.79 | 0.0073 | |
| C/T ratio | 1.0 (1.0–1.0) | 1.0 (1.0–1.0) | 0.40 | 0.065 | |
| SUVmax | 8.4 (5.9–13.5) | 8.9 (4.9–13.8) | 0.86 | 0.016 | |
| cT, T1/T2/T3 | 40 (48.2%)/29 (35.0%)/14 (16.8%) | 38 (45.8%)/31 (37.4%)/14 (16.8%) | 0.94 | 0.057 | |
| cN, N0/N1/N2 | 41 (49.4%)/31 (37.4%)/11 (13.2%) | 47 (56.6%)/25 (30.1%)/11 (13.3%) | 0.59 | 0.17 | |
| Side, right/left | 52 (62.7%)/31 (37.3%) | 45 (54.2%)/38 (45.8%) | 0.35 | 0.18 | |
| Lobe, upper/lower | 48 (57.8%)/35 (42.2%) | 49 (59.0%)/34 (41.0%) | 1.00 | 0.027 | |
| Pathological and Postoperative variables | Histology Adenocarcinoma Squamous cell carcinoma Others | 58 (69.9%) 15 (18.1%) 10 (12.0%) | 60 (72.3%) 13 (15.7%) 10 (12.0%) | 0.92 | 0.070 |
| Pathologic tumor size, cm | 3.0 (2.5–4.0) | 3.0 (2.2–4.0) | 0.59 | 0.031 | |
| Pathologic invasive size, cm | 3.0 (2.0–4.0) | 2.7 (2.0–3.5) | 0.73 | 0.024 | |
| Lymphatic invasion | 63 (75.9%) | 57 (68.7%) | 0.39 | 0.17 | |
| Vascular invasion | 66 (79.5%) | 64 (77.1%) | 0.85 | 0.064 | |
| Pleural invasion | 39 (47.0%) | 43 (51.8%) | 0.64 | 0.10 | |
| Number of dissected LNs | 20 (16–26) | 17 (13–25) | 0.026 | 0.28 | |
| Median number of dissected metastasis LNs | 3 (2–6) | 2 (1–4) | 0.014 | 0.33 | |
| Pathologic N status N1 N2 | 37 (44.6%) 46 (55.4%) | 49 (59.0%) 34 (41.0%) | 0.087 | 0.32 | |
| Detail of N2 status aN2 in lobe-specific LND field bN2 in systematic LND field | c43 (51.8%) c11 (13.2%) | 34 (41.0%) 0 (0%) | 0.21 0.0007 | 0.21 0.55 | |
| Adjuvant chemotherapy | 44 (53.0%) | 48 (57.8%) | 0.64 | 0.10 | |
| Recurrence | 40 (48.2%) | 45 (54.2%) | 0.60 | 0.13 | |
| Recurrence pattern Locoregional Distant Locoregional and Distant | 9 (22.5%) 20 (50.0%) 11 (27.5%) | 14 (31.1%) 18 (40.0%) 13 (28.9%) | 0.59 | 0.18 | |
| Lymph node relapse | 12 (14.5%) | 17 (20.5%) | 0.41 | 0.18 | |
Values are median (interquartile range) or n (%). SD, standardized difference.
aN2 in lobe-specific LND field is defined as N2 lymph node that could be dissected by lobe-specific LND
bN2 in systematic LND field is defined as N2 lymph node that could not be dissected by lobe-specific LND but could be dissected by systematic LND
c8 patients had both N2 lymph node in lobe-specific LND and systematic LND fields
Among the propensity score-matched patients, the median number of dissected lymph nodes was 20 (interquartile range, 16–26) in the systematic LND group and 17 (interquartile range, 13–25) in the lobe-specific LND group (P = 0.026). N2 lymph node metastasis occurred in 46 (55.4%) patients in the systematic LND group and 34 (41%) patients in the lobe-specific LND group. Among these, 11 patients (13.2%) in the systematic LND group had lymph node metastasis in the ‘systematic LND field’ that lobe-specific LND could not dissect.
Comparable CIRs were identified in patients who underwent systematic LND compared with those who underwent lobe-specific LND (5-year CIR rates, 62.1 vs. 60.1%, respectively; P = 0.57; Fig. 3A). In addition, comparable results in CICSD were observed between patients who underwent systematic LND compared with those who underwent lobe-specific LND (5-year CICSD rates, 27.9 vs. 23.3%, respectively; P = 0.91; Fig. 3B). The propensity score-adjusted multivariable analysis for CIR revealed that prognosis associated with systematic LND was not significantly different to those obtained using lobe-specific LND (hazard ratio, 1.17; 95% confidence interval, 0.82–1.67; P = 0.37; Table 3). Furthermore, the propensity score-adjusted multivariable analysis for CICSD revealed that prognosis associated with systematic LND was not significantly different to those obtained using lobe-specific LND (hazard ratio, 1.07; 95% confidence interval, 0.57–2.01; P = 0.83; Table 3).
