-
PDF
- Split View
-
Views
-
Cite
Cite
Kazuma Sasamura, Takashi Soyano, Takuyo Kozuka, Takeshi Yuasa, Shinya Yamamoto, Junji Yonese, Masahiko Oguchi, Ryoichi Yoshimura, Yasuo Yoshioka, Outcomes of intensity-modulated radiation therapy for intermediate- or high-risk prostate cancer: a single-institutional study, Japanese Journal of Clinical Oncology, Volume 52, Issue 2, February 2022, Pages 170–178, https://doi.org/10.1093/jjco/hyab167
Close - Share Icon Share
Abstract
There are few reports from Japan about the outcomes of intensity-modulated radiation therapy for localized prostate cancer. This study was aimed at assessing the efficacy and toxicity of intensity-modulated radiation therapy in patients with intermediate- or high-risk prostate cancer.
We conducted a review of the data, retrieved from our institutional database, of patients who had received intensity-modulated radiation therapy for localized prostate cancer at a radiation dose of 78 Gy in 39 fractions. Data of 201 patients with intermediate-risk prostate cancer and 311 patients with high-risk prostate cancer were analyzed.
The median follow-up period after the completion of intensity-modulated radiation therapy was 100 months (range, 24–154). The rates of cause-specific survival, overall survival, metastasis-free survival and biochemical recurrence-free survival in the intermediate-risk patients were 99, 95, 95 and 94% at 5 years and 99, 91, 90 and 86% at 8 years, respectively; the corresponding rates in the high-risk patients were 100, 97, 91 and 84% at 5 years and 96, 92, 84 and 76% at 8 years, respectively. The crude incidence of late grade 2–3 genitourinary toxicity was 28.1%, and that of late grade 3 genitourinary toxicity was 2.0%. The crude incidence of late grade 2 gastrointestinal toxicity was 5.1%, and there were no cases of late grade 3 gastrointestinal toxicity.
Our data demonstrated that intensity-modulated radiation therapy is effective for patients with localized intermediate-risk or high-risk prostate cancer while having minimal toxicity.
Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy among men in developed countries (1–3). Methods for definitive treatment of prostate cancer include surgery and radiation therapy (RT), with or without androgen deprivation therapy (ADT). A number of RT modalities have been developed for patients with localized prostate cancer, including external beam RT (EBRT), low-dose rate brachytherapy and high-dose rate brachytherapy. Several retrospective and a few prospective studies have shown a dose–response relationship in RT of prostate cancer (4–9). In the history of EBRT, 3-dimensional conformal RT (3D-CRT) has replaced 2-dimensional RT, enabling dose-escalation to achieve better outcomes. Next, intensity-modulated RT (IMRT), an advanced form of 3D-CRT, was developed, which can deliver non-uniform beam intensities to an irregular target volume to create a highly sculpted dose distribution. IMRT enables safe delivery of increased doses of radiation to the target (prostate gland and seminal vesicles) with concurrent dose reductions to the adjacent normal tissues (e.g. rectum and bladder) (10). A number of studies worldwide have reported on the effectiveness and toxicities of IMRT for localized prostate cancer. However, there are very few reports of large-scale trials from Japan on the outcomes of IMRT using a uniform prescription dose in patients with intermediate- and high-risk prostate cancer. The purpose of this study conducted at a single institution in Japan was to evaluate the long-term effectiveness and toxicities of IMRT in patients with localized prostate cancer.
Patients and methods
Patients
This study was conducted with the approval of the institutional review board of out institute (approved number: 2020-GA-1214). For this study, low risk was defined by a pretreatment serum prostate-specific antigen (PSA) level of <10 ng/mL, Gleason score of ≤6 and stage T1c to T2a cancer, intermediate risk by a PSA level of ≥10 but <20 ng/mL, Gleason score of 7 or stage T2b to T2c cancer, and high risk by a PSA level of ≥20 ng/mL, Gleason score of ≥8 or stage T3 to T4 cancer. The T stage was assessed in accordance with the classification proposed by the Union for International Cancer Control 8th edition, 2017. Between May 2007 and August 2014, 682 patients with clinically localized prostate cancer with the histological subtype of adenocarcinoma were treated by IMRT in fractions of 2.0 Gy. We excluded 77 patients who were scheduled prescribing a total dose of less than 78 Gy from the analysis; thus, low-risk patients, all of whom had been prescribed a radiation dose of less than 78 Gy, were excluded from the study. Additionally, we excluded 2 patients whose irradiation was suspended in the middle because they had to treat other illness quickly. In addition, we excluded 6 patients who had received high-intensity focused ultrasound or holmium enucleation of the prostate prior to the RT from the analysis. We excluded 7 patients who had been diagnosed as having castration-resistant prostate cancer before the start of RT. Furthermore, we excluded 4 patients for whom adequate information on clinical staging was not available. We also excluded 14 patients who were lost to follow-up within 2 years after the end of RT. We also excluded 60 patients who were inserted a fiducial gold marker 1–2 weeks prior to RT planning. We conducted an analysis of the data of the remaining 512 patients, whose clinical characteristics are shown in Table 1. The authors note that the follow-up of 216 of the 512 patients had already lost at the timing of this study survey in 2021, whose median follow-up period was 87 months.
