-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroyuki Adachi, Aya Saito, Yasushi Shintani, Jiro Okami, Hiroyuki Ito, Takashi Ohtsuka, Takeshi Mori, Shun-ichi Watanabe, Masayuki Chida, Shunsuke Endo, Ryoichi Nakanishi, Mitsutaka Kadokura, Hidemi Suzuki, Etsuo Miyaoka, Ichiro Yoshino, Hiroshi Date, Japanese Joint Committee of Lung Cancer Registry, Is adjuvant chemotherapy for completely resected p-stage IA (>2 cm) and stage IB non-small-cell lung cancer beneficial for elderly patients? A large, retrospective cohort study based on real-world data from Japan, Japanese Journal of Clinical Oncology, Volume 53, Issue 12, December 2023, Pages 1191–1200, https://doi.org/10.1093/jjco/hyad116
Close - Share Icon Share
Abstract
The efficacy of tegafur-uracil as adjuvant chemotherapy for patients with completely resected stage I non-small-cell lung cancer is proven; however, its efficacy for elderly patients remains unclear. Herein, we evaluated the effectiveness of adjuvant chemotherapy for elderly patients with completely resected stage I non-small-cell lung cancer based on real-world Japanese data using propensity score matching.
This retrospective study extracted data from a nationwide registry study, performed in 2016, on patients ≥75 years who underwent lobectomy with mediastinal nodal dissection for non-small-cell lung cancer in 2010 and were diagnosed with p-stage IA (>2 cm) or stage IB non-small-cell lung cancer. We classified the 1294 patients into two groups—Group A, postoperative adjuvant chemotherapy (n = 295, 22.8%) and Group N, no adjuvant chemotherapy (n = 999, 77.2%)—and analyzed differences in postoperative overall survival between groups.
Group A showed no advantage in overall survival over Group N as a whole (hazard ratio: 0.824 [95% confidence interval: 0.631–1.076]), in p-stage IA (hazard ratio: 0.617 [95% confidence interval: 0.330–1.156]) and in p-stage IB (hazard ratio: 0.806 [95% confidence interval: 0.597–1.088]) subsets. Even after propensity score matching, Group A showed no significant advantage in overall survival over Group N as a whole (hazard ratio: 0.975 [95% confidence interval: 0.688–1.381]), in p-stage IA (hazard ratio: 1.390 [95% confidence interval: 0.539–3.586]) and in p-stage IB (hazard ratio: 0.922 [95% confidence interval: 0.633–1.343]).
adjuvant chemotherapy for completely resected p-stage IA (>2 cm) and stage IB non-small-cell lung cancer showed no benefit for recommendation for elderly patients; considering the risk of adverse events, we do not recommend adjuvant chemotherapy for elderly patients.
Introduction
Lung cancer is the most frequent cause of cancer-related deaths worldwide (1). Surgery is the main treatment for non-small-cell lung cancer (NSCLC); however, in approximately half of all patients with NSCLC and in 20–30% of patients with pathologic TNM stage I (hereafter, p-stage I) NSCLC who undergo curative intent surgery, postoperative recurrence resulting in death has been observed (2,3). Adjuvant chemotherapy (ACT) has been developed to improve the prognosis of patients with locally advanced stage II–III NSCLC (4). In Western countries, ACT is not recommended for patients with postoperative early-stage NSCLC; however, in Japan (5), according to the results of the trials of Kato et al. (6) and Hamada et al. (7), administration of adjuvant tegafur-uracil (UFT) is recommended for patients with p-stage IA with a tumor size of >2 cm and with p-stage IB. However, ACT also carries the risk of adverse effects (AEs). Previous studies showed that grade ≥ 3 AEs occurred in 75–85% of patients with the use of cisplatin-doublet (8,9) and 1.3% of patients with the use of UFT (10). These AEs can affect patient activities, and in the worst-case scenario, lead to death owing to toxicity. Indeed, in Shukuya et al.’s (11) large-scale, real-world, observational study, ~30% of patients with p-stage IA with tumor size >2 cm or with p-stage IB disease were administered adjuvant UFT; this proportion was small, owing to the risk of adverse events. For elderly patients with generally impaired organ functions, ACT is possibly more harmful than beneficial given its toxicity; therefore, these patients are typically excluded from clinical trials concerning chemotherapy. Physicians sometimes do not administer ACT solely because of patients’ advanced age, despite the rising incidence of lung cancer in patients aged >75 years (12). Contrastingly, human life expectancy has increased with recent developments in medical technology. In 2019, the Japanese Ministry of Health, Labour and Welfare reported that the life expectancy of Japanese individuals at 75 and 80 years of age was 12.63 and 9.42 years, respectively, in men and 16.25 and 12.28 years, respectively, in women (13), indicating that elderly patients can expect to live comparatively longer if their disease is not recurrent.
Thus, whether ACT, especially UFT, is beneficial or harmful for the treatment of elderly patients with p-stage I NSCLC remains elusive. Therefore, we aimed to evaluate the influence of ACT on elderly patients with completely resected p-stage IA (>2 cm) and p-stage IB NSCLC based on real-world Japanese data, using the propensity score (PS) matching method to eliminate selection bias as much as possible.
Methods
Patients
In 2016, the Japanese Joint Committee of Lung Cancer Registry conducted a nationwide registry study. The registry followed the ethical guidelines for epidemiological studies, and the review board of Osaka University Hospital approved this study (Approval No. 15321). The institutional review boards of all participating institutions then approved the registry, and further, this retrospective study using registered data from the participating institutions. This registry included patients with a pathological diagnosis of any primary lung cancer who underwent surgery with curative intent between 1 January and 31 December 2010. Participating institutions were required to register all eligible patients, and 18973 patients from 297 institutes were included (2). The median follow-up period after surgery in censored patients was 66.5 months. The inclusion criteria of the present study were as follows: (i) patients aged ≥75 years at the time of surgery; (ii) those who underwent lobectomy/bilobectomy or pneumonectomy with mediastinal lymph node dissection; and (iii) those who were pathologically diagnosed with stage IA NSCLC with a maximum tumor diameter of >2.0 cm or stage IB NSCLC (according to the 7th edition of the TNM staging system for lung cancer issued by the International Union Against Cancer (14), because the registry could not accumulate data on the invasive diameter of the pathological tumor). The exclusion criteria were as follows: (a) patients who received any induction therapy; (b) those who underwent postoperative radiation therapy; (c) those who had multiple lung cancers treated within 5 years of surgery; (d) those who were pathologically diagnosed with adenocarcinoma in situ, salivary ground-type tumors, or carcinoid tumors; and (e) those without data concerning the introduction of ACT. The patients were divided into two groups according to ACT administration (group A, ACT administered; group N, ACT not administered).