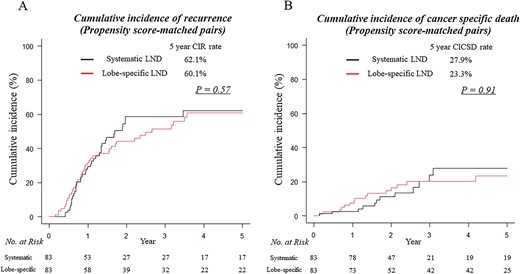
CIR (A) and CICSD curves (B) for patients who underwent lobectomy with systematic mediastinal LND (black lines) and lobectomy with lobe-specific mediastinal LND (red lines) in propensity score-matched group.
Propensity score-adjusted multivariable analysis for CIR and CICSD in patients with N1 positive NSCLC
| Variables . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| CIR | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.17 | 0.82–1.67 | 0.37 |
| CICSD | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.07 | 0.57–2.01 | 0.83 |
| Variables . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| CIR | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.17 | 0.82–1.67 | 0.37 |
| CICSD | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.07 | 0.57–2.01 | 0.83 |
Propensity scores for surgical procedure were calculated using preoperative variables that included age, sex, whole tumor size, solid component size, SUV max, clinical T, clinical N, Histology, pathological whole tumor size and pathological invasive size. CIR, cumulative incidence of recurrence; CICSD, cumulative incidence of cancer-specific death.
Propensity score-adjusted multivariable analysis for CIR and CICSD in patients with N1 positive NSCLC
| Variables . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| CIR | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.17 | 0.82–1.67 | 0.37 |
| CICSD | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.07 | 0.57–2.01 | 0.83 |
| Variables . | Hazard ratio . | 95% confidence interval . | P value . |
|---|---|---|---|
| CIR | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.17 | 0.82–1.67 | 0.37 |
| CICSD | |||
| Propensity score | – | – | – |
| Extent of LND: systematic LND (vs. lobe-specific LND) | 1.07 | 0.57–2.01 | 0.83 |
Propensity scores for surgical procedure were calculated using preoperative variables that included age, sex, whole tumor size, solid component size, SUV max, clinical T, clinical N, Histology, pathological whole tumor size and pathological invasive size. CIR, cumulative incidence of recurrence; CICSD, cumulative incidence of cancer-specific death.
Discussion
Our study analyzed the oncological outcomes of patients with N1-positive NSCLC undergoing lobectomy with systematic mediastinal LND versus lobectomy with lobe-specific mediastinal LND using a multicenter database with adjustment for preoperative factors to minimize the effect of patient selection bias. We demonstrated that systematic mediastinal LND allows the harvest of more lymph nodes and provides more appropriate pathological staging than lobe-specific mediastinal LND alone. However, conversely, the extent of LND was not associated with survival outcomes in the treatment of N1-positive lung cancer.
There remain several controversies regarding the extent of LND. In 1951, Cahan from Memorial Sloan Kettering Cancer Center, reporting on complete mediastinal lymphadenectomy, found that some patients experienced long-term survival when the positive regional lymph nodes were removed (10). In a subsequent review, the authors commented that complete mediastinal LND led to a ‘more favorable long-term survival’ (11). Thus, systematic LND has been considered the standard care for lung cancer resection. However, recently, as a minimally invasive surgical technique, the concept of lobe-specific mediastinal LND has been established. Several retrospective institutional studies have demonstrated detailed nodal spread patterns in surgically resected NSCLC (5–7). These studies suggest that lobe-specific mediastinal LND might be an acceptable approach and hold comparable oncologic outcomes with systematic LND. Therefore, lobe-specific mediastinal LND has been chosen more frequently and has become a more popular surgical option. To provide strong evidence justifying lobe-specific LND for lung cancer, a prospective randomized controlled trial (JCOG1413) is ongoing in Japan (12).
However, the significance and appropriate extent of mediastinal LND in patients with more aggressive tumors has not been established. Inevitably, N1-positive tumors may be more malignant than N0 and have more extensive lymph node metastasis in the mediastinal field. Most of the papers on lobe-specific LND have focused on clinical N0, and the significance of LND in N1-positive NSCLC has not yet been elucidated (5–7). ‘How much does the extent of lymphadenectomy affect oncological outcomes in the treatment of N1-positive NSCLC?’ ‘Which is the appropriate extent of lymphadenectomy in the treatment of N1-positive NSCLC, systematic or lobe-specific mediastinal LND?’ Few studies are evaluating this topic. Therefore, we planned this study and analyzed 359 consecutive patients (lobectomy with systematic and lobectomy with lobe-specific LND) with N1-positive NSCLC registered in a multicenter database using propensity score-matched analysis.
