-
PDF
- Split View
-
Views
-
Cite
Cite
Tatsuya Ioka, Junji Furuse, Akira Fukutomi, Junki Mizusawa, Satoaki Nakamura, Nobuyoshi Hiraoka, Yoshinori Ito, Hiroshi Katayama, Makoto Ueno, Masafumi Ikeda, Kazuya Sugimori, Naohiro Okano, Kyoko Shimizu, Hiroaki Yanagimoto, Takuji Okusaka, Masato Ozaka, Akiko Todaka, Shoji Nakamori, Kazutoshi Tobimatsu, Naohiro Sata, Yohei Kawashima, Ayumu Hosokawa, Taketo Yamaguchi, Hiroyuki Miyakawa, Hiroki Hara, Nobumasa Mizuno, Hiroshi Ishii, Hepatobiliary and Pancreatic Oncology Group (HBPOG) of Japan Clinical Oncology Group (JCOG), Randomized phase II study of chemoradiotherapy with versus without induction chemotherapy for locally advanced pancreatic cancer: Japan Clinical Oncology Group trial, JCOG1106, Japanese Journal of Clinical Oncology, Volume 51, Issue 2, February 2021, Pages 235–243, https://doi.org/10.1093/jjco/hyaa198
Close - Share Icon Share
Abstract
Chemoradiotherapy is a treatment option for locally advanced pancreatic cancer. However, the efficacy of induction chemotherapy prior to chemoradiotherapy is uncertain. The aim of this randomized, multicentre phase II study is to evaluate the efficacy and safety of chemoradiotherapy with and without induction chemotherapy to determine the significance of induction chemotherapy.
Patients with locally advanced pancreatic cancer were randomly assigned to the chemoradiotherapy arm (Arm A) or induction chemotherapy followed by the chemoradiotherapy arm (Arm B). Patients in Arm A underwent radiotherapy with concurrent S-1. Patients in Arm B received induction gemcitabine for 12 weeks, and thereafter, only patients with controlled disease underwent the same chemoradiotherapy as Arm A. After chemoradiotherapy, gemcitabine was continued until disease progression or unacceptable toxicity in both arms. The primary endpoint was overall survival.
Amongst 102 patients enrolled, 100 were eligible for efficacy assessment. The probability of survival was greater in Arm B in the first 12 months, but the trend was reversed in the following periods (1-year survival 66.7 vs. 69.3%, 2-year survival 36.9 vs. 18.9%). The hazard ratio was 1.255 (95% confidence interval 0.816–1.930) in favour of Arm A. Gastrointestinal toxicity was slightly more frequent and three treatment-related deaths occurred in Arm A.
This study suggested that the chemoradiotherapy using S-1 alone had more promising efficacy with longer-term survival, compared with induction gemcitabine followed by chemoradiotherapy for locally advanced pancreatic cancer.
The study was registered at the UMIN Clinical Trials Registry as UMIN000006811.
Introduction
Pancreatic cancer (PC) is one of the leading causes of cancer-related death in the world (1). The prognosis of this disease is extremely poor, with a 5-year survival rate of <5%. Surgical resection offers the only chance for cure, although approximately half of patients already have metastases at the time of diagnosis (2). Nearly one-third of patients are diagnosed with locally advanced pancreatic cancer (LAPC) due to vascular invasion, particularly of the superior mesenteric artery or the celiac axis. Systemic chemotherapy is the mainstay of treatment for unresectable PC consisting of both LAPC and metastatic PC. Gemcitabine has been the standard treatment for over than a decade. Recently, FOLFIRINOX (leucovorin and fluorouracil plus irinotecan plus oxaliplatin) and gemcitabine plus nab-paclitaxel (GnP) have demonstrated a remarkable survival benefit compared with gemcitabine alone in patients with metastatic PC (3,4). These two regimens are now widely used for patients with unresectable PC with good performance status (PS), although their effectiveness in LAPC has not been confirmed in a randomized controlled trial.
Chemoradiotherapy (CRT) is also a treatment option for LAPC. Two randomized trials comparing CRT with gemcitabine alone have been reported; however, the results of these trials were contradictory (5,6). Therefore, the role of radiotherapy remains controversial, although there remains a possibility that CRT can improve long-term survival of patients with LAPC (6). Some retrospective studies have suggested that the use of induction chemotherapy prior to CRT could improve survival (7,8). Approximately 30% of patients with LAPC have occult metastases that progress rapidly within a few months. Administration of induction chemotherapy may help to identify those patients and spare them from potentially toxic radiotherapy. However, the efficacy of this strategy has not been confirmed.
The Hepatobiliary and Pancreatic Oncology Group (HBPOG) of Japan Clinical Oncology Group (JCOG) thus conducted a randomized phase II study to evaluate the efficacy and safety of CRT with and without induction chemotherapy to determine the significance of induction chemotherapy. The most promising CRT regimen is to be compared with systemic chemotherapy in a subsequent phase III trial.
Methods
Patients
The eligibility criteria for inclusion were as follows: histologically or cytologically proven adenocarcinoma of the pancreas; diagnosis of clinical stage III (T4N0-1 and M0) lesion according to the Union for International Cancer Control TNM version 7 by imaging (laparoscopy was not required); all pancreatic lesions and lymph node metastases included in a square radiation field (10 cm × 10 cm); no obvious direct invasion of the digestive tract; no active gastroduodenal ulcer and no ascites or pleural effusion. Patients also had to satisfy the following criteria: age between 20 and 80 years; Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1; no history of surgical resection for PC; no history of chemotherapy or radiotherapy for PC or other malignancies; adequate bone marrow, hepatic and renal function (leucocyte count ≥3500/mm3; haemoglobin ≥9.0 g/dl; platelet count ≥100 000/mm3; albumin ≥3.0 g/dl; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤100 IU/l (≤150 IU/l in patients with biliary drainage); total bilirubin ≤2.0 mg/dl (≤3.0 mg/dl in patients with biliary drainage); creatinine ≤1.2 mg/dl; creatinine clearance ≥50 ml/min). Written informed consent from all participants was also required. Patients were not eligible if they had any of the following conditions: active infection; pulmonary fibrosis or interstitial pneumonia; watery diarrhoea; severe complications, such as heart failure, renal failure and uncontrolled diabetes; active concomitant malignancy and psychiatric disorders. Furthermore, the following patients were also excluded: those undergoing treatment with flucytosine, phenytoin or warfarin potassium; women who were pregnant, breastfeeding or of childbearing potential and men who were willing to conceive a child.