The following clinicopathological variables were analyzed: age, sex, performance status scored according to the Eastern Cooperative Oncology Group (ECOG-PS) (15), smoking history, preoperative serum carcino-embryonic antigen (CEA) levels, comorbidities, surgical procedure, operative time, extent of lymph node dissection, number of dissected lymph nodes, presence of combined resection of other tissues, postoperative complications (grade 3 or more according to the Clavien–Dindo classification), histology, presence of lymphatic or vessel invasion or both, presence of pleural invasion, p–T factor, p-N factor, p-stage, presence of driver oncogenes (epidermal growth factor receptor [EGFR] mutation, anaplastic lymphoma kinase [ALK] rearrangement), and ACT. Pathological T, N and stage were diagnosed according to the 7th edition of the TNM staging system (14). The Charlson comorbidity index (CCI) was calculated using the comorbidity factors according to Charlson et al. (16) (the factors of peripheral vascular disease and digestive ulcer were not included owing to the lack of these data). The extent of lymph node dissection was defined as lobe-specific, systematic or radical (additional dissection or sampling of the bilateral mediastinal or supraclavicular lymph nodes to systematic dissection areas). ACT agents were classified as oral drugs (almost all patients used UFT, whereas only a few used tegafur/gimeracil/oteracil [S-1]), cisplatin doublets or others (single intravenous agents).
Statistical analysis
Overall survival (OS) was defined as the period between the date of surgical resection and death due to any cause.
The PS-matching method was used to adjust for confounding variables, thereby reducing bias. The PS was calculated using logistic regression based on 18 perioperative factors—age, sex, ECOG-PS, CEA level, CCI, surgical procedure, operative time, extent of lymph node dissection, postoperative complications, histology, lymphatic invasion, vessel invasion, pleural invasion, p–T factor, p-N factor, p-stage, EGFR mutation and ALK rearrangement—that were thought to be potentially associated with ACT selection. Patients in Groups A and N were matched in a 1:1 ratio according to PS using a greedy algorithm with a caliper width equal to 0.2 of the standard deviation of the logit of the PS.
Clinicopathological variables were compared using the Mann–Whitney U and Chi-square tests. In addition, survival curves were estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using a Cox proportional hazards model. All analyses were performed using IBM SPSS Statistics 25 (IBM Corp., New York, NY, USA) and SAS 9.4 software programs (SAS Inc, Cary, NC, USA), with P < 0.05 considered statistically significant.
Results
Among the 18973 patients entered into the nationwide registry study (2), 1294 patients were enrolled and analyzed in this study. Of these, 779 were men and 515 women, with a median age of 78 years (range, 75–91 years). The median follow-up period was 60.5 months (range 0.5–81.5 months). According to ACT administration, 295 patients were classified into group A, while 999 patients were classified into group N. The characteristics and postoperative outcomes of each group are summarized in Table 1 (detailed data concerning comorbidities and postoperative complications are shown in Supplemental Table S1), and the postoperative OS curves of each group are shown in Fig. 1. The patients in Group A were significantly younger, had lower CCI scores and had fewer postoperative complications than those in group N, indicating that the general condition of the patients in group A was better than that of the patients in group N. In contrast, patients in group A underwent more extensive nodal dissection, had more pleural invasion and had higher p–T status and p-stage, indicating that patients in group A had more aggressive disease than those in group N. Driver oncogenes were also found more frequently in group A. Despite ACT administration, patients in group A did not show a significant advantage in OS compared to patients in group N as a whole (hazard ratio [HR] 0.824 [95% CI: 0.631–1.076], P = 0.153). Furthermore, in the subset analysis, patients in group A did not show a significant advantage in OS compared to patients in group N in both p-stage IA and p-stage IB subsets (HR 0.617 [95% CI: 0.330–1.156], P = 0.127; and HR 0.806 [95% CI: 0.597–1.088], P = 0.157, respectively).
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 1294) . | Group A (N = 295) . | Group N (N = 999) . | . |
| Age (years; median [range]) | 78.0 [75–91] | 77.0 [75–86] | 78.0 [75–91] | <0.001 |
| Sex (male) | 779 (60.2) | 177 (60.0) | 602 (60.3) | 0.946 |
| ECOG-PS (0 or 1) | 1218 (94.1) | 281 (95.3) | 937 (93.8) | 0.400 |
| Smoking history | 782 (60.4) | 168 (56.9) | 614 (61.5) | 0.373 |
| CEA (> 5 ng/ml) | 393 (30.4) | 98 (33.2) | 295 (29.5) | 0.279 |
| CCI | 0.003 | |||
| 0 | 161 (12.4) | 34 (11.5) | 127 (12.7) | |
| 1–2 | 860 (66.5) | 218 (73.9) | 642 (64.3) | |
| ≥ 3 | 273 (21.1) | 43 (14.6) | 230 (23.0) | |
| Surgical procedure | 0.549 | |||
| Lobectomy/bi-lobectomy | 1289 (99.6) | 294 (99.7) | 995 (99.6) | |
| Pneumonectomy | 5 (0.4) | 1 (1.5) | 4 (1.0) | |
| Operation time (minutes; median [range]) | 200 [67–763] | 208 [67–439] | 199 [72–763] | 0.073 |
| Extent of LN dissection | 0.019 | |||
| Lobe-specific | 696 (53.8) | 137 (46.5) | 559 (56.0) | |
| Systematic | 463 (35.8) | 129 (43.7) | 334 (33.4) | |
| Radical | 27 (2.1) | 6 (2.0) | 21 (2.1) | |