| Characteristic . | All patients . | Intermediate-risk patients . | High-risk patients . |
|---|---|---|---|
| No. of patients | 512 | 201 | 311 |
| Age (years) | |||
| Median | 71 | 70 | 71 |
| Range | 49–84 | 49–83 | 53–84 |
| T classification | |||
| T1 | 135 | 88 | 47 |
| T2 | 206 | 113 | 93 |
| T3 | 167 | 0 | 167 |
| T4 | 4 | 0 | 4 |
| Gleason score | |||
| ≤6 | 27 | 23 | 4 |
| 7 | 252 | 178 | 74 |
| ≥8 | 233 | 0 | 233 |
| Pretreatment PSA (ng/ml) | |||
| <10 | 225 | 126 | 99 |
| 10-20 | 159 | 75 | 84 |
| ≥20 | 128 | 0 | 128 |
| Median | 11.3 | 8.1 | 16.0 |
| Range | 1.9–499.0 | 2.4–19.6 | 1.9–499.0 |
| Androgen deprivation therapy | |||
| ≥2 years | 74 | 7 | 67 |
| 12 months | 183 | 36 | 147 |
| 6 months | 126 | 50 | 76 |
| None | 129 | 108 | 21 |
| Range (years) | 0–11.4 | 0–5.1 | 0–11.4 |
| Follow-up (months) | |||
| Median | 100 | 99 | 100 |
| Range | 24–154 | 24–149 | 24–154 |
| Characteristic . | All patients . | Intermediate-risk patients . | High-risk patients . |
|---|---|---|---|
| No. of patients | 512 | 201 | 311 |
| Age (years) | |||
| Median | 71 | 70 | 71 |
| Range | 49–84 | 49–83 | 53–84 |
| T classification | |||
| T1 | 135 | 88 | 47 |
| T2 | 206 | 113 | 93 |
| T3 | 167 | 0 | 167 |
| T4 | 4 | 0 | 4 |
| Gleason score | |||
| ≤6 | 27 | 23 | 4 |
| 7 | 252 | 178 | 74 |
| ≥8 | 233 | 0 | 233 |
| Pretreatment PSA (ng/ml) | |||
| <10 | 225 | 126 | 99 |
| 10-20 | 159 | 75 | 84 |
| ≥20 | 128 | 0 | 128 |
| Median | 11.3 | 8.1 | 16.0 |
| Range | 1.9–499.0 | 2.4–19.6 | 1.9–499.0 |
| Androgen deprivation therapy | |||
| ≥2 years | 74 | 7 | 67 |
| 12 months | 183 | 36 | 147 |
| 6 months | 126 | 50 | 76 |
| None | 129 | 108 | 21 |
| Range (years) | 0–11.4 | 0–5.1 | 0–11.4 |
| Follow-up (months) | |||
| Median | 100 | 99 | 100 |
| Range | 24–154 | 24–149 | 24–154 |
| Characteristic . | All patients . | Intermediate-risk patients . | High-risk patients . |
|---|---|---|---|
| No. of patients | 512 | 201 | 311 |
| Age (years) | |||
| Median | 71 | 70 | 71 |
| Range | 49–84 | 49–83 | 53–84 |
| T classification | |||
| T1 | 135 | 88 | 47 |
| T2 | 206 | 113 | 93 |
| T3 | 167 | 0 | 167 |
| T4 | 4 | 0 | 4 |
| Gleason score | |||
| ≤6 | 27 | 23 | 4 |
| 7 | 252 | 178 | 74 |
| ≥8 | 233 | 0 | 233 |
| Pretreatment PSA (ng/ml) | |||
| <10 | 225 | 126 | 99 |
| 10-20 | 159 | 75 | 84 |
| ≥20 | 128 | 0 | 128 |
| Median | 11.3 | 8.1 | 16.0 |
| Range | 1.9–499.0 | 2.4–19.6 | 1.9–499.0 |
| Androgen deprivation therapy | |||
| ≥2 years | 74 | 7 | 67 |
| 12 months | 183 | 36 | 147 |
| 6 months | 126 | 50 | 76 |
| None | 129 | 108 | 21 |
| Range (years) | 0–11.4 | 0–5.1 | 0–11.4 |
| Follow-up (months) | |||
| Median | 100 | 99 | 100 |
| Range | 24–154 | 24–149 | 24–154 |
| Characteristic . | All patients . | Intermediate-risk patients . | High-risk patients . |
|---|---|---|---|
| No. of patients | 512 | 201 | 311 |
| Age (years) | |||
| Median | 71 | 70 | 71 |
| Range | 49–84 | 49–83 | 53–84 |
| T classification | |||
| T1 | 135 | 88 | 47 |
| T2 | 206 | 113 | 93 |
| T3 | 167 | 0 | 167 |
| T4 | 4 | 0 | 4 |
| Gleason score | |||
| ≤6 | 27 | 23 | 4 |
| 7 | 252 | 178 | 74 |
| ≥8 | 233 | 0 | 233 |
| Pretreatment PSA (ng/ml) | |||
| <10 | 225 | 126 | 99 |
| 10-20 | 159 | 75 | 84 |
| ≥20 | 128 | 0 | 128 |
| Median | 11.3 | 8.1 | 16.0 |
| Range | 1.9–499.0 | 2.4–19.6 | 1.9–499.0 |
| Androgen deprivation therapy | |||
| ≥2 years | 74 | 7 | 67 |
| 12 months | 183 | 36 | 147 |
| 6 months | 126 | 50 | 76 |
| None | 129 | 108 | 21 |
| Range (years) | 0–11.4 | 0–5.1 | 0–11.4 |
| Follow-up (months) | |||
| Median | 100 | 99 | 100 |
| Range | 24–154 | 24–149 | 24–154 |
Treatment
The treatment planning was conducted using the Eclipse treatment planning system (Varian Medical Systems, Palo Alto, CA, USA). For the treatment planning, 1 hour after glycerin enema and collection of urine, computed tomography (CT) and magnetic resonance imaging scanning were carried out on the same day. For non-T3b cases, the clinical target volume (CTV) was defined as the prostate gland (and any extracapsular lesion) plus a 5-mm margin, then the proximal seminal vesicles were added, and finally, the rectum and bladder were eliminated. For T3b cases, the CTV was defined as the prostate (and any extracapsular lesion) plus a 5-mm margin, and then the seminal vesicles were added, and finally, the rectum was eliminated. The planning target volume (PTV) was defined as the CTV plus a 5-mm margin. The modified PTV was defined as PTV minus the rectum. None of the patients received pelvic lymph node irradiation. The patients’ rectum wall and bladder wall were contoured as the organs at risk (OARs). The thickness of the rectum wall and the bladder wall were contoured as 4 mm. In addition, if the small intestine or colon existed near the PTV, these organs were also contoured as OARs. For the dose calculation, we used the anisotropic analytical algorithm. IMRT was delivered with 10-MV photon beams.