The study protocol was approved by the JCOG Protocol Review Committee and the institutional review board of each participating institution and the study was performed in accordance with the guidelines of the Declaration of Helsinki and the Ethical Guidelines for Clinical Research of Japan. The JCOG Data and Safety Monitoring Committee, which is a standing committee, monitored patient safety and the progress of the trial. Pathological diagnoses for all enrolled patients were reviewed centrally by a study-specific pathology panel consisting of three pathologists. This study was registered at the UMIN Clinical Trials Registry as UMIN000006811 (http://www.umin.ac.jp/ctr/index.htm).
Randomization
After confirmation of the eligibility criteria at the JCOG Data Center, the patients were randomized to either the CRT arm (Arm A) or induction chemotherapy followed by CRT arm (Arm B), using a minimization method including a random component to balance the adjustment factors of institution and carbohydrate antigen 19-9 (CA19-9) level (<1000 or ≥1000 IU/ml). Neither the investigators nor the patients were blinded to treatment allocation.
Treatment
Patients assigned to Arm A received concurrent radiotherapy with S-1, an oral fluoropyrimidine derivative, at first. After CRT, maintenance chemotherapy with gemcitabine was started within 4 weeks after discontinuing CRT. Patients assigned to Arm B received 12 weeks of induction chemotherapy with gemcitabine prior to CRT. Subsequently, patients without distant metastasis and whose tumour remained to be included in a square radiation field (10 cm × 10 cm) received S-1 and concurrent radiotherapy within 4 weeks after the last dose of induction chemotherapy. After CRT, maintenance chemotherapy with gemcitabine was only given to patients who were without disease progression and had experienced limited toxic effects during induction chemotherapy.
Induction chemotherapy with gemcitabine
Gemcitabine at a dose of 1000 mg/m2 was delivered intravenously over 30 minutes on days 1, 8 and 15, followed by a 1-week rest (one cycle). This administration of gemcitabine was repeated every 4 weeks. Induction chemotherapy was continued for up to 12 weeks.
S-1 and concurrent radiotherapy
The treatment schedule of CRT was based on that adopted in a previous multicentre phase II study conducted in Japan (9). Radiotherapy was delivered with ≥6-MV photons, using a multiple (three or more) field technique. Intensity-modulated radiation therapy was not permitted in this study. The radiation field was defined by computed tomography-assisted treatment planning as follows: primary tumour and metastatic lymph nodes with short-axis diameter of ≥1 cm identified on computed tomography were contoured as gross tumour volumes. The clinical target volume (CTV) included the primary tumour with a 0.5-cm margin and metastatic lymph nodes. Prophylactic irradiation of regional lymph nodes was not performed. The definition of planning target volume (PTV) included the CTV with a 1-cm margin laterally and a 1- to 2-cm margin in the craniocaudal direction to take into account respiratory organ motion and daily set-up error. The reference point for the radiation dose was set at the centre of the PTV. The spinal cord dose was maintained at <45 Gy. The volume of liver to receive 30 Gy was required to be <40%, and the volume to receive 20 Gy was required to be <67%. The volume of both kidneys to receive 18 Gy was required to be <35%. Radiation treatment plan was monitored centrally by two radiation oncologists as a part of the radiotherapy quality control programme by the JCOG Radiotherapy Committee. A total dose of 50.4 Gy was delivered in 28 fractions over 5.5 weeks. S-1 was administered orally twice a day at a dose of 80 mg/m2 per day on the day of irradiation (Monday through Friday) according to the body surface area (BSA) as follows: 80 mg for BSA <1.25 m2; 100 mg for 1.25 m2 ≤ BSA < 1.50 m2 and 120 mg for 1.50 m2 ≤ BSA.
Maintenance chemotherapy with gemcitabine
The treatment schedule of maintenance gemcitabine was the same as the induction phase schedule. However, for patients in Arm B, gemcitabine was started at the same dose and according to the same schedule as the last dosing of induction chemotherapy.
Protocol-specified treatment modifications or delays were permitted in the event of predefined treatment-related adverse events (AEs). Protocol treatment was discontinued for the following reasons: treatment deemed to be ineffective because of disease progression; difficulties continuing treatment due to AEs, such as pneumonitis, grade 4 non-haematological toxicity or other medical conditions, as determined by the investigators; if CRT could not be started within 56 days of induction chemotherapy (for Arm B); if maintenance chemotherapy could not be started within 56 days after CRT; requirement for a further dose reduction, following a second reduction of gemcitabine during maintenance chemotherapy; patient refusal to continue the protocol treatment.
Assessments
Physical examination and laboratory tests were performed at least on each of drug administration day during induction and maintenance chemotherapy and at least weekly during CRT. All AEs were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), ver. 4.0. We evaluated abdominal computed tomography scans, chest radiography (or chest computed tomography scans) and tumour markers, including CA19-9, at the completion of induction chemotherapy (for Arm B), at the completion of CRT and every 6 weeks during maintenance chemotherapy.
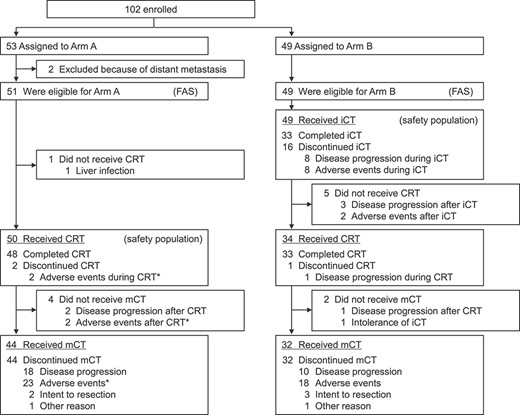
Patient flow chart. iCT, induction chemotherapy; mCT, maintenance chemotherapy. Asterisk indicates one treatment-related death.