| Detail unknown | 108 (8.3) | 23 (7.8) | 85 (8.5) | |
| Number of dissected LN (≥ 6) | 1210 (93.5) | 268 (90.8) | 942 (94.3) | 0.183 |
| Combined resection of other tissues | 16 (1.2) | 3 (1.0) | 13 (1.3) | 0.271 |
| Postoperative complication (≥ G3) | 145 (11.2) | 16 (5.4) | 129 (12.9) | < 0.001 |
| Histology | 0.477 | |||
| Adenocarcinoma | 874 (67.5) | 210 (71.2) | 664 (66.5) | |
| Squamous cell carcinoma | 334 (25.8) | 64 (21.7) | 270 (27.0) | |
| Others | 86 (6.7) | 21 (7.1) | 65 (6.5) | |
| Lymphatic invasion | 316 (24.4) | 83 (28.1) | 233 (23.3) | 0.097 |
| Vessel invasion | 369 (28.5) | 96 (32.5) | 273 (27.3) | 0.065 |
| Pleural invasion | 377 (29.1) | 100 (33.9) | 277 (27.7) | 0.007 |
| pT | < 0.001 | |||
| T1b | 504 (38.9) | 75 (25.4) | 429 (42.9) | |
| T2a | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| p-stage | < 0.001 | |||
| IA | 504 (38.9) | 75 (12.7) | 429 (42.9) | |
| IB | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| EGFR mutation | 0.011 | |||
| Negative | 283 (21.9) | 80 (27.1) | 203 (20.3) | |
| Positive | 175 (13.5) | 46 (15.6) | 129 (12.9) | |
| Ex21L858R | 86 | 20 | 66 | |
| Ex19del | 69 | 19 | 50 | |
| Others | 20 | 7 | 13 | |
| Unknown | 836 (64.6) | 169 (57.3) | 667 (66.8) | |
| ALK rearrangement | 0.183 | |||
| Negative | 50 (3.9) | 15 (5.1) | 35 (3.5) | |
| Positive | 2 (0.2) | 1 (0.3) | 1 (0.1) | |
| Unknown | 1242 (95.9) | 279 (94.6) | 963 (96.4) | |
| ACT agents | < 0.001 | |||
| None | 999 (77.2) | - | 999 (100.0) | |
| Cisplatin doublet | 14 (1.1) | 14 (4.7) | - | |
| Oral drug | 274 (21.2) | 274 (92.9) | - | |
| Others | 7 (0.5) | 7 (2.4) | - | |
| F/U period (month; median [range]) | 60.5 [0.5–81.5] | 62.5 [0.5–80.5] | 60.5 [0.5–80.5] | < 0.001 |
| Postoperative recurrence | < 0.001 | |||
| None | 1065 (82.3) | 221 (74.9) | 844 (84.5) | |
| Locoregional | 104 (8.0) | 30 (10.2) | 74 (7.4) | |
| Distant | 125 (9.7) | 44 (14.9) | 81 (8.1) | |
| Outcome | 0.591 | |||
| Alive | 975 (75.3) | 226 (76.6) | 749 (75.0) | |
| Cancer-related death | 125 (9.7) | 35 (11.9) | 90 (9.0) | |
| Non-cancer-related death | 171 (13.2) | 32 (10.8) | 139 (13.9) | |
| Death with unknown cause | 23 (1.8) | 2 (0.7) | 21 (2.1) | |
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 1294) . | Group A (N = 295) . | Group N (N = 999) . | . |
| Age (years; median [range]) | 78.0 [75–91] | 77.0 [75–86] | 78.0 [75–91] | <0.001 |
| Sex (male) | 779 (60.2) | 177 (60.0) | 602 (60.3) | 0.946 |
| ECOG-PS (0 or 1) | 1218 (94.1) | 281 (95.3) | 937 (93.8) | 0.400 |
| Smoking history | 782 (60.4) | 168 (56.9) | 614 (61.5) | 0.373 |
| CEA (> 5 ng/ml) | 393 (30.4) | 98 (33.2) | 295 (29.5) | 0.279 |
| CCI | 0.003 | |||
| 0 | 161 (12.4) | 34 (11.5) | 127 (12.7) | |
| 1–2 | 860 (66.5) | 218 (73.9) | 642 (64.3) | |
| ≥ 3 | 273 (21.1) | 43 (14.6) | 230 (23.0) | |
| Surgical procedure | 0.549 | |||
| Lobectomy/bi-lobectomy | 1289 (99.6) | 294 (99.7) | 995 (99.6) | |
| Pneumonectomy | 5 (0.4) | 1 (1.5) | 4 (1.0) | |
| Operation time (minutes; median [range]) | 200 [67–763] | 208 [67–439] | 199 [72–763] | 0.073 |
| Extent of LN dissection | 0.019 | |||
| Lobe-specific | 696 (53.8) | 137 (46.5) | 559 (56.0) | |
| Systematic | 463 (35.8) | 129 (43.7) | 334 (33.4) | |
| Radical | 27 (2.1) | 6 (2.0) | 21 (2.1) | |
| Detail unknown | 108 (8.3) | 23 (7.8) | 85 (8.5) | |
| Number of dissected LN (≥ 6) | 1210 (93.5) | 268 (90.8) | 942 (94.3) | 0.183 |
| Combined resection of other tissues | 16 (1.2) | 3 (1.0) | 13 (1.3) | 0.271 |
| Postoperative complication (≥ G3) | 145 (11.2) | 16 (5.4) | 129 (12.9) | < 0.001 |
| Histology | 0.477 | |||
| Adenocarcinoma | 874 (67.5) | 210 (71.2) | 664 (66.5) | |
| Squamous cell carcinoma | 334 (25.8) | 64 (21.7) | 270 (27.0) | |
| Others | 86 (6.7) | 21 (7.1) | 65 (6.5) | |
| Lymphatic invasion | 316 (24.4) | 83 (28.1) | 233 (23.3) | 0.097 |
| Vessel invasion | 369 (28.5) | 96 (32.5) | 273 (27.3) | 0.065 |
| Pleural invasion | 377 (29.1) | 100 (33.9) | 277 (27.7) | 0.007 |
| pT | < 0.001 | |||
| T1b | 504 (38.9) | 75 (25.4) | 429 (42.9) | |
| T2a | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| p-stage | < 0.001 | |||
| IA | 504 (38.9) | 75 (12.7) | 429 (42.9) | |
| IB | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| EGFR mutation | 0.011 | |||
| Negative | 283 (21.9) | 80 (27.1) | 203 (20.3) | |
| Positive | 175 (13.5) | 46 (15.6) | 129 (12.9) | |
| Ex21L858R | 86 | 20 | 66 | |
| Ex19del | 69 | 19 | 50 | |
| Others | 20 | 7 | 13 | |
| Unknown | 836 (64.6) | 169 (57.3) | 667 (66.8) | |
| ALK rearrangement | 0.183 | |||
| Negative | 50 (3.9) | 15 (5.1) | 35 (3.5) | |
| Positive | 2 (0.2) | 1 (0.3) | 1 (0.1) | |
| Unknown | 1242 (95.9) | 279 (94.6) | 963 (96.4) | |
| ACT agents | < 0.001 | |||
| None | 999 (77.2) | - | 999 (100.0) | |
| Cisplatin doublet | 14 (1.1) | 14 (4.7) | - | |
| Oral drug | 274 (21.2) | 274 (92.9) | - | |
| Others | 7 (0.5) | 7 (2.4) | - | |
| F/U period (month; median [range]) | 60.5 [0.5–81.5] | 62.5 [0.5–80.5] | 60.5 [0.5–80.5] | < 0.001 |
| Postoperative recurrence | < 0.001 | |||
| None | 1065 (82.3) | 221 (74.9) | 844 (84.5) | |
| Locoregional | 104 (8.0) | 30 (10.2) | 74 (7.4) | |
| Distant | 125 (9.7) | 44 (14.9) | 81 (8.1) | |
| Outcome | 0.591 | |||
| Alive | 975 (75.3) | 226 (76.6) | 749 (75.0) | |
| Cancer-related death | 125 (9.7) | 35 (11.9) | 90 (9.0) | |
| Non-cancer-related death | 171 (13.2) | 32 (10.8) | 139 (13.9) | |
| Death with unknown cause | 23 (1.8) | 2 (0.7) | 21 (2.1) | |
All values are presented as numbers (percentage) or as medians [ranges] where indicated.