We administered a total dose of 78 Gy in 39 fractions to 95% of the modified PTV. Optimization was performed using dose-volume constraints. The parameters that were used to produce the desired dose distribution were as follows. As for the modified PTV, the dose covering 99% of the target volume (D99%) should be more than 95% of the prescription dose. Basically, the maximum dose heterogeneity allowable in the modified PTV should be 10%. The mean dose of the modified PTV should be less than 105% of the prescription dose. In the overlap between PTV and rectum, D99% should be more than 85.2% of the prescription dose and should be less than 91.5% of the prescription dose. As for the prostate, the minimum dose should be more than 99.5% of the prescription dose. As for the rectum wall, the constraints was set that no greater than 60% of the volume should receive >40 Gy (V40 Gy < 60%), that no greater than 35% of the volume should receive >60 Gy (V60 Gy < 35%), that no greater than 25% of the volume should receive >70 Gy (V70 Gy < 25%) and that no greater than 2% of the volume should receive >78 Gy (V78 Gy < 2%). As for the bladder wall, the constraints were set that no greater than 60% of the volume should receive >40 Gy (V40 Gy < 60%) and that no greater than 35% of the volume should receive >70 Gy (V70 Gy < 35%). In addition, the limits that no greater than 0.5 mL of the colon volume should receive >65 Gy (V65 Gy < 0.5 mL) and that no greater than 0.5 mL of the small intestine wall volume should receive >60 Gy (V60 Gy < 0.5 mL) were also used.
The IMRT was delivered using the Clinac 21EX (Varian Medical Systems, Palo Alto, CA, USA) linear accelerator. All patients were treated in the supine position. For fixation, we used a thermoplastic shell. We used 2D-matching with an on-board imager for verifying the patient position at each irradiation. In addition, from July 2008, we used cone-beam CT for verifying the patient position a few times during the irradiation sessions. For every treatment fraction, patients were positioned based on the positions of the pelvic bone. Patients were encouraged to defecate before irradiation with or without purgatives and to collect urine 1 hour before irradiation.
ADT consisted of a luteinizing hormone-releasing hormone agonist and antiandrogen (i.e. combined androgen blockade). ADT was used at the discretion of the attending urologist for the intermediate- and high-risk patients. Our general principle has now been established as administering 6 months of neoadjuvant ADT for the intermediate-risk patients, or as administering ADT for 2 years in total, including 6 months of neoadjuvant, for the high-risk patients. However, in not a small number of cases especially treated before about 2010, duration of ADT was less than 2 years for the high-risk patients. This was because the evidence had not well permeated the clinical practice or some urologists expected that the escalated dose of 78 Gy would compensate for shortening the duration of ADT. In addition, ADT was discontinued if the patient requested to do so because of adverse events of ADT.
Analysis
The actuarial rates of overall survival, cause-specific survival, metastasis-free survival and biochemical recurrence-free survival, as well as the cumulative incidences of late toxicities, were calculated using the Kaplan–Meier method. Biochemical failure was defined as the nadir value of PSA plus 2 ng/mL, in accordance with the recommendations by the Radiation Therapy Oncology Group/American Society for Therapeutic Radiology and Oncology Phoenix Consensus Conference. Physician-reported toxicity was prospectively evaluated during RT and at each follow-up visit and the evaluation was enrolled in our institutional database. Adverse events were evaluated using the Common Terminology Criteria for Adverse Events, version 4.0. P values of <0.05 were considered statistically significant. We performed all the statistical analyses using IBM SPSS Statistics version 22.0.
Results
Clinical outcomes
The median follow-up period after the completion of IMRT was 100 months (range, 24–154). For the 512 patients overall, the actuarial rates of cause-specific survival, overall survival, metastasis-free survival and biochemical recurrence-free survival were 100, 97, 92 and 88% at 5 years and 98, 92, 87 and 80% at 8 years, respectively. The corresponding rates for the 201 intermediate-risk patients were 99, 95, 95 and 94% at 5 years and 99, 91, 90 and 86% at 8 years, respectively (Fig. 1A–D), and those for the 311 high-risk patients were 100, 97, 91 and 84% at 5 years and 96, 92, 84 and 76% at 8 years (Fig. 1A–D). Biochemical failure was observed in 102 of the 512 patients (20%). Clinical recurrence or metastasis was observed in 42 of the 512 patients (8%). Forty-one patients died of causes other than prostate cancer, whereas 12 died of prostate cancer, including 2 intermediate- and 10 high-risk patients. Kaplan–Meier curves of the biochemical recurrence-free survival rate classified by the more detailed risk classification by the National Comprehensive Cancer Network (NCCN) (11), including favorable intermediate-risk, unfavorable intermediate-risk, NCCN-high-risk and NCCN-very-high-risk, are shown in Fig. 2. The biochemical recurrence-free survival rate of the NCCN-very-high-risk patients was significantly worse than that of the NCCN-high-risk patients (P ≤ 0.001). Kaplan–Meier curves of the high-risk patients classified by the use and duration of ADT are shown in Fig. 3. In the high-risk patients, use of ADT was significantly associated with an improved biochemical recurrence-free survival rate (P = 0.004). In the high-risk patients, more than 2 years of ADT tended to improve the biochemical recurrence-free survival rate, but this difference was not statistically significant (P = 0.101). In the intermediate-risk patients, use of ADT was not significantly associated with a biochemical recurrence-free survival rate (P = 0.502).
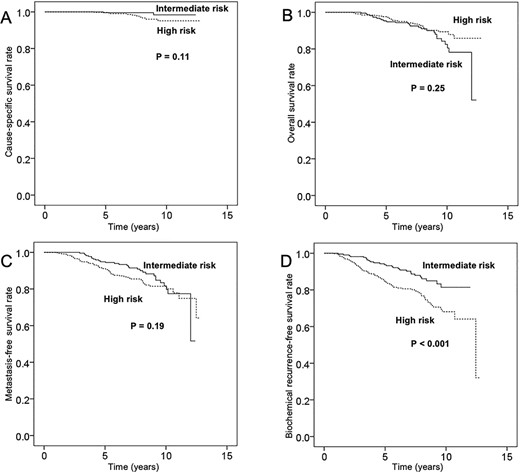
Rates of (A) cause-specific survival, (B) overall survival, (C) metastasis-free survival and (D) biochemical recurrence-free survival in patients with intermediate-risk and high-risk localized prostate cancer.