Endpoints
The primary endpoint was overall survival (OS). The secondary endpoints were progression-free survival (PFS), distant metastasis-free survival (DMFS), CA19-9 response and the incidences of AEs. OS was calculated from the date of randomization until the date of death from any cause. PFS was measured from randomization until the date of disease progression or death in patients without disease progression. DMFS was the time from randomization to the date of appearance of distant metastasis or death in patients without distant metastasis. CA19-9 response was assessed for patients with a pretreatment CA19-9 level of >100 U/ml and a positive response was defined as a reduction by >50% from the pretreatment level (9).
Statistical analysis
This study adopted Liu’s selection design (10). We assumed that the 1-year survival would be 60% for one regimen and over 70% for the other regimen. In this study, the total sample size required to ensure a minimum probability of 90% of correctly selecting the more effective regimen was 100 patients. Treatment in Arm B would be expected to be less toxic because the administration of induction chemotherapy may spare some of the patients from potentially toxic radiotherapy. Therefore, the decision rule was set as follows: Arm B would be considered more promising if the point estimate of the hazard ratio (HR) of OS for Arm B to Arm A was <1.186. We planned the primary analysis at 1 year after enrolment completion and the final analysis 1 year after the primary analysis to confirm long-term outcomes.
OS and PFS were estimated using the Kaplan–Meier method. HRs of treatment effects were estimated by using the Cox regression model. All of the analyses were performed based on the full analysis set from all randomized patients, excluding ineligible patients, using SAS version 9.2 (SAS Institute, Cary, NC).
Results
Patient characteristics and treatment delivery
From December 2011 to September 2013, 102 patients were enrolled from 26 institutions in HBPOG of JCOG. Fifty-three patients were assigned to Arm A and 49 patients were assigned to Arm B (Fig. 1). However, two patients in Arm A were found to be ineligible due to the finding of distant metastasis before the start of protocol treatment, and one patient in Arm A could not commence treatment due to liver infection. Therefore, 100 patients were included in the full analysis set and 99 patients were included in the safety population.
Baseline characteristics of the full analysis set were mostly comparable in the two treatment arms (Table 1), although there were more patients of ECOG PS 0 in Arm A than in Arm B (69 vs. 57%). Pathological diagnosis was reviewed in 98 patients, and 94 patients (96%) were confirmed to have pancreatic adenocarcinoma (including adenocarcinoma suspected) (Supplementary Table S1).
| . | Arm A . | Arm B . |
|---|---|---|
| (n = 51) | (n = 49) | |
| Age (years) | ||
| Median (range) | 65 (38–78) | 67 (28–80) |
| Gender, n (%) | ||
| Male | 22 (43) | 23 (47) |
| Female | 29 (57) | 26 (53) |
| ECOG PS, n (%) | ||
| 0 | 35 (69) | 28 (57) |
| 1 | 16 (31) | 21 (43) |
| Tumor location, n (%) | ||
| Head | 29 (57) | 25 (51) |
| Body or tail | 22 (43) | 24 (49) |
| Nodal statusa, n (%) | ||
| N0 | 40 (78) | 36 (73) |
| N1 | 11 (22) | 13 (27) |
| Biliary drainage, n (%) | ||
| No | 36 (71) | 35 (71) |
| Yes | 15 (29) | 14 (29) |
| CA19-9 (IU/ml), n (%) | ||
| < 1000 | 39 (76) | 37 (76) |
| ≥ 1000 | 12 (24) | 12 (24) |
| . | Arm A . | Arm B . |
|---|---|---|
| (n = 51) | (n = 49) | |
| Age (years) | ||
| Median (range) | 65 (38–78) | 67 (28–80) |
| Gender, n (%) | ||
| Male | 22 (43) | 23 (47) |
| Female | 29 (57) | 26 (53) |
| ECOG PS, n (%) | ||
| 0 | 35 (69) | 28 (57) |
| 1 | 16 (31) | 21 (43) |
| Tumor location, n (%) | ||
| Head | 29 (57) | 25 (51) |
| Body or tail | 22 (43) | 24 (49) |
| Nodal statusa, n (%) | ||
| N0 | 40 (78) | 36 (73) |
| N1 | 11 (22) | 13 (27) |
| Biliary drainage, n (%) | ||
| No | 36 (71) | 35 (71) |
| Yes | 15 (29) | 14 (29) |
| CA19-9 (IU/ml), n (%) | ||
| < 1000 | 39 (76) | 37 (76) |
| ≥ 1000 | 12 (24) | 12 (24) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
aAccording to the Union for International Cancer Control TNM version 7.