ACT, adjuvant chemotherapy; ALK, anaplastic lymphoma kinase; CEA, carcino-embryonic antigen; CCI, Charlson comorbidity index; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; F/U, follow-up; LN, lymph node
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 1294) . | Group A (N = 295) . | Group N (N = 999) . | . |
| Age (years; median [range]) | 78.0 [75–91] | 77.0 [75–86] | 78.0 [75–91] | <0.001 |
| Sex (male) | 779 (60.2) | 177 (60.0) | 602 (60.3) | 0.946 |
| ECOG-PS (0 or 1) | 1218 (94.1) | 281 (95.3) | 937 (93.8) | 0.400 |
| Smoking history | 782 (60.4) | 168 (56.9) | 614 (61.5) | 0.373 |
| CEA (> 5 ng/ml) | 393 (30.4) | 98 (33.2) | 295 (29.5) | 0.279 |
| CCI | 0.003 | |||
| 0 | 161 (12.4) | 34 (11.5) | 127 (12.7) | |
| 1–2 | 860 (66.5) | 218 (73.9) | 642 (64.3) | |
| ≥ 3 | 273 (21.1) | 43 (14.6) | 230 (23.0) | |
| Surgical procedure | 0.549 | |||
| Lobectomy/bi-lobectomy | 1289 (99.6) | 294 (99.7) | 995 (99.6) | |
| Pneumonectomy | 5 (0.4) | 1 (1.5) | 4 (1.0) | |
| Operation time (minutes; median [range]) | 200 [67–763] | 208 [67–439] | 199 [72–763] | 0.073 |
| Extent of LN dissection | 0.019 | |||
| Lobe-specific | 696 (53.8) | 137 (46.5) | 559 (56.0) | |
| Systematic | 463 (35.8) | 129 (43.7) | 334 (33.4) | |
| Radical | 27 (2.1) | 6 (2.0) | 21 (2.1) | |
| Detail unknown | 108 (8.3) | 23 (7.8) | 85 (8.5) | |
| Number of dissected LN (≥ 6) | 1210 (93.5) | 268 (90.8) | 942 (94.3) | 0.183 |
| Combined resection of other tissues | 16 (1.2) | 3 (1.0) | 13 (1.3) | 0.271 |
| Postoperative complication (≥ G3) | 145 (11.2) | 16 (5.4) | 129 (12.9) | < 0.001 |
| Histology | 0.477 | |||
| Adenocarcinoma | 874 (67.5) | 210 (71.2) | 664 (66.5) | |
| Squamous cell carcinoma | 334 (25.8) | 64 (21.7) | 270 (27.0) | |
| Others | 86 (6.7) | 21 (7.1) | 65 (6.5) | |
| Lymphatic invasion | 316 (24.4) | 83 (28.1) | 233 (23.3) | 0.097 |
| Vessel invasion | 369 (28.5) | 96 (32.5) | 273 (27.3) | 0.065 |
| Pleural invasion | 377 (29.1) | 100 (33.9) | 277 (27.7) | 0.007 |
| pT | < 0.001 | |||
| T1b | 504 (38.9) | 75 (25.4) | 429 (42.9) | |
| T2a | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| p-stage | < 0.001 | |||
| IA | 504 (38.9) | 75 (12.7) | 429 (42.9) | |
| IB | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| EGFR mutation | 0.011 | |||
| Negative | 283 (21.9) | 80 (27.1) | 203 (20.3) | |
| Positive | 175 (13.5) | 46 (15.6) | 129 (12.9) | |
| Ex21L858R | 86 | 20 | 66 | |
| Ex19del | 69 | 19 | 50 | |
| Others | 20 | 7 | 13 | |
| Unknown | 836 (64.6) | 169 (57.3) | 667 (66.8) | |
| ALK rearrangement | 0.183 | |||
| Negative | 50 (3.9) | 15 (5.1) | 35 (3.5) | |
| Positive | 2 (0.2) | 1 (0.3) | 1 (0.1) | |
| Unknown | 1242 (95.9) | 279 (94.6) | 963 (96.4) | |
| ACT agents | < 0.001 | |||
| None | 999 (77.2) | - | 999 (100.0) | |
| Cisplatin doublet | 14 (1.1) | 14 (4.7) | - | |
| Oral drug | 274 (21.2) | 274 (92.9) | - | |
| Others | 7 (0.5) | 7 (2.4) | - | |
| F/U period (month; median [range]) | 60.5 [0.5–81.5] | 62.5 [0.5–80.5] | 60.5 [0.5–80.5] | < 0.001 |
| Postoperative recurrence | < 0.001 | |||
| None | 1065 (82.3) | 221 (74.9) | 844 (84.5) | |
| Locoregional | 104 (8.0) | 30 (10.2) | 74 (7.4) | |
| Distant | 125 (9.7) | 44 (14.9) | 81 (8.1) | |
| Outcome | 0.591 | |||
| Alive | 975 (75.3) | 226 (76.6) | 749 (75.0) | |
| Cancer-related death | 125 (9.7) | 35 (11.9) | 90 (9.0) | |
| Non-cancer-related death | 171 (13.2) | 32 (10.8) | 139 (13.9) | |
| Death with unknown cause | 23 (1.8) | 2 (0.7) | 21 (2.1) | |
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 1294) . | Group A (N = 295) . | Group N (N = 999) . | . |
| Age (years; median [range]) | 78.0 [75–91] | 77.0 [75–86] | 78.0 [75–91] | <0.001 |
| Sex (male) | 779 (60.2) | 177 (60.0) | 602 (60.3) | 0.946 |
| ECOG-PS (0 or 1) | 1218 (94.1) | 281 (95.3) | 937 (93.8) | 0.400 |
| Smoking history | 782 (60.4) | 168 (56.9) | 614 (61.5) | 0.373 |
| CEA (> 5 ng/ml) | 393 (30.4) | 98 (33.2) | 295 (29.5) | 0.279 |
| CCI | 0.003 | |||
| 0 | 161 (12.4) | 34 (11.5) | 127 (12.7) | |
| 1–2 | 860 (66.5) | 218 (73.9) | 642 (64.3) | |
| ≥ 3 | 273 (21.1) | 43 (14.6) | 230 (23.0) | |
| Surgical procedure | 0.549 | |||
| Lobectomy/bi-lobectomy | 1289 (99.6) | 294 (99.7) | 995 (99.6) | |
| Pneumonectomy | 5 (0.4) | 1 (1.5) | 4 (1.0) | |
| Operation time (minutes; median [range]) | 200 [67–763] | 208 [67–439] | 199 [72–763] | 0.073 |