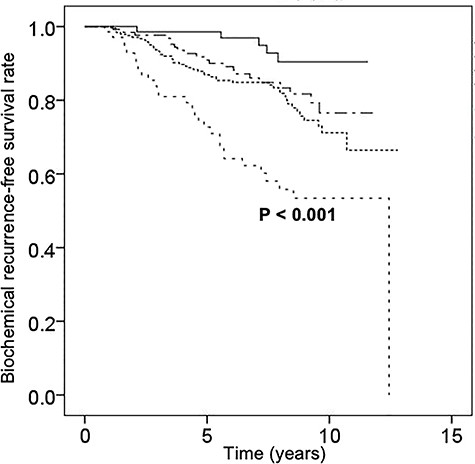
Kaplan–Meier curves of biochemical recurrence-free survival in patients with favorable intermediate-risk prostate cancer (n = 74, continuous straight line), with unfavorable intermediate-risk prostate cancer (n = 127, uneven dotted line), with National Comprehensive Cancer Network (NCCN)-high-risk prostate cancer (n = 242, even dotted line) and with NCCN-very-high-risk prostate cancer (n = 69, spaced even-dotted line).
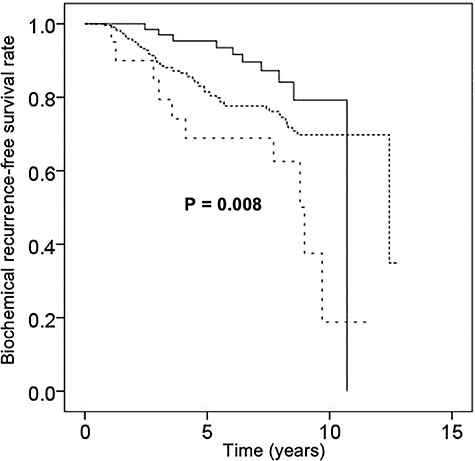
Kaplan–Meier curves of biochemical recurrence-free survival in patients with high-risk prostate cancer with the use of ADT of more than 2 years (n = 67, continuous straight line), with the use of ADT of less than 2 years (n = 223, even dotted line) and without the use of ADT (n = 21, spaced even-dotted line).
Toxicity
A summary of adverse events is listed in Table 2. Acute urinary toxicities consisted mostly of urinary frequency/urgency and urinary tract obstruction. The total dose was reduced to 74 Gy in one patient due to a grade 2 urinary pain that developed during IMRT course. Acute gastrointestinal toxicity was represented mostly by anal pain. There were no cases of grade3, 4 or 5 acute toxicity pertaining to either system. Grade 2 acute toxicity occurred in 307 patients (60%), including 172 patients (34%) with urinary frequency/urgency, 83 patients (16%) with urinary tract obstruction and 77 patients (15%) with anal pain.
| Toxicity . | Acute toxicity Grade . | Late toxicity Grade . | ||||
|---|---|---|---|---|---|---|
| 2 . | 3 . | 4 . | 2 . | 3 . | 4 . | |
| Genitourinary toxicity | ||||||
| Hematuria | - | - | - | 8 (2) | 6 (1) | 0 |
| Urinary incontinence | - | - | - | 2 (0) | 0 | 0 |
| Urinary frequency/urgency | 172 (34) | 0 | 0 | 75 (15) | 0 | 0 |
| Urinary tract obstruction | 83 (16) | 0 | 0 | 59 (11) | 4 (1) | 0 |
| Urinary tract pain | 8 (2) | 0 | 0 | - | - | - |
| Gastrointestinal toxicity | ||||||
| Anal pain | 77 (15) | 0 | 0 | 3 (1) | 0 | 0 |
| Proctitis | 17 (3) | 0 | 0 | 14 (3) | 0 | 0 |
| Rectal hemorrhage | 3 (1) | 0 | 0 | 9 (2) | 0 | 0 |
| Toxicity . | Acute toxicity Grade . | Late toxicity Grade . | ||||
|---|---|---|---|---|---|---|
| 2 . | 3 . | 4 . | 2 . | 3 . | 4 . | |
| Genitourinary toxicity | ||||||
| Hematuria | - | - | - | 8 (2) | 6 (1) | 0 |
| Urinary incontinence | - | - | - | 2 (0) | 0 | 0 |
| Urinary frequency/urgency | 172 (34) | 0 | 0 | 75 (15) | 0 | 0 |
| Urinary tract obstruction | 83 (16) | 0 | 0 | 59 (11) | 4 (1) | 0 |
| Urinary tract pain | 8 (2) | 0 | 0 | - | - | - |
| Gastrointestinal toxicity | ||||||
| Anal pain | 77 (15) | 0 | 0 | 3 (1) | 0 | 0 |
| Proctitis | 17 (3) | 0 | 0 | 14 (3) | 0 | 0 |
| Rectal hemorrhage | 3 (1) | 0 | 0 | 9 (2) | 0 | 0 |
Values represent the patient numbers (percentages). Grade: the Common Terminology Criteria for Adverse Events, version 4.0.