| . | Arm A . | Arm B . |
|---|---|---|
| (n = 51) | (n = 49) | |
| Age (years) | ||
| Median (range) | 65 (38–78) | 67 (28–80) |
| Gender, n (%) | ||
| Male | 22 (43) | 23 (47) |
| Female | 29 (57) | 26 (53) |
| ECOG PS, n (%) | ||
| 0 | 35 (69) | 28 (57) |
| 1 | 16 (31) | 21 (43) |
| Tumor location, n (%) | ||
| Head | 29 (57) | 25 (51) |
| Body or tail | 22 (43) | 24 (49) |
| Nodal statusa, n (%) | ||
| N0 | 40 (78) | 36 (73) |
| N1 | 11 (22) | 13 (27) |
| Biliary drainage, n (%) | ||
| No | 36 (71) | 35 (71) |
| Yes | 15 (29) | 14 (29) |
| CA19-9 (IU/ml), n (%) | ||
| < 1000 | 39 (76) | 37 (76) |
| ≥ 1000 | 12 (24) | 12 (24) |
| . | Arm A . | Arm B . |
|---|---|---|
| (n = 51) | (n = 49) | |
| Age (years) | ||
| Median (range) | 65 (38–78) | 67 (28–80) |
| Gender, n (%) | ||
| Male | 22 (43) | 23 (47) |
| Female | 29 (57) | 26 (53) |
| ECOG PS, n (%) | ||
| 0 | 35 (69) | 28 (57) |
| 1 | 16 (31) | 21 (43) |
| Tumor location, n (%) | ||
| Head | 29 (57) | 25 (51) |
| Body or tail | 22 (43) | 24 (49) |
| Nodal statusa, n (%) | ||
| N0 | 40 (78) | 36 (73) |
| N1 | 11 (22) | 13 (27) |
| Biliary drainage, n (%) | ||
| No | 36 (71) | 35 (71) |
| Yes | 15 (29) | 14 (29) |
| CA19-9 (IU/ml), n (%) | ||
| < 1000 | 39 (76) | 37 (76) |
| ≥ 1000 | 12 (24) | 12 (24) |
ECOG PS, Eastern Cooperative Oncology Group performance status.
aAccording to the Union for International Cancer Control TNM version 7.
Amongst 51 eligible patients in Arm A, 50 (98%) underwent CRT and 44 (86%) underwent maintenance chemotherapy. However, amongst 49 eligible patients in Arm B, all patients underwent induction chemotherapy, 34 (69%) underwent CRT and 32 (65%) underwent maintenance chemotherapy. At the data cut-off point (October 2015), there were no patients who continued the protocol treatment. Maintenance chemotherapy was terminated early in two patients in Arm A and three patients in Arm B, as they had to undergo surgery because their tumours were assessed as resectable. The median number of cycles of maintenance gemcitabine was 5.5 cycles in Arm A and 5.0 cycles in Arm B. The median relative dose intensity (the proportion of the administered cumulative dose relative to the planned cumulative dose) of maintenance gemcitabine for up to 24 weeks was 60% in Arm A and 56% in Arm B.
Safety
Table 2 shows the AEs through all protocol treatment in the safety population. The most frequently observed grade 3 or 4 AEs observed in Arm A (≥5% of subjects) were anaemia, anorexia and nausea. While those most frequently observed in Arm B were liver dysfunction (increased alkaline phosphatase, AST and ALT) and biliary infection. Three treatment-related deaths, interstitial pneumonitis due to chemotherapy, gastroduodenal haemorrhage, biliary infection, occurred only in Arm A.
Adverse events through all protocol treatment in the safety population (CTCAE version 4.0)
| Variable . | Arm A (n = 50), % . | Arm B (n = 49), % . | ||||
|---|---|---|---|---|---|---|
| . | All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . |
| Haematological | ||||||
| White blood cell decreased | 94 | 60 | 2 | 94 | 59 | 2 |
| Anaemia | 100 | 14 | 4 | 98 | 10 | 2 |
| Platelet count decreased | 100 | 8 | 2 | 94 | 8 | 6 |
| Neutrophil count decreased | 92 | 46 | 8 | 96 | 39 | 18 |
| Non-haematological | ||||||
| Blood bilirubin increased | 30 | 8 | 0 | 29 | 8 | 0 |
| ALP increased | 66 | 0 | 0 | 71 | 16 | 0 |
| AST increased | 88 | 12 | 2 | 78 | 27 | 2 |
| ALT increased | 94 | 14 | 0 | 80 | 18 | 4 |
| Creatinine increased | 8 | 2 | 0 | 14 | 0 | 0 |
| Fatigue | 66 | 8 | — | 65 | 4 | — |
| Fever | 18 | 0 | 0 | 24 | 0 | 0 |
| Urticaria | 8 | 0 | — | 14 | 0 | — |
| Anorexia | 88 | 16 | 0 | 76 | 4 | 0 |
| Constipation | 44 | 0 | 0 | 49 | 2 | 0 |
| Diarrhoea | 46 | 6 | 0 | 37 | 4 | 0 |
| Nausea | 80 | 8 | — | 63 | 2 | — |
| Vomiting | 50 | 2 | 0 | 33 | 4 | 0 |
| Mucositis oral | 12 | 0 | 0 | 14 | 0 | 0 |
| Dysgeusia | 34 | — | — | 24 | — | — |
| Pneumonitis | 6 | 0 | 4a | 4 | 2 | 0 |
| Gastric/duodenal ulcer | 6 | 6 | 0 | 8 | 4 | 0 |
| Gastric/duodenal haemorrhage | 10 | 8 | 2a | 12 | 6 | 0 |
| Biliary infection | 20 | 18 | 2a | 27 | 27 | 0 |
| Variable . | Arm A (n = 50), % . | Arm B (n = 49), % . | ||||
|---|---|---|---|---|---|---|
| . | All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . |
| Haematological | ||||||
| White blood cell decreased | 94 | 60 | 2 | 94 | 59 | 2 |
| Anaemia | 100 | 14 | 4 | 98 | 10 | 2 |
| Platelet count decreased | 100 | 8 | 2 | 94 | 8 | 6 |
| Neutrophil count decreased | 92 | 46 | 8 | 96 | 39 | 18 |
| Non-haematological | ||||||
| Blood bilirubin increased | 30 | 8 | 0 | 29 | 8 | 0 |
| ALP increased | 66 | 0 | 0 | 71 | 16 | 0 |
| AST increased | 88 | 12 | 2 | 78 | 27 | 2 |
| ALT increased | 94 | 14 | 0 | 80 | 18 | 4 |
| Creatinine increased | 8 | 2 | 0 | 14 | 0 | 0 |
| Fatigue | 66 | 8 | — | 65 | 4 | — |
| Fever | 18 | 0 | 0 | 24 | 0 | 0 |
| Urticaria | 8 | 0 | — | 14 | 0 | — |
| Anorexia | 88 | 16 | 0 | 76 | 4 | 0 |
| Constipation | 44 | 0 | 0 | 49 | 2 | 0 |
| Diarrhoea | 46 | 6 | 0 | 37 | 4 | 0 |
| Nausea | 80 | 8 | — | 63 | 2 | — |
| Vomiting | 50 | 2 | 0 | 33 | 4 | 0 |
| Mucositis oral | 12 | 0 | 0 | 14 | 0 | 0 |
| Dysgeusia | 34 | — | — | 24 | — | — |
| Pneumonitis | 6 | 0 | 4a | 4 | 2 | 0 |
| Gastric/duodenal ulcer | 6 | 6 | 0 | 8 | 4 | 0 |
| Gastric/duodenal haemorrhage | 10 | 8 | 2a | 12 | 6 | 0 |
| Biliary infection | 20 | 18 | 2a | 27 | 27 | 0 |
Events with a frequency of more than 10% or high-grade events (grades 3 and 4) are listed.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
aThree treatment-related deaths occurred in Arm A (pneumonitis, gastroduodenal haemorrhage, biliary infection).