| Extent of LN dissection | 0.019 | |||
| Lobe-specific | 696 (53.8) | 137 (46.5) | 559 (56.0) | |
| Systematic | 463 (35.8) | 129 (43.7) | 334 (33.4) | |
| Radical | 27 (2.1) | 6 (2.0) | 21 (2.1) | |
| Detail unknown | 108 (8.3) | 23 (7.8) | 85 (8.5) | |
| Number of dissected LN (≥ 6) | 1210 (93.5) | 268 (90.8) | 942 (94.3) | 0.183 |
| Combined resection of other tissues | 16 (1.2) | 3 (1.0) | 13 (1.3) | 0.271 |
| Postoperative complication (≥ G3) | 145 (11.2) | 16 (5.4) | 129 (12.9) | < 0.001 |
| Histology | 0.477 | |||
| Adenocarcinoma | 874 (67.5) | 210 (71.2) | 664 (66.5) | |
| Squamous cell carcinoma | 334 (25.8) | 64 (21.7) | 270 (27.0) | |
| Others | 86 (6.7) | 21 (7.1) | 65 (6.5) | |
| Lymphatic invasion | 316 (24.4) | 83 (28.1) | 233 (23.3) | 0.097 |
| Vessel invasion | 369 (28.5) | 96 (32.5) | 273 (27.3) | 0.065 |
| Pleural invasion | 377 (29.1) | 100 (33.9) | 277 (27.7) | 0.007 |
| pT | < 0.001 | |||
| T1b | 504 (38.9) | 75 (25.4) | 429 (42.9) | |
| T2a | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| p-stage | < 0.001 | |||
| IA | 504 (38.9) | 75 (12.7) | 429 (42.9) | |
| IB | 790 (61.1) | 220 (74.6) | 570 (57.1) | |
| EGFR mutation | 0.011 | |||
| Negative | 283 (21.9) | 80 (27.1) | 203 (20.3) | |
| Positive | 175 (13.5) | 46 (15.6) | 129 (12.9) | |
| Ex21L858R | 86 | 20 | 66 | |
| Ex19del | 69 | 19 | 50 | |
| Others | 20 | 7 | 13 | |
| Unknown | 836 (64.6) | 169 (57.3) | 667 (66.8) | |
| ALK rearrangement | 0.183 | |||
| Negative | 50 (3.9) | 15 (5.1) | 35 (3.5) | |
| Positive | 2 (0.2) | 1 (0.3) | 1 (0.1) | |
| Unknown | 1242 (95.9) | 279 (94.6) | 963 (96.4) | |
| ACT agents | < 0.001 | |||
| None | 999 (77.2) | - | 999 (100.0) | |
| Cisplatin doublet | 14 (1.1) | 14 (4.7) | - | |
| Oral drug | 274 (21.2) | 274 (92.9) | - | |
| Others | 7 (0.5) | 7 (2.4) | - | |
| F/U period (month; median [range]) | 60.5 [0.5–81.5] | 62.5 [0.5–80.5] | 60.5 [0.5–80.5] | < 0.001 |
| Postoperative recurrence | < 0.001 | |||
| None | 1065 (82.3) | 221 (74.9) | 844 (84.5) | |
| Locoregional | 104 (8.0) | 30 (10.2) | 74 (7.4) | |
| Distant | 125 (9.7) | 44 (14.9) | 81 (8.1) | |
| Outcome | 0.591 | |||
| Alive | 975 (75.3) | 226 (76.6) | 749 (75.0) | |
| Cancer-related death | 125 (9.7) | 35 (11.9) | 90 (9.0) | |
| Non-cancer-related death | 171 (13.2) | 32 (10.8) | 139 (13.9) | |
| Death with unknown cause | 23 (1.8) | 2 (0.7) | 21 (2.1) | |
All values are presented as numbers (percentage) or as medians [ranges] where indicated.
ACT, adjuvant chemotherapy; ALK, anaplastic lymphoma kinase; CEA, carcino-embryonic antigen; CCI, Charlson comorbidity index; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; F/U, follow-up; LN, lymph node

Postoperative overall survival in groups A and N. (A) As a whole, (B) in p-stage IA, (C) in p-stage IB subset. group A, received postoperative adjuvant chemotherapy; and group N, did not receive preoperative adjuvant chemotherapy.
PS matching between groups A and N was conducted to eliminate selection bias caused by confounding factors, and 290 cases in each group were matched (Supplemental Table S2 and Supplemental Fig. S1 show the summary of statistics and the histogram for PS in each group, respectively). The patient characteristics after matching are shown in Table 2. After PS matching, no significant differences in these characteristics were observed between groups. The postoperative OS curves after PS matching are shown in Fig. 2. Even after PS matching, patients in group A did not show a significant advantage in OS compared to patients in group N as a whole (HR 0.975 [95% CI: 0.688–1.381], P = 0.885), in p-stage IA subset (HR 1.390 [95% CI: 0.539–3.586], P = 0.496) or in p-stage IB subset (HR 0.922 [95% CI: 0.633–1.343], P = 0.673). We performed another subset analysis, stratified according to the agent used for ACT in the PS-matched cohort. In both cohorts, the administration of intravenous agents, including cisplatin doublet, did not improve postoperative OS compared to that in the no-ACT and/or oral drug administration cohort (Fig. 3). Concerning the presence of EGFR mutation, patients in group A did not show a significant advantage in OS compared to patients in group N in either the EGFR mutant subset or EGFR wild/unknown subset in the PS-matched cohort (Supplemental Fig. S2).