| Toxicity . | Acute toxicity Grade . | Late toxicity Grade . | ||||
|---|---|---|---|---|---|---|
| 2 . | 3 . | 4 . | 2 . | 3 . | 4 . | |
| Genitourinary toxicity | ||||||
| Hematuria | - | - | - | 8 (2) | 6 (1) | 0 |
| Urinary incontinence | - | - | - | 2 (0) | 0 | 0 |
| Urinary frequency/urgency | 172 (34) | 0 | 0 | 75 (15) | 0 | 0 |
| Urinary tract obstruction | 83 (16) | 0 | 0 | 59 (11) | 4 (1) | 0 |
| Urinary tract pain | 8 (2) | 0 | 0 | - | - | - |
| Gastrointestinal toxicity | ||||||
| Anal pain | 77 (15) | 0 | 0 | 3 (1) | 0 | 0 |
| Proctitis | 17 (3) | 0 | 0 | 14 (3) | 0 | 0 |
| Rectal hemorrhage | 3 (1) | 0 | 0 | 9 (2) | 0 | 0 |
| Toxicity . | Acute toxicity Grade . | Late toxicity Grade . | ||||
|---|---|---|---|---|---|---|
| 2 . | 3 . | 4 . | 2 . | 3 . | 4 . | |
| Genitourinary toxicity | ||||||
| Hematuria | - | - | - | 8 (2) | 6 (1) | 0 |
| Urinary incontinence | - | - | - | 2 (0) | 0 | 0 |
| Urinary frequency/urgency | 172 (34) | 0 | 0 | 75 (15) | 0 | 0 |
| Urinary tract obstruction | 83 (16) | 0 | 0 | 59 (11) | 4 (1) | 0 |
| Urinary tract pain | 8 (2) | 0 | 0 | - | - | - |
| Gastrointestinal toxicity | ||||||
| Anal pain | 77 (15) | 0 | 0 | 3 (1) | 0 | 0 |
| Proctitis | 17 (3) | 0 | 0 | 14 (3) | 0 | 0 |
| Rectal hemorrhage | 3 (1) | 0 | 0 | 9 (2) | 0 | 0 |
Values represent the patient numbers (percentages). Grade: the Common Terminology Criteria for Adverse Events, version 4.0.
Late urinary toxicity consisted mostly of urinary frequency/urgency and urinary tract obstruction. The most frequent late gastrointestinal toxicity was rectal bleeding. There were no cases of grade 4 or 5 late toxicity pertaining to either organ system. Grade 3 late toxicity occurred in 10 patients, including hematuria in 6 patients (1%) and urinary tract obstruction in 4 patients (1%). Grade 2 late toxicity occurred in 159 patients (31%), including 75 patients (15%) with urinary frequency/urgency and 58 patients (11%) with urinary tract obstruction. The actuarial incidence rate of late grade 2 or 3 genitourinary toxicity was 26% at 5 years and 29% at 8 years, and that of late grade 3 genitourinary toxicity was 1% at 5 years and 1% at 8 years (Fig. 4A). The actuarial incidence rate of late grade 2 or 3 gastrointestinal toxicity was 5% at 5 years and 6% at 8 years, and that of late grade 3 gastrointestinal toxicity was 0 at 5 years and 0 at 8 years (Fig. 4B). The multivariate analysis for predictors of grade 2 or higher late GU and GI toxicities is shown in Table 3. Age ≥ 70 years was a significant predictor of both GU and GI late toxicity of grade 2 or higher. Acute GU toxicity of grade 2 or higher was significant predictors of late GU toxicity of grade 2. Acute GI toxicity of grade 1 or higher was significant predictors of late GI toxicity of grade 2.
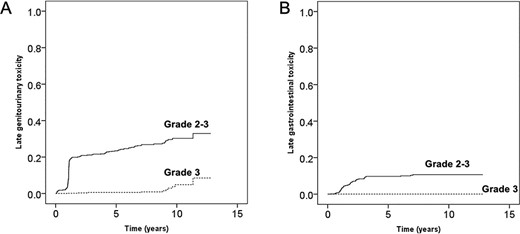
Cumulative incidence rate of grade 2 or higher late toxicity. Grading was based on the Common Terminology Criteria for Adverse Events, version 4.0. (A) Genitourinary toxicity. (B) Gastrointestinal toxicity.
| . | Late GU ≥ grade 2 . | Late GI ≥ grade 2 . |
|---|---|---|
| Age > 70 | P < 0.001 | P = 0.049 |
| ADT | P = 0.823 | P = 0.406 |
| Acute GU toxicity (≥grade 2) | P < 0.001 | P = 0.694 |
| Acute GI toxicity (≥grade 1) | P = 0.753 | P = 0.016 |
| Mean dose of bladder wall (>35 Gy) | P = 0.464 | P = 0.449 |
| Mean dose of rectum wall (>43 Gy) | P = 0.150 | P = 0.574 |
| . | Late GU ≥ grade 2 . | Late GI ≥ grade 2 . |
|---|---|---|
| Age > 70 | P < 0.001 | P = 0.049 |
| ADT | P = 0.823 | P = 0.406 |
| Acute GU toxicity (≥grade 2) | P < 0.001 | P = 0.694 |
| Acute GI toxicity (≥grade 1) | P = 0.753 | P = 0.016 |
| Mean dose of bladder wall (>35 Gy) | P = 0.464 | P = 0.449 |
| Mean dose of rectum wall (>43 Gy) | P = 0.150 | P = 0.574 |
Abbreviations: ADT: androgen deprivation therapy; GI: gastrointestinal; GU: genitourinary.
| . | Late GU ≥ grade 2 . | Late GI ≥ grade 2 . |
|---|---|---|
| Age > 70 | P < 0.001 | P = 0.049 |
| ADT | P = 0.823 | P = 0.406 |
| Acute GU toxicity (≥grade 2) | P < 0.001 | P = 0.694 |
| Acute GI toxicity (≥grade 1) | P = 0.753 | P = 0.016 |
| Mean dose of bladder wall (>35 Gy) | P = 0.464 | P = 0.449 |
| Mean dose of rectum wall (>43 Gy) | P = 0.150 | P = 0.574 |
| . | Late GU ≥ grade 2 . | Late GI ≥ grade 2 . |
|---|---|---|
| Age > 70 | P < 0.001 | P = 0.049 |
| ADT | P = 0.823 | P = 0.406 |
| Acute GU toxicity (≥grade 2) | P < 0.001 | P = 0.694 |
| Acute GI toxicity (≥grade 1) | P = 0.753 | P = 0.016 |
| Mean dose of bladder wall (>35 Gy) | P = 0.464 | P = 0.449 |
| Mean dose of rectum wall (>43 Gy) | P = 0.150 | P = 0.574 |
Abbreviations: ADT: androgen deprivation therapy; GI: gastrointestinal; GU: genitourinary.