Adverse events through all protocol treatment in the safety population (CTCAE version 4.0)
| Variable . | Arm A (n = 50), % . | Arm B (n = 49), % . | ||||
|---|---|---|---|---|---|---|
| . | All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . |
| Haematological | ||||||
| White blood cell decreased | 94 | 60 | 2 | 94 | 59 | 2 |
| Anaemia | 100 | 14 | 4 | 98 | 10 | 2 |
| Platelet count decreased | 100 | 8 | 2 | 94 | 8 | 6 |
| Neutrophil count decreased | 92 | 46 | 8 | 96 | 39 | 18 |
| Non-haematological | ||||||
| Blood bilirubin increased | 30 | 8 | 0 | 29 | 8 | 0 |
| ALP increased | 66 | 0 | 0 | 71 | 16 | 0 |
| AST increased | 88 | 12 | 2 | 78 | 27 | 2 |
| ALT increased | 94 | 14 | 0 | 80 | 18 | 4 |
| Creatinine increased | 8 | 2 | 0 | 14 | 0 | 0 |
| Fatigue | 66 | 8 | — | 65 | 4 | — |
| Fever | 18 | 0 | 0 | 24 | 0 | 0 |
| Urticaria | 8 | 0 | — | 14 | 0 | — |
| Anorexia | 88 | 16 | 0 | 76 | 4 | 0 |
| Constipation | 44 | 0 | 0 | 49 | 2 | 0 |
| Diarrhoea | 46 | 6 | 0 | 37 | 4 | 0 |
| Nausea | 80 | 8 | — | 63 | 2 | — |
| Vomiting | 50 | 2 | 0 | 33 | 4 | 0 |
| Mucositis oral | 12 | 0 | 0 | 14 | 0 | 0 |
| Dysgeusia | 34 | — | — | 24 | — | — |
| Pneumonitis | 6 | 0 | 4a | 4 | 2 | 0 |
| Gastric/duodenal ulcer | 6 | 6 | 0 | 8 | 4 | 0 |
| Gastric/duodenal haemorrhage | 10 | 8 | 2a | 12 | 6 | 0 |
| Biliary infection | 20 | 18 | 2a | 27 | 27 | 0 |
| Variable . | Arm A (n = 50), % . | Arm B (n = 49), % . | ||||
|---|---|---|---|---|---|---|
| . | All grade . | Grade 3 . | Grade 4 . | All grade . | Grade 3 . | Grade 4 . |
| Haematological | ||||||
| White blood cell decreased | 94 | 60 | 2 | 94 | 59 | 2 |
| Anaemia | 100 | 14 | 4 | 98 | 10 | 2 |
| Platelet count decreased | 100 | 8 | 2 | 94 | 8 | 6 |
| Neutrophil count decreased | 92 | 46 | 8 | 96 | 39 | 18 |
| Non-haematological | ||||||
| Blood bilirubin increased | 30 | 8 | 0 | 29 | 8 | 0 |
| ALP increased | 66 | 0 | 0 | 71 | 16 | 0 |
| AST increased | 88 | 12 | 2 | 78 | 27 | 2 |
| ALT increased | 94 | 14 | 0 | 80 | 18 | 4 |
| Creatinine increased | 8 | 2 | 0 | 14 | 0 | 0 |
| Fatigue | 66 | 8 | — | 65 | 4 | — |
| Fever | 18 | 0 | 0 | 24 | 0 | 0 |
| Urticaria | 8 | 0 | — | 14 | 0 | — |
| Anorexia | 88 | 16 | 0 | 76 | 4 | 0 |
| Constipation | 44 | 0 | 0 | 49 | 2 | 0 |
| Diarrhoea | 46 | 6 | 0 | 37 | 4 | 0 |
| Nausea | 80 | 8 | — | 63 | 2 | — |
| Vomiting | 50 | 2 | 0 | 33 | 4 | 0 |
| Mucositis oral | 12 | 0 | 0 | 14 | 0 | 0 |
| Dysgeusia | 34 | — | — | 24 | — | — |
| Pneumonitis | 6 | 0 | 4a | 4 | 2 | 0 |
| Gastric/duodenal ulcer | 6 | 6 | 0 | 8 | 4 | 0 |
| Gastric/duodenal haemorrhage | 10 | 8 | 2a | 12 | 6 | 0 |
| Biliary infection | 20 | 18 | 2a | 27 | 27 | 0 |
Events with a frequency of more than 10% or high-grade events (grades 3 and 4) are listed.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CTCAE, Common Terminology Criteria for Adverse Events.
aThree treatment-related deaths occurred in Arm A (pneumonitis, gastroduodenal haemorrhage, biliary infection).