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 580) . | Group A (N = 290) . | Group N (N = 290) . | . |
| Age | 1.000 | |||
| ≤ 80 years | 500 (86.2) | 250 (86.2) | 250 (86.2) | |
| > 80 years | 80 (13.8) | 40 (13.8) | 40 (13.8) | |
| Sex (male) | 346 (59.7) | 174 (60.0) | 172 (59.3) | 0.933 |
| ECOG-PS (0 or 1) | 553 (95.3) | 276 (95.2) | 277 (95.5) | 1.000 |
| CEA (> 5 ng/ml) | 198 (34.1) | 98 (33.8) | 100 (34.5) | 0.930 |
| CCI | 0.449 | |||
| 0 | 65 (11.2) | 34 (11.7) | 31 (10.7) | |
| 1–2 | 440 (75.9) | 214 (73.8) | 226 (77.9) | |
| ≥ 3 | 75 (12.9) | 42 (14.5) | 33 (11.4) | |
| Surgical procedure | 1.000 | |||
| Lobectomy/bi-lobectomy | 578 (99.7) | 289 (99.7) | 289 (99.7) | |
| Pneumonectomy | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Operation time | 0.540 | |||
| < 240 min | 382 (65.9) | 187 (64.5) | 195 (67.2) | |
| ≥ 240 min | 198 (34.1) | 103 (35.5) | 95 (32.8) | |
| Extent of LN dissection | 0.946 | |||
| Lobe-specific | 267 (46.0) | 134 (46.1) | 133 (45.9) | |
| Systematic | 252 (43.5) | 127 (43.8) | 125 (43.1) | |
| Others | 61 (10.5) | 29 (0.1) | 32 (11.0) | |
| Postoperative complication (≥ G3) | 28 (4.7) | 16 (5.5) | 12 (4.1) | 0.562 |
| Histology | 0.282 | |||
| Adenocarcinoma | 399 (68.8) | 206 (71.0) | 193 (66.6) | |
| Non-adenocarcinoma | 181 (31.2) | 84 (29.0) | 97 (33.4) | |
| Lymphatic invasion | 321 (55.3) | 157 (54.1) | 164 (56.6) | 0.835 |
| Vessel invasion | 306 (52.8) | 150 (51.7) | 156 (53.8) | 0.842 |
| Pleural invasion | 201 (34.7) | 97 (33.4) | 104 (35.9) | 0.699 |
| pT | 1.000 | |||
| T1b | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| T2a | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| p-stage | 1.000 | |||
| IA | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| IB | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| EGFR mutation | 86 (14.8) | 46 (15.9) | 40 (13.8) | 0.559 |
| ALK rearrangement | 2 (0.3) | 1 (0.3) | 1 (0.3) | 1.000 |
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 580) . | Group A (N = 290) . | Group N (N = 290) . | . |
| Age | 1.000 | |||
| ≤ 80 years | 500 (86.2) | 250 (86.2) | 250 (86.2) | |
| > 80 years | 80 (13.8) | 40 (13.8) | 40 (13.8) | |
| Sex (male) | 346 (59.7) | 174 (60.0) | 172 (59.3) | 0.933 |
| ECOG-PS (0 or 1) | 553 (95.3) | 276 (95.2) | 277 (95.5) | 1.000 |
| CEA (> 5 ng/ml) | 198 (34.1) | 98 (33.8) | 100 (34.5) | 0.930 |
| CCI | 0.449 | |||
| 0 | 65 (11.2) | 34 (11.7) | 31 (10.7) | |
| 1–2 | 440 (75.9) | 214 (73.8) | 226 (77.9) | |
| ≥ 3 | 75 (12.9) | 42 (14.5) | 33 (11.4) | |
| Surgical procedure | 1.000 | |||
| Lobectomy/bi-lobectomy | 578 (99.7) | 289 (99.7) | 289 (99.7) | |
| Pneumonectomy | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Operation time | 0.540 | |||
| < 240 min | 382 (65.9) | 187 (64.5) | 195 (67.2) | |
| ≥ 240 min | 198 (34.1) | 103 (35.5) | 95 (32.8) | |
| Extent of LN dissection | 0.946 | |||
| Lobe-specific | 267 (46.0) | 134 (46.1) | 133 (45.9) | |
| Systematic | 252 (43.5) | 127 (43.8) | 125 (43.1) | |
| Others | 61 (10.5) | 29 (0.1) | 32 (11.0) | |
| Postoperative complication (≥ G3) | 28 (4.7) | 16 (5.5) | 12 (4.1) | 0.562 |
| Histology | 0.282 | |||
| Adenocarcinoma | 399 (68.8) | 206 (71.0) | 193 (66.6) | |
| Non-adenocarcinoma | 181 (31.2) | 84 (29.0) | 97 (33.4) | |
| Lymphatic invasion | 321 (55.3) | 157 (54.1) | 164 (56.6) | 0.835 |
| Vessel invasion | 306 (52.8) | 150 (51.7) | 156 (53.8) | 0.842 |
| Pleural invasion | 201 (34.7) | 97 (33.4) | 104 (35.9) | 0.699 |
| pT | 1.000 | |||
| T1b | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| T2a | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| p-stage | 1.000 | |||
| IA | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| IB | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| EGFR mutation | 86 (14.8) | 46 (15.9) | 40 (13.8) | 0.559 |
| ALK rearrangement | 2 (0.3) | 1 (0.3) | 1 (0.3) | 1.000 |
All values are presented as numbers (percentage) or as medians [ranges] where indicated.
ACT, adjuvant chemotherapy; ALK, anaplastic lymphoma kinase; CEA, carcino-embryonic antigen; CCI, Charlson comorbidity index; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; LN, lymph node
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 580) . | Group A (N = 290) . | Group N (N = 290) . | . |
| Age | 1.000 | |||
| ≤ 80 years | 500 (86.2) | 250 (86.2) | 250 (86.2) | |
| > 80 years | 80 (13.8) | 40 (13.8) | 40 (13.8) | |
| Sex (male) | 346 (59.7) | 174 (60.0) | 172 (59.3) | 0.933 |