Discussion
For the radical treatment of localized prostate cancer, several retrospective studies have shown better biochemical control rates and lower frequencies of grade ≥ 2 gastrointestinal toxicity with IMRT than with 3D-CRT (12–16). While there are some reports of the long-term clinical outcomes of IMRT for localized prostate cancer, e.g. the Memorial Sloan-Kettering Cancer Center reported the 10-year outcomes of high-dose IMRT (17); reports on the long-term outcomes of IMRT from Japan have been few until date. There are some studies (18–30) reporting the long-term outcomes of IMRT in Japan and overseas with a fraction size of 2.0 Gy or less (Table 4). However, none of these researchers except Tanaka et al. included more than 500 patients in their study cohorts in Japan. We were therefore prompted to conduct the current study, which included 512 patients, with a median follow-up period of 8.3 years.
| . | . | . | . | . | . | . | Late toxicity ≥ . | Late toxicity . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Grade 2a (%) . | Grade 3a (%) . | ||
| Author . | No. of patients . | Risk group . | Median follow-up (months) . | Total dose (Gy)/no. of fx . | Androgen deprivation therapy (%) . | Biochemical control rate (%) (at years) . | GU . | GI . | GU . | GI . |
| Kobayashi (20) | 169 | Intermediate | 43 | 76/38 | 100 | 95.9 (5 years) | 8.9 | 5.5 | 0.3 | 0 |
| 133 | High | 100 | 87.2 (5 years) | |||||||
| Takeda (21) | 36 | Intermediate | 60 | 76–80/38–40 | 87.9 | 100 (5 years) | 6.4 | 5.7 | NA | 1.4 |
| 105 | High | 82.2 (5 years) | ||||||||
| Tanaka (22) | 205 | Low | 50 | 74/37 | 59.2 | 98.9 (3 years), 95.7 (5 years) | 11.4 | 4.3 | 0.3 | 2.3 |
| 450 | Intermediate | 74–76/37–38 | 94.9 (3 years), 91.4 (5 years) | |||||||
| 436 | High | 78/39 | 95.9 (3 years), 91.4 (5 years)b | |||||||
| Tomita (23) | 16 | Low | 102 | 74/37 | 100 | 100 (10 years) | 13.0 | 11.5 | 1.4 | 1.9 |
| 45 | Intermediate | 78/39 | 100 | 84 (10 years) | ||||||
| 147 | High | 90 (10 years)b | ||||||||
| Aizawa (24) | 268 | High | 114 | 78/39 | 100 | 72.7 (10 years)b | 19.4 | 5.2 | 3.7 | 2.4 |
| Aizawa (25) | 106 | Intermediate | 96 | 70–78/35–39 | 100 | 93.2 (5 years), 88.7 (10 years) | 15.1 | 6.7 | NA | 0 |
| Hoffman (28) | 29 | Low | 66 | 75.6/42 | 22.8 | NA | 14.9 | 5 | 1 | 1 |
| 71 | Intermediate | |||||||||
| 1 | High | |||||||||
| Pollack (29) | 101 | Intermediate | 68 | 76/38 | 19.8 | 78.6 (5 years) | 47.7 | 22.5 | 3.3 | 2 |
| 51 | High | 100 | ||||||||
| CHHiP (30) | 157 | Low | 63 | 74/37 | 96.8 | 96.7 (5 years) | 9.1c | 13.7c | 0.4 | 0 |
| 779 | Intermediate | 86.8 (5 years) | ||||||||
| 129 | High | 86.5 (5 years) | ||||||||
| Present study | 201 | Intermediate | 100 | 78/39 | 46.3 | 93.6 (5 years), 85.8 (8 years) | 26.6 | 4.7 | 1.8 | 0 |
| 311 | High | 93.2 | 83.7 (5 years), 75.9 (8 years) | |||||||
| . | . | . | . | . | . | . | Late toxicity ≥ . | Late toxicity . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Grade 2a (%) . | Grade 3a (%) . | ||
| Author . | No. of patients . | Risk group . | Median follow-up (months) . | Total dose (Gy)/no. of fx . | Androgen deprivation therapy (%) . | Biochemical control rate (%) (at years) . | GU . | GI . | GU . | GI . |
| Kobayashi (20) | 169 | Intermediate | 43 | 76/38 | 100 | 95.9 (5 years) | 8.9 | 5.5 | 0.3 | 0 |
| 133 | High | 100 | 87.2 (5 years) | |||||||
| Takeda (21) | 36 | Intermediate | 60 | 76–80/38–40 | 87.9 | 100 (5 years) | 6.4 | 5.7 | NA | 1.4 |
| 105 | High | 82.2 (5 years) | ||||||||
| Tanaka (22) | 205 | Low | 50 | 74/37 | 59.2 | 98.9 (3 years), 95.7 (5 years) | 11.4 | 4.3 | 0.3 | 2.3 |
| 450 | Intermediate | 74–76/37–38 | 94.9 (3 years), 91.4 (5 years) | |||||||
| 436 | High | 78/39 | 95.9 (3 years), 91.4 (5 years)b | |||||||
| Tomita (23) | 16 | Low | 102 | 74/37 | 100 | 100 (10 years) | 13.0 | 11.5 | 1.4 | 1.9 |
| 45 | Intermediate | 78/39 | 100 | 84 (10 years) | ||||||
| 147 | High | 90 (10 years)b | ||||||||
| Aizawa (24) | 268 | High | 114 | 78/39 | 100 | 72.7 (10 years)b | 19.4 | 5.2 | 3.7 | 2.4 |
| Aizawa (25) | 106 | Intermediate | 96 | 70–78/35–39 | 100 | 93.2 (5 years), 88.7 (10 years) | 15.1 | 6.7 | NA | 0 |
| Hoffman (28) | 29 | Low | 66 | 75.6/42 | 22.8 | NA | 14.9 | 5 | 1 | 1 |
| 71 | Intermediate | |||||||||
| 1 | High | |||||||||
| Pollack (29) | 101 | Intermediate | 68 | 76/38 | 19.8 | 78.6 (5 years) | 47.7 | 22.5 | 3.3 | 2 |
| 51 | High | 100 | ||||||||
| CHHiP (30) | 157 | Low | 63 | 74/37 | 96.8 | 96.7 (5 years) | 9.1c | 13.7c | 0.4 | 0 |
| 779 | Intermediate | 86.8 (5 years) | ||||||||
| 129 | High | 86.5 (5 years) | ||||||||
| Present study | 201 | Intermediate | 100 | 78/39 | 46.3 | 93.6 (5 years), 85.8 (8 years) | 26.6 | 4.7 | 1.8 | 0 |
| 311 | High | 93.2 | 83.7 (5 years), 75.9 (8 years) | |||||||
Abbreviation: NA: not available.