Efficacy
We performed the primary analysis in October 2014 when 65 deaths had been reported and median follow-up time for survivors was 16.9 months. The 1-year survival was 66.7% in Arm A and 69.3% in Arm B. The HR for death was 1.157 (95% confidence interval [CI] 0.708–1.893). According to the pre-specified decision rule (HR 1.157 < 1.186), Arm B was to be selected as a more promising regimen. However, the 2-year OS, with immature data at the primary analysis, was 32.1% in Arm A and 18.7% in Arm B. Therefore, we decided to defer the definitive conclusion until the data, including long-term outcomes, were confirmed at the final analysis.
The final analysis was performed in October 2015 based on 85 deaths. The median follow-up duration for survivors was 26.9 months. The 1-year survival of the two arms was unchanged, and the 2-year OS was 36.9% in Arm A and 18.9% in Arm B. The median OS was 19.0 months (95% CI 15.0–20.6) in Arm A and 17.2 months (95% CI 12.6–20.3) in Arm B. The HR was 1.255 (95% CI 0.816–1.930) in favour of Arm A (Fig. 2). In a univariate analysis of OS according to the main clinical factors at inclusion, ECOG PS 1 was suggested to be a prognostic factor for poor survival (P = 0.053). However, the HR on multivariable analysis adjusting for patient characteristics remained in favour of Arm A (1.186, 95% CI, 0.747–1.882) (Table 3). In the subgroup analysis of survival, the following factors were associated with poorer short-term outcomes in Arm A than in Arm B: age ≥ 65 years, ECOG PS of 1, the usage of biliary drainage and regional lymph node metastasis (N1) (Supplementary Figs S1–S7).
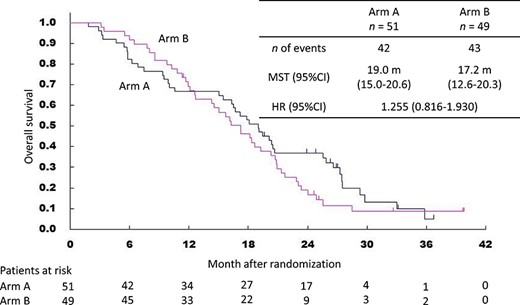
Kaplan–Meier curves for overall survival. CI, confidence interval; HR, hazard ratio; MST, median survival time.
| Variable . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value (Two-sided) . | HR (95% CI) . | P value (Two-sided) . |
| Treatment arm (Arm A vs. Arm B) | 1.255 (0.816–1.930) | 0.30 | 1.186 (0.747–1.882) | 0.47 |
| Age (years old) (<65 vs. ≥65) | 1.035 (0.673–1.593) | 0.87 | 0.985 (0.629–1.542) | 0.95 |
| Gender (male vs. female) | 1.163 (0.757–1.788) | 0.49 | 1.231 (0.786–1.928) | 0.36 |
| ECOG PS (0 vs. 1) | 1.540 (0.994–2.386) | 0.053 | 1.704 (0.998–2.912) | 0.051 |
| Biliary drainage (no vs. yes) | 1.091 (0.679–1.752) | 0.72 | 0.929 (0.501–1.724) | 0.82 |
| CA19-9 (IU/ml) (<1000 vs. ≥1000) | 1.282 (0.786–2.091) | 0.32 | 1.127 (0.655–1.939) | 0.66 |
| Tumor location (head vs. body or tail) | 0.941 (0.613–1.444) | 0.78 | 0.715 (0.394–1.298) | 0.27 |
| Nodal status (N0 vs. N1) | 1.009 (0.610–1.669) | 0.97 | 1.106 (0.625–1.957) | 0.73 |
| Variable . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value (Two-sided) . | HR (95% CI) . | P value (Two-sided) . |
| Treatment arm (Arm A vs. Arm B) | 1.255 (0.816–1.930) | 0.30 | 1.186 (0.747–1.882) | 0.47 |
| Age (years old) (<65 vs. ≥65) | 1.035 (0.673–1.593) | 0.87 | 0.985 (0.629–1.542) | 0.95 |
| Gender (male vs. female) | 1.163 (0.757–1.788) | 0.49 | 1.231 (0.786–1.928) | 0.36 |
| ECOG PS (0 vs. 1) | 1.540 (0.994–2.386) | 0.053 | 1.704 (0.998–2.912) | 0.051 |
| Biliary drainage (no vs. yes) | 1.091 (0.679–1.752) | 0.72 | 0.929 (0.501–1.724) | 0.82 |
| CA19-9 (IU/ml) (<1000 vs. ≥1000) | 1.282 (0.786–2.091) | 0.32 | 1.127 (0.655–1.939) | 0.66 |
| Tumor location (head vs. body or tail) | 0.941 (0.613–1.444) | 0.78 | 0.715 (0.394–1.298) | 0.27 |
| Nodal status (N0 vs. N1) | 1.009 (0.610–1.669) | 0.97 | 1.106 (0.625–1.957) | 0.73 |
CI, confidence interval; HR, hazard ratio.