| ECOG-PS (0 or 1) | 553 (95.3) | 276 (95.2) | 277 (95.5) | 1.000 |
| CEA (> 5 ng/ml) | 198 (34.1) | 98 (33.8) | 100 (34.5) | 0.930 |
| CCI | 0.449 | |||
| 0 | 65 (11.2) | 34 (11.7) | 31 (10.7) | |
| 1–2 | 440 (75.9) | 214 (73.8) | 226 (77.9) | |
| ≥ 3 | 75 (12.9) | 42 (14.5) | 33 (11.4) | |
| Surgical procedure | 1.000 | |||
| Lobectomy/bi-lobectomy | 578 (99.7) | 289 (99.7) | 289 (99.7) | |
| Pneumonectomy | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Operation time | 0.540 | |||
| < 240 min | 382 (65.9) | 187 (64.5) | 195 (67.2) | |
| ≥ 240 min | 198 (34.1) | 103 (35.5) | 95 (32.8) | |
| Extent of LN dissection | 0.946 | |||
| Lobe-specific | 267 (46.0) | 134 (46.1) | 133 (45.9) | |
| Systematic | 252 (43.5) | 127 (43.8) | 125 (43.1) | |
| Others | 61 (10.5) | 29 (0.1) | 32 (11.0) | |
| Postoperative complication (≥ G3) | 28 (4.7) | 16 (5.5) | 12 (4.1) | 0.562 |
| Histology | 0.282 | |||
| Adenocarcinoma | 399 (68.8) | 206 (71.0) | 193 (66.6) | |
| Non-adenocarcinoma | 181 (31.2) | 84 (29.0) | 97 (33.4) | |
| Lymphatic invasion | 321 (55.3) | 157 (54.1) | 164 (56.6) | 0.835 |
| Vessel invasion | 306 (52.8) | 150 (51.7) | 156 (53.8) | 0.842 |
| Pleural invasion | 201 (34.7) | 97 (33.4) | 104 (35.9) | 0.699 |
| pT | 1.000 | |||
| T1b | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| T2a | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| p-stage | 1.000 | |||
| IA | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| IB | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| EGFR mutation | 86 (14.8) | 46 (15.9) | 40 (13.8) | 0.559 |
| ALK rearrangement | 2 (0.3) | 1 (0.3) | 1 (0.3) | 1.000 |
| . | No. of patients (%) . | P Value . | ||
|---|---|---|---|---|
| . | Total (N = 580) . | Group A (N = 290) . | Group N (N = 290) . | . |
| Age | 1.000 | |||
| ≤ 80 years | 500 (86.2) | 250 (86.2) | 250 (86.2) | |
| > 80 years | 80 (13.8) | 40 (13.8) | 40 (13.8) | |
| Sex (male) | 346 (59.7) | 174 (60.0) | 172 (59.3) | 0.933 |
| ECOG-PS (0 or 1) | 553 (95.3) | 276 (95.2) | 277 (95.5) | 1.000 |
| CEA (> 5 ng/ml) | 198 (34.1) | 98 (33.8) | 100 (34.5) | 0.930 |
| CCI | 0.449 | |||
| 0 | 65 (11.2) | 34 (11.7) | 31 (10.7) | |
| 1–2 | 440 (75.9) | 214 (73.8) | 226 (77.9) | |
| ≥ 3 | 75 (12.9) | 42 (14.5) | 33 (11.4) | |
| Surgical procedure | 1.000 | |||
| Lobectomy/bi-lobectomy | 578 (99.7) | 289 (99.7) | 289 (99.7) | |
| Pneumonectomy | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Operation time | 0.540 | |||
| < 240 min | 382 (65.9) | 187 (64.5) | 195 (67.2) | |
| ≥ 240 min | 198 (34.1) | 103 (35.5) | 95 (32.8) | |
| Extent of LN dissection | 0.946 | |||
| Lobe-specific | 267 (46.0) | 134 (46.1) | 133 (45.9) | |
| Systematic | 252 (43.5) | 127 (43.8) | 125 (43.1) | |
| Others | 61 (10.5) | 29 (0.1) | 32 (11.0) | |
| Postoperative complication (≥ G3) | 28 (4.7) | 16 (5.5) | 12 (4.1) | 0.562 |
| Histology | 0.282 | |||
| Adenocarcinoma | 399 (68.8) | 206 (71.0) | 193 (66.6) | |
| Non-adenocarcinoma | 181 (31.2) | 84 (29.0) | 97 (33.4) | |
| Lymphatic invasion | 321 (55.3) | 157 (54.1) | 164 (56.6) | 0.835 |
| Vessel invasion | 306 (52.8) | 150 (51.7) | 156 (53.8) | 0.842 |
| Pleural invasion | 201 (34.7) | 97 (33.4) | 104 (35.9) | 0.699 |
| pT | 1.000 | |||
| T1b | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| T2a | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| p-stage | 1.000 | |||
| IA | 150 (25.9) | 75 (25.9) | 75 (25.9) | |
| IB | 430 (74.1) | 215 (74.1) | 215 (74.1) | |
| EGFR mutation | 86 (14.8) | 46 (15.9) | 40 (13.8) | 0.559 |
| ALK rearrangement | 2 (0.3) | 1 (0.3) | 1 (0.3) | 1.000 |
All values are presented as numbers (percentage) or as medians [ranges] where indicated.
ACT, adjuvant chemotherapy; ALK, anaplastic lymphoma kinase; CEA, carcino-embryonic antigen; CCI, Charlson comorbidity index; ECOG-PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; LN, lymph node
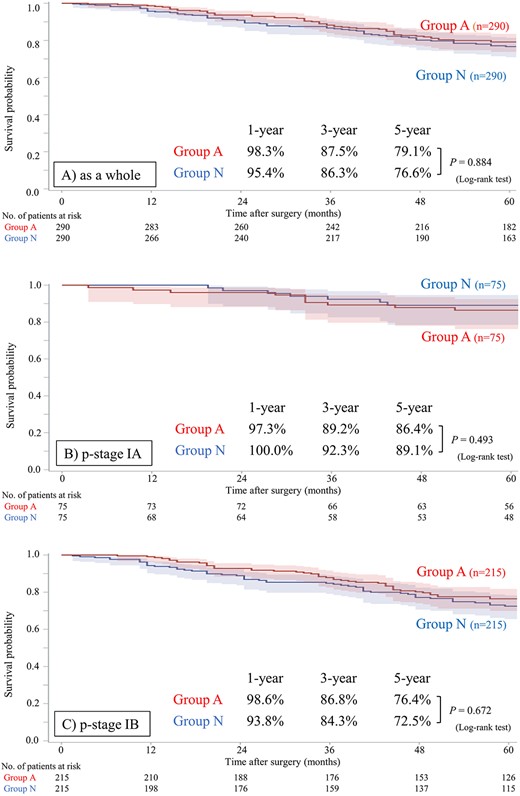
Postoperative overall survival in groups A and N after propensity score matching. (A) As a whole, (B) in p-stage IA, (C) in p-stage IB subset. group A, received postoperative adjuvant chemotherapy; and group N, no adjuvant chemotherapy.
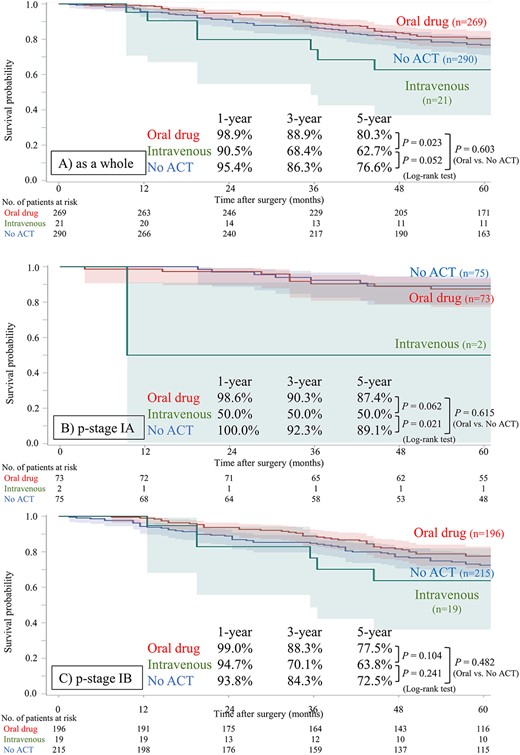
Postoperative overall survival stratified according to the agent for adjuvant chemotherapy after propensity score matching. (A) As a whole, (B) in p-stage IA, (C) in p-stage IB subset. The intravenous group includes patients who were administrated cisplatin-doublet or a single intravenous agent.