aCrude rate.
bOnly high-risk groups following the National Comprehensive Cancer Network classification.
cEstimated cumulative incidence at 5 years.
| . | . | . | . | . | . | . | Late toxicity ≥ . | Late toxicity . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Grade 2a (%) . | Grade 3a (%) . | ||
| Author . | No. of patients . | Risk group . | Median follow-up (months) . | Total dose (Gy)/no. of fx . | Androgen deprivation therapy (%) . | Biochemical control rate (%) (at years) . | GU . | GI . | GU . | GI . |
| Kobayashi (20) | 169 | Intermediate | 43 | 76/38 | 100 | 95.9 (5 years) | 8.9 | 5.5 | 0.3 | 0 |
| 133 | High | 100 | 87.2 (5 years) | |||||||
| Takeda (21) | 36 | Intermediate | 60 | 76–80/38–40 | 87.9 | 100 (5 years) | 6.4 | 5.7 | NA | 1.4 |
| 105 | High | 82.2 (5 years) | ||||||||
| Tanaka (22) | 205 | Low | 50 | 74/37 | 59.2 | 98.9 (3 years), 95.7 (5 years) | 11.4 | 4.3 | 0.3 | 2.3 |
| 450 | Intermediate | 74–76/37–38 | 94.9 (3 years), 91.4 (5 years) | |||||||
| 436 | High | 78/39 | 95.9 (3 years), 91.4 (5 years)b | |||||||
| Tomita (23) | 16 | Low | 102 | 74/37 | 100 | 100 (10 years) | 13.0 | 11.5 | 1.4 | 1.9 |
| 45 | Intermediate | 78/39 | 100 | 84 (10 years) | ||||||
| 147 | High | 90 (10 years)b | ||||||||
| Aizawa (24) | 268 | High | 114 | 78/39 | 100 | 72.7 (10 years)b | 19.4 | 5.2 | 3.7 | 2.4 |
| Aizawa (25) | 106 | Intermediate | 96 | 70–78/35–39 | 100 | 93.2 (5 years), 88.7 (10 years) | 15.1 | 6.7 | NA | 0 |
| Hoffman (28) | 29 | Low | 66 | 75.6/42 | 22.8 | NA | 14.9 | 5 | 1 | 1 |
| 71 | Intermediate | |||||||||
| 1 | High | |||||||||
| Pollack (29) | 101 | Intermediate | 68 | 76/38 | 19.8 | 78.6 (5 years) | 47.7 | 22.5 | 3.3 | 2 |
| 51 | High | 100 | ||||||||
| CHHiP (30) | 157 | Low | 63 | 74/37 | 96.8 | 96.7 (5 years) | 9.1c | 13.7c | 0.4 | 0 |
| 779 | Intermediate | 86.8 (5 years) | ||||||||
| 129 | High | 86.5 (5 years) | ||||||||
| Present study | 201 | Intermediate | 100 | 78/39 | 46.3 | 93.6 (5 years), 85.8 (8 years) | 26.6 | 4.7 | 1.8 | 0 |
| 311 | High | 93.2 | 83.7 (5 years), 75.9 (8 years) | |||||||
| . | . | . | . | . | . | . | Late toxicity ≥ . | Late toxicity . | ||
|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | . | . | . | Grade 2a (%) . | Grade 3a (%) . | ||
| Author . | No. of patients . | Risk group . | Median follow-up (months) . | Total dose (Gy)/no. of fx . | Androgen deprivation therapy (%) . | Biochemical control rate (%) (at years) . | GU . | GI . | GU . | GI . |
| Kobayashi (20) | 169 | Intermediate | 43 | 76/38 | 100 | 95.9 (5 years) | 8.9 | 5.5 | 0.3 | 0 |
| 133 | High | 100 | 87.2 (5 years) | |||||||
| Takeda (21) | 36 | Intermediate | 60 | 76–80/38–40 | 87.9 | 100 (5 years) | 6.4 | 5.7 | NA | 1.4 |
| 105 | High | 82.2 (5 years) | ||||||||
| Tanaka (22) | 205 | Low | 50 | 74/37 | 59.2 | 98.9 (3 years), 95.7 (5 years) | 11.4 | 4.3 | 0.3 | 2.3 |
| 450 | Intermediate | 74–76/37–38 | 94.9 (3 years), 91.4 (5 years) | |||||||
| 436 | High | 78/39 | 95.9 (3 years), 91.4 (5 years)b | |||||||
| Tomita (23) | 16 | Low | 102 | 74/37 | 100 | 100 (10 years) | 13.0 | 11.5 | 1.4 | 1.9 |
| 45 | Intermediate | 78/39 | 100 | 84 (10 years) | ||||||
| 147 | High | 90 (10 years)b | ||||||||
| Aizawa (24) | 268 | High | 114 | 78/39 | 100 | 72.7 (10 years)b | 19.4 | 5.2 | 3.7 | 2.4 |
| Aizawa (25) | 106 | Intermediate | 96 | 70–78/35–39 | 100 | 93.2 (5 years), 88.7 (10 years) | 15.1 | 6.7 | NA | 0 |
| Hoffman (28) | 29 | Low | 66 | 75.6/42 | 22.8 | NA | 14.9 | 5 | 1 | 1 |
| 71 | Intermediate | |||||||||
| 1 | High | |||||||||
| Pollack (29) | 101 | Intermediate | 68 | 76/38 | 19.8 | 78.6 (5 years) | 47.7 | 22.5 | 3.3 | 2 |
| 51 | High | 100 | ||||||||
| CHHiP (30) | 157 | Low | 63 | 74/37 | 96.8 | 96.7 (5 years) | 9.1c | 13.7c | 0.4 | 0 |
| 779 | Intermediate | 86.8 (5 years) | ||||||||
| 129 | High | 86.5 (5 years) | ||||||||
| Present study | 201 | Intermediate | 100 | 78/39 | 46.3 | 93.6 (5 years), 85.8 (8 years) | 26.6 | 4.7 | 1.8 | 0 |
| 311 | High | 93.2 | 83.7 (5 years), 75.9 (8 years) | |||||||
Abbreviation: NA: not available.