| Variable . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value (Two-sided) . | HR (95% CI) . | P value (Two-sided) . |
| Treatment arm (Arm A vs. Arm B) | 1.255 (0.816–1.930) | 0.30 | 1.186 (0.747–1.882) | 0.47 |
| Age (years old) (<65 vs. ≥65) | 1.035 (0.673–1.593) | 0.87 | 0.985 (0.629–1.542) | 0.95 |
| Gender (male vs. female) | 1.163 (0.757–1.788) | 0.49 | 1.231 (0.786–1.928) | 0.36 |
| ECOG PS (0 vs. 1) | 1.540 (0.994–2.386) | 0.053 | 1.704 (0.998–2.912) | 0.051 |
| Biliary drainage (no vs. yes) | 1.091 (0.679–1.752) | 0.72 | 0.929 (0.501–1.724) | 0.82 |
| CA19-9 (IU/ml) (<1000 vs. ≥1000) | 1.282 (0.786–2.091) | 0.32 | 1.127 (0.655–1.939) | 0.66 |
| Tumor location (head vs. body or tail) | 0.941 (0.613–1.444) | 0.78 | 0.715 (0.394–1.298) | 0.27 |
| Nodal status (N0 vs. N1) | 1.009 (0.610–1.669) | 0.97 | 1.106 (0.625–1.957) | 0.73 |
| Variable . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| . | HR (95% CI) . | P value (Two-sided) . | HR (95% CI) . | P value (Two-sided) . |
| Treatment arm (Arm A vs. Arm B) | 1.255 (0.816–1.930) | 0.30 | 1.186 (0.747–1.882) | 0.47 |
| Age (years old) (<65 vs. ≥65) | 1.035 (0.673–1.593) | 0.87 | 0.985 (0.629–1.542) | 0.95 |
| Gender (male vs. female) | 1.163 (0.757–1.788) | 0.49 | 1.231 (0.786–1.928) | 0.36 |
| ECOG PS (0 vs. 1) | 1.540 (0.994–2.386) | 0.053 | 1.704 (0.998–2.912) | 0.051 |
| Biliary drainage (no vs. yes) | 1.091 (0.679–1.752) | 0.72 | 0.929 (0.501–1.724) | 0.82 |
| CA19-9 (IU/ml) (<1000 vs. ≥1000) | 1.282 (0.786–2.091) | 0.32 | 1.127 (0.655–1.939) | 0.66 |
| Tumor location (head vs. body or tail) | 0.941 (0.613–1.444) | 0.78 | 0.715 (0.394–1.298) | 0.27 |
| Nodal status (N0 vs. N1) | 1.009 (0.610–1.669) | 0.97 | 1.106 (0.625–1.957) | 0.73 |
CI, confidence interval; HR, hazard ratio.
The final analysis of PFS was based on 94 events amongst 100 patients. The median PFS was 10.1 months (95% CI 6.0–12.5) in Arm A and 10.4 months (95% CI 7.0–13.6) in Arm B. The HR was 1.03 (95% CI 0.69–1.55) (Fig. 3). The 1- and 2-year PFS were 39.2% and 8.6% in Arm A and 46.6% and 4.2% in Arm B. The analysis of DMFS was based on 93 events. The median DMFS was 11.0 months (95% CI 6.0–15.9) in Arm A and 11.4 months (95% CI 7.2–13.6) in Arm B. The HR was 1.20 (95% CI 0.79–1.80) (Fig. 4). The 1- and 2-year DMFS were 45.1% and 14.8% in Arm A and 48.7% and 4.2% in Arm B.
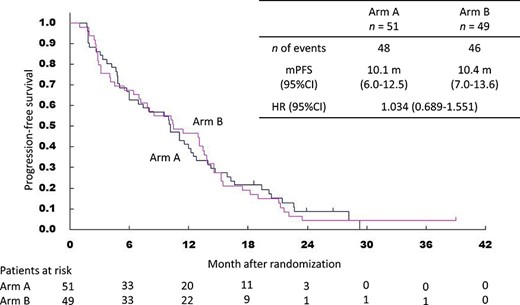
Kaplan–Meier curves for progression-free survival. mPFS, median progression-free survival time.
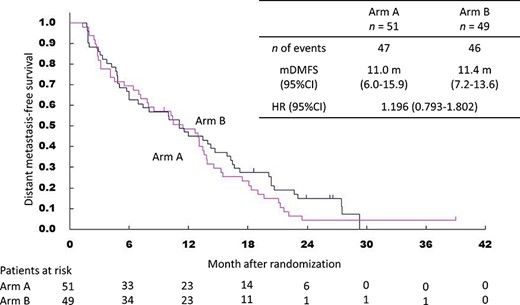
Kaplan–Meier curves for distant metastasis-free survival. mDMFS, median distant metastasis-free survival time.
Amongst the patients with pretreatment CA19-9 level of >100 U/ml, the CA19-9 response rates were 67.6% (25/37) in Arm A and 63.3% (19/30) in Arm B.
Discussion
This is the first randomized trial to evaluate the role of induction chemotherapy prior to CRT compared with CRT alone for the treatment of LAPC. Arm B with induction chemotherapy of gemcitabine was less toxic than Arm A without induction chemotherapy, because gastrointestinal toxicity was slightly more frequent and three treatment-related deaths occurred in Arm A, and three treatment-related deaths were observed only in Arm A. Regarding liver dysfunction in Arm B, there are some possibilities such as liver toxicity due to gemcitabine and higher incidence of biliary infection in Arm B due to earlier disease progression in gemcitabine treatment. Although short-term survival was favourable and toxicity could be reduced, the use of induction chemotherapy did not lead to sufficient long-term survival. Therefore, we suggest that induction chemotherapy, at least with gemcitabine, is not a promising regimen for LAPC.
Amongst patients who underwent induction chemotherapy, 31% did not receive CRT mainly due to disease progression during or after induction chemotherapy. This suggests that induction chemotherapy could spare patients with rapidly progressive disease who are suitable for systemic chemotherapy from potentially toxic and ineffective radiotherapy, which might contribute to favourable short-term survival and reduced toxicity.
However, induction chemotherapy followed by CRT was inferior to CRT alone in terms of long-term OS. There are some possible reasons for this finding: first, gemcitabine is inadequate for induction chemotherapy. We could not use a more effective chemotherapeutic regimen, such as FOLFIRINOX and GnP, because no regimen had been approved in Japan when this trial was conducted. Induction chemotherapy with these two regimens should be investigated in the future. Second, there is a possibility that some patients with LAPC are suitable for undergoing systemic chemotherapy and others for undergoing CRT, because the survival curves of the two arms crossed each other at around the 1-year point. This phenomenon may suggest the existence of two subgroups in which the treatment effect would differ. From the results of the subgroup analysis, potentially toxic CRT might be inappropriate for patients aged ≥65 years old or those with an ECOG PS of 1. Moreover, patients with regional lymph node metastasis might also be suitable for undergoing systemic chemotherapy because they are likely to have occult metastases. However, because of the limited number of patients in our subanalysis, this hypothesis could not be evaluated and future studies are required to corroborate this hypothesis.