Discussion
We conducted a retrospective study using the Japanese Joint Committee of Lung Cancer Registry to evaluate the effects of postoperative ACT with UFT on the OS of elderly patients with p-stage IA (>2 cm) or p-stage IB NSCLC, using PS matching to eliminate selection bias. Our results showed that ACT had no advantage in the postoperative OS of elderly patients with p-stage I NSCLC.
Japanese guidelines, based on the results of Kato et al. (6) and Hamada et al. (7), recommend the administration of UFT as ACT for patients with completely resected p-stage IA (>2 cm) and p-stage IB NSCLC (17). Contrastingly, in Western countries, the administration of ACT for patients with p-stage I NSCLC is not recommended (4) based on the results of a meta-analysis conducted by Pignon et al. (18). Kato et al. (6) conducted prospective randomized controlled trials (RCTs) on 999 patients with completely resected stage I NSCLC and demonstrated the efficacy of adjuvant UFT administration in patients with T2 disease, i.e. a tumor size of >3 cm and/or with pleural invasion (5-year OS; 85% in UFT group vs. 74% in control group, P = 0.005). Additionally, that study demonstrated the low frequency of severe AEs; none of the patients who were administered adjuvant UFT experienced grade ≥ 3 leukopenia, and only 1% of the patients experienced grade ≥ 3 nausea/vomiting. Thereafter, Hamada et al. (7) conducted a meta-analysis with six RCTs (6,19–23) when the TNM staging system for the 7th edition was under revision; they reported an improvement in OS with adjuvant UFT administration in patients with completely resected p-stage IA (>2 cm) NSCLC (5-year OS: 88% in UFT group vs. 82% in the control group, P = 0.011). However, all the aforementioned trials concerning UFT were conducted with patients younger than 75 years, and the efficacy of adjuvant UFT for elderly patients with p-stage I NSCLC remains uncertain.
This large-cohort retrospective study based on Japanese real-world data revealed that administration of ACT was not associated with improvement of postoperative OS in patients older than 75 years with p-stage IA (>2 cm) and p-stage IB NSCLC, despite the recommendation of Japanese guidelines for administration of ACT using UFT, regardless of patients’ age (17). We assume that the discrepancy between Harada et al.’s (7) results and ours was partially due to the difference in the remaining natural lifetime. Naturally, younger patients have a relatively long life expectancy if their diseases do not recur, and the influence of ACT which prevents disease recurrence on postoperative OS may be more effective. In contrast, elderly patients have shorter expected remaining natural lifetime than younger patients, regardless of whether they experience recurrence or not, which may decrease the efficacy of ACT on OS compared to that in younger patients. Other factors, such as incomplete administration regimen may influence the discrepancy. According to Kato et al. (6), as mentioned above, the frequency of severe AEs (≥G3) in patients who were administered adjuvant UFT is low. However, they also reported that 17 and 13% of patients who were administered adjuvant UFT experienced G1-2 anorexia and nausea/vomiting, respectively. Elderly patients have generally impaired organ functions compared to young patients; thus, these non-severe AEs may greatly affect general condition and, consequently, readily lead to discontinuation of ACT, and in worse cases, lead to worsened general condition and decreased remaining lifetime. We consider that these factors intricately influence the postoperative course of elderly patients and counteract the effect of UFT on improving OS. In addition, our findings revealed that neither administration of UFT nor intravenous agents, including cisplatin doublet, contributed to the improvement of postoperative survival for elderly patients. Some previous studies were conducted to evaluate the superiority of intravenous agents over UFT in the p-stage IB cohort; however, no evidence of the superiority of intravenous agents over UFT was reported (24,25). According to these results, despite UFT administration being tolerable in postoperative elderly patients considering the results of Kunitoh et al. who examined a cohort including 10% of patients aged >75 years (10), we consider that ACT administration for elderly patients with completely resected p-stage IA (>2 cm) and stage IB NSCLC is not recommended.
Recently, the ADAURA trial was conducted to evaluate the efficacy of osimertinib as an ACT agent for patients with completely resected p-stage IB–IIIA NSCLC harboring EGFR mutation, and it showed remarkable results (26). Osimertinib may improve postoperative OS even for elderly patients with p-stage I disease (it is not currently approved for p-stage I disease in Japan); however, its cost-effectiveness should be considered. According to the 2017 annual report of the Japanese Association for Thoracic Surgery, there are ~900 cases/year of patients older than 75 years with completely resected p-stage IB adenocarcinoma (27). Assuming that 50% of patients harbor EGFR mutations and 30% of these patients receive ACT with osimertinib, the cost amounts to ~USD 24 million/year. Almost 80–90% of this cost is defrayed out of the National Treasury of the Japanese Medical System, making the use of this agent as an adjuvant for elderly patients a cause for concern.
The current study has some limitations. First, its retrospective design was a major limitation. Although we used the PS matching method to minimize selection bias, complete bias elimination was impossible. Additionally, we used the CCI (although the score was calculated excluding two factors) to assess the preoperative comorbid status. However, the CCI has been reported to be a prospectively applicable method, and its usefulness has not been verified in a retrospective setting. Second, we were unable to analyze data concerning the details of agents used including drug delivery and toxicity, important factors in evaluating the efficacy of ACT, because these data were not recorded in the nationwide registry. Third, the status regarding the presence/absence of driver oncogenes, especially EGFR mutations, was reported for only ~40% of the patients. Recent studies have reported differences in the efficacy of ACT between EGFR mutation-positive and negative patients (28,29), which may have affected our results. Despite these limitations, we believe that our study can help researchers considerably and influence the future treatment of stage I NSCLC. To the best of our knowledge, ours is the first study to investigate the benefit of ACT for elderly patients with p-stage I NSCLC using large-scale, real-world data. To confirm the efficacy of ACT for elderly patients, well-designed prospective RCTs are needed in the future.
To conclude, our results suggest that ACT for completely resected p-stage IA (>2 cm) and stage IB NSCLC has no benefit to be recommended for elderly patients. Considering the risk of AEs, even if tolerable in some patients, we do not recommend ACT for elderly patients with stage I NSCLC.
Acknowledgements
The authors wish to thank all the contributors at the participating institutions. This work was supported by the Japan Lung Cancer Society, the Japanese Association for Chest Surgery, the Japanese Respiratory Society, the Japan Society for Respiratory Endoscopy and the Japanese Association for Thoracic Surgery.
Conflict of interest statement
None declared.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial or not-for-profit sectors.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.