aCrude rate.
bOnly high-risk groups following the National Comprehensive Cancer Network classification.
cEstimated cumulative incidence at 5 years.
The clinical outcomes of the patients in our study were satisfactory. The 5-year biochemical control rates of 94 and 84% in the intermediate- and high-risk patients, 46 and 92% of whom had also received ADT, respectively, seemed comparable to other reports from Japan and overseas. The rates of grade 3 late toxicities, including GU (1.9%) and for GI (0.0%) toxicities, were acceptably low and comparable to that reported in the literature. However, there appeared to be room for improvement in regard to grade 2 late toxicities, including GU (28%) and GI (5%) toxicities, even though the rates were within the range reported in the literature (Table 4). This problem is mentioned again below.
Kobayashi et al. (20) reported the outcomes of IMRT at a prescribed dose of 76 Gy administered in 38 fractions in patients with for localized prostate cancer. They implanted fiducial gold markers into the prostate gland in all patients. The crude incidence rates of late grade 2–3 genitourinary toxicity and gastrointestinal toxicity were 8.9 and 5.5%, respectively. Takeda et al. (21) reported the outcomes of IMRT at a prescribed dose of 76-80 Gy administered in 38–40 fractions in patients with localized prostate cancer. They also implanted fiducial gold markers into the prostate in all patients. The crude incidence rates of late grade 2–3 genitourinary toxicity and gastrointestinal toxicity were 6.4 and 5.7%, respectively. As compared with these two studies, the crude rates of late grade 2–3 genitourinary toxicity seemed comparatively worse in our study (30.0%). We speculated that one of the reasons could be that we did not use fiducial gold markers for the patients in the current study. Some authors have reported that image guidance on the basis of fiducial gold markers provided a higher precision of prostate localization and consequently, lower toxicity (31). Therefore, we have decided, in principle, to use fiducial gold markers for all patients in our clinical practice. We propose to conduct a detailed comparison between IMRT with and without fiducial gold markers in the future, after the results become clear.
Use of ADT in combination with EBRT at a relatively lower radiation dose (65–70 Gy) has been clearly shown to improve the therapeutic outcomes in patients with intermediate- or high-risk prostate cancer (32,33). However, some researchers had suggested that such benefit of addition of ADT needs to be confirmed again in the context of combination with EBRT at a higher radiation dose (≥74 Gy) using modern techniques, such as IMRT. Zumsteg et al. (34) reported that short-term ADT significantly improved the biochemical relapse rate, distant metastasis rate and prostate cancer-specific mortality rate when administered in combination with high doses (≥81 Gy) of EBRT in patients with intermediate-risk prostate cancer. Bolla et al. (35) reported that addition of 6 months of ADT significantly improved the biochemical relapse rate and clinical progression-free survival when administered in combination with EBRT at a high dose (70–78 Gy) in patients with intermediate-risk prostate cancer. Kobayashi et al. (20) reported the outcomes of 169 intermediate-risk patients treated by IMRT at a prescribed dose of 76 Gy in 38 fractions plus ADT. They reported a high biochemical control rate of 95.9% at 5 years. In the present study, ADT was administered to only 46.2% of 201 intermediate-risk patients, but the biochemical control rate was still sufficiently high (93.6%) at 5 years. Takeda’s report (21) lends support to our results; they administered ADT to only 67% of their patients treated by IMRT, but they achieved a biochemical control rate of 100% at 5 years, although the number of patients in their study was small (36 intermediate-risk patients). These findings might suggest that, although the role of ADT is still important, not all intermediate-risk patients may need ADT in this high-dose IMRT era. The next step would be to identify the subgroup of intermediate-risk patients in whom ADT addition could be omitted.
The major limitations of this study can be attributed to its retrospective nature. Especially, the duration of ADT was not uniform or adhered to the current evidence strictly, i.e. more than 70% of the high-risk patients did not receive enough long-tern ADT (2 years or more). In addition, not a small number of patients had already been lost for follow-up at the timing of this study survey. Such heterogeneity of the patients and treatments should be considered as a possible bias of this study and may have affected the results significantly. However, the authors believe that this study makes an important contribution, because it provides long-term outcome data for localized prostate cancer patients treated by IMRT in Japan. As compared with other previous reports from Japan, we adopted a uniform dose of 78 Gy administered in 39 fractions for our study, with the study conducted on the largest number of patients (512 patients) so far, with a relatively long median follow-up period of 8.3 years.
Recently, moderately hypofractionated RT has been introduced as a new standard treatment option for prostate cancer utilizing IMRT. Moderate hypofractionation is defined as use of a fraction size of between 2.4 and 3.4 Gy (36), and its non-inferiority to conventional fractionation (1.8–2.0 Gy/fraction) has been demonstrated in several randomized phase 3 trials (30,37–39). We expect that the findings of the current study will provide useful information as one of the standard references for the treatment outcomes of IMRT using conventional fractionation for prostate cancer patients in Japan and may be referred to as a historical control in comparison to hypofractionated RT for localized prostate cancer.
In conclusion, we presented clinical data after IMRT in patients with intermediate- or high-risk prostate cancer. Our data showed that IMRT is effective for patients with localized intermediate- or high-risk prostate cancer. In addition, the incidence of severe adverse events was also relatively low.