CRT alone also showed favourable long-term DMFS, as well as long-term OS, although PFS was similar. These results suggest that the treatment effect of CRT is not only limited to local tumour control but also includes prevention of distant metastasis, which leads to long-term survival. Furthermore, the use of induction chemotherapy may allow the development of micro metastasis due to the delay of the start of CRT. Therefore, induction chemotherapy clinical trials should investigate efficient chemotherapeutic drugs and also determine the optimal duration of treatment.
The LAP07 phase 3 randomized trial did not show any survival benefit of CRT compared with chemotherapy in patients with LAPC after 4 months of induction chemotherapy (11). However, CRT showed favourable PFS and was associated with a significantly longer period without treatment. Furthermore, gemcitabine with or without erlotinib were not considered the optimal regimen for induction chemotherapy and a relatively long period (4 months) of induction chemotherapy may identify patients who are suitable for continuing the same regimen as induction chemotherapy. Therefore, we believe that CRT remains a treatment option for patients with LAPC.
In our study, S-1-based concurrent radiotherapy showed favourable efficacy, which is consistent with the results of previous trials (9,12,13). Although the incidence of grade 3/4 gastrointestinal toxicity appeared to be higher with CRT alone than in the induction chemotherapy arm, this CRT regimen is considered a promising, well-tolerated regimen that can be recommended as a test arm for a subsequent phase III trial for comparison with systemic chemotherapy.
Gemcitabine has been treated as a standard treatment for LAPC, but FOLFIRINOX and GnP, which showed superior efficacy over gemcitabine in patients with metastatic disease (3,4), may also benefit patients with LAPC. Therefore, we started another randomized phase II trial to evaluate the efficacy and safety of these two regimens and determine the most promising chemotherapy regimen for LAPC (JCOG1407, UMIN000023143). In the future, we will conduct a phase III trial to compare gemcitabine, S-1-based concurrent radiation and a selected chemotherapy regimen in JCOG1407.
Some reports have demonstrated survival benefits of surgical resection in patients with initially unresectable PC after intensive chemotherapy and/or CRT, so called conversion surgery (14–16). In this study, two patients in Arm A and three patients in Arm B underwent surgery because their tumours were assessed as resectable. The rate of conversion surgery in this study was 5 of 102 (4.9%) patients was not high, compared with recent reports, because conversion surgery was not often considered in this study. In the future, it is necessary to investigate the clinical significance of conversion surgery, and the data of conversion surgery are collected in the JCOG 1407.
In conclusion, this study suggested that the CRT using S-1 alone had more promising efficacy with longer-term survival, compared with induction gemcitabine followed by CRT for LAPC. We selected S-1-based concurrent radiotherapy without induction chemotherapy for a subsequent phase III trial to compare systemic chemotherapy.
Supplementary Material
Supplementary material can be found at Japanese Journal of Clinical Oncology online.
Authors’ contributions
Akira Fukutomi, Junji Furuse, Junki Mizusawa, Satoaki Nakamura, Yoshinori Ito, Hiroshi Katayama and Hiroshi Ishii contributed to the conception and design of the study. Akira Fukutomi, Junji Furuse, Tatsuya Ioka, Makoto Ueno, Masafumi Ikeda, Kazuya Sugimori, Naohiro Okano, Kyoko Shimizu, Hiroaki Yanagimoto, Takuji Okusaka, Masato Ozaka, Akiko Todaka, Shoji Nakamori, Kazutoshi Tobimatsu, Naohiro Sata, TM, Ayumu Hosokawa, Taketo Yamaguchi, Hiroyuki Miyakawa, Hiroki Hara, Nobumasa Mizuno and Hiroshi Ishii contributed to the acquisition of data. Shoji Nakamori and Yoshinori Ito contributed to the quality control of radiotherapy. Nobuyoshi Hiraoka contributed to the central review of pathological diagnosis. Akira Fukutomi, Junji Furuse, Junki Mizusawa, Hiroshi Katayama and Hiroki Hara analysed and interpreted the data. All authors read and approved the final manuscript.
Acknowledgements
We thank the patients, their families and all the investigators who participated in the study for their contribution. We also thank the members of the JCOG Data Center and JCOG Operations Office for their support in preparing the manuscript (Dr Kenichi Nakamura), data management (Ms Ayaka Nakano, Ms Kyoko Hasegawa) and oversight of the study management (Dr Haruhiko Fukuda). We are grateful to the members of the Central Pathology Review Committee (Dr Nobuyoshi Hiraoka, National Cancer Center Hospital; Dr Noriyoshi Fukushima, Jichi Medical University; Dr Tomoko Mitsuhashi, Hokkaido University Hospital).
Funding
The National Cancer Center Research and Development Fund [23-A-16, 23-A-22, 26-A-4, 29-A-3]; Health and Labour Sciences Research Grants for Clinical Cancer Research [H23-006] from the Ministry of Health, Labour and Welfare of Japan and by AMED under Grant Number JP15ck0106084.
Conflict of interest statement
Akira Fukutomi received honoraria from Eli Lilly, Sawai Pharmaceutical, Taiho Pharmaceutical and Yakult Honsha, and research funding from Taiho Pharmaceutical. Junji Furuse received honoraria and research funding from Eisai, Eli Lilly, Kyowa Pharmaceutical Industry, Taiho Pharmaceutical and Yakult Honsha, and research funding from Mochida Pharmaceutical. Tatsuya Ioka received research funding and fees for promotional materials from Taiho Pharmaceutical. Masafumi Ikeda received honoraria from Eisai, Eli Lilly and Taiho Pharmaceutical. Takuji Okusaka received research funding from Eli Lilly and Pfizer. Masato Ozaka received honoraria from Taiho Pharmaceutical. Shoji Nakamori received research funding from Eisai. Ayumu Hosokawa received honoraria and research funding from Taiho Pharmaceutical. Nobumasa Mizuno received research funding from Eisai, Taiho Pharmaceutical, Takeda Pharmaceutical and Yakult Honsha. The remaining authors declare no conflict of interest.