In all cohorts, the systematic LND groups had worse oncologic outcomes than the lobe-specific LND group. However, each patient group differed regarding clinicopathological variables. First, to minimize patient selection bias, we used propensity score-matched analyses. In our matched model, CIR and CICSD were comparable in the systematic and lobe-specific LND groups. In addition, we used multivariable analyses and found that the prognosis associated with systematic LND was comparable with those obtained using lobe-specific LND. We must admit that there may be selection bias in this study. However, when assessing the results of these analyses, we believe that the extent of mediastinal LND could not affect the oncological outcome in the treatment of N1-positive NSCLC.
Some surgeons believe that the main role of LND is to prevent understaging and not to secure the local control of cancer. More lymph nodes dissected elevates staging accuracy, thus suitably upstaging the patients who would have otherwise been incorrectly included among lower-stage patients. This finding is known as the Will Rogers phenomenon or stage migration (13). When lymph node sampling is inadequate, the true N stage remains unrecognized, producing a false downstaging. In this study, the median number of dissected lymph nodes was 20 and 17 in the systematic and lobe-specific LND, respectively, following propensity score matching. The systematic LND group detected more N2 lymph node metastasis (55.4 vs. 41%, P = 0.087). However, finally, the two groups experienced comparable overall recurrence (48.2 vs. 54.2%, P = 0.60) and lymph node relapse (14.5 vs. 20.5%, P = 0.41). We believe that the main role of LND is to prevent understaging rather than obtaining better oncological outcomes in N1 lung cancer treatment. In addition, the current guidelines recommend postoperative chemotherapy when N1 is positive (14). Namely, N1 positive and N2 positive do not make a big difference in postoperative treatment strategies. Both groups received adjuvant chemotherapy at a comparable frequency (53 vs. 57.8%). The extent of mediastinal LND might not strongly affect postoperative management in N1-positive NSCLC treatment.
The major limitation of this study is its retrospective design, which allowed surgeon selection bias in both groups. Given the lack of randomization, the potential for surgeon bias exists in terms of patient selection for one approach over another. To address these issues, we performed our survival analysis using CIR and CICSD rather than overall survival and recurrence-free survival to focus more clearly on the oncological outcomes of the two groups. Furthermore, we added propensity score-matched analyses and multivariable analyses to minimize the differences in patient characteristics. The sample size of this study was relatively small for a propensity score-matched cohort study; thus, definitive conclusions regarding the appropriate extent of lymphadenectomy for N1-positive NSCLC could not be established. We also used a multicenter database integrating data from three Japanese facilities to enlarge the sample size to the maximum extent possible. This database does not include data on co-morbid medical conditions, surgical approach, intraoperative outcomes, postoperative complications and mortality and postoperative treatment, whether the clinical diagnosis of N is a definitive pathological diagnosis, and the presence or absence of an intraoperative diagnosis. This insufficient information is our limitation. We plan to conduct another large cohort analysis to elucidate the oncologic outcomes of systematic and lobe-specific LND, including data on these factors. Clinical staging (=cN status) is more important than pathological staging (=pN status) for surgeons. However, in this study, we did perform several analyses based on pN status because clinical diagnosis is not easy and false positives and false negatives can easily occur. And, the definition of lobe-specific LND varies on studies.
Extensive LND might be disadvantageous in terms of postoperative complications. Although the database in this study did not include data on complications, several reports have demonstrated that a larger extent of mediastinal LND might increase postoperative morbidity and mortality rates (6). These results suggest that complications can arise from injury of the bronchial arteries and nerves, recurrent nerves, laryngeal nerves, and the thoracic duct and lymphatic backflow, resulting in ischemic tissue changes, pulmonary edema and pneumonia, as well as respiratory distress syndrome. We believe that if postoperative morbidity does increase, extensive mediastinal LND should be carefully considered.
In conclusion, this multicenter, propensity score-matched analysis suggested that the extent of mediastinal LND could affect accurate pathological staging; however, it was not associated with survival outcome in the treatment of N1-positive lung cancer. Further investigations on the appropriate extent of LND for the treatment of NSCLC will be needed.
Conflict of interest statement
None declared.
Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
List of abbreviations
NSCLC, non-small cell lung cancer
LND, lymph node dissection
HRCT, high-resolution computed tomography
FDG-PET, 18F-fluorodeoxyglucose-positron emission tomography
SUVmax, maximum standard uptake value
CIR, cumulative incidence of recurrence
CICSD, cumulative incidence of cancer-specific death
Data availability
The data that support the findings of this study are openly available.


