-
PDF
- Split View
-
Views
-
Cite
Cite
Toshiaki Suzuki, Sayo Kawai, Makoto Ueno, Yingsong Lin, Shogo Kikuchi, Treatment patterns in pancreatic cancer patients based on a hospital claims database in Japan, Japanese Journal of Clinical Oncology, Volume 51, Issue 2, February 2021, Pages 228–234, https://doi.org/10.1093/jjco/hyaa183
Close - Share Icon Share
Abstract
Pancreatic cancer treatment is evolving, but few studies have examined a nationwide trend in the treatment patterns. The purpose of this study was to clarify real-world treatment patterns for pancreatic cancer in Japan.
This retrospective study examined the treatment patterns among 68 479 patients, who had pancreatic cancer diagnosis in Medical Data Vision claims database from 2010 to 2018. We extracted relevant data on treatment options, including chemotherapy, surgery and their combination. For patients who had undergone chemotherapy, we sought to analyse the use of different chemotherapy regimens. In addition, we examined the trend in treatment patterns by age group (<59, 60–69, 70–79, ≥80).
The trend in treatment options of pancreatic cancer remained stable from 2010 to 2018, with chemotherapy being the most common therapeutic option and surgery performed in approximately half that of chemotherapy. On the other hand, the use of chemotherapy regimen had changed during the same period. Although gemcitabine was the most commonly administrated single-agent regimen in 2010, gemcitabine + nab-paclitaxel was the most frequently used therapeutic agent in 2018. In the older age groups (age ≥80), the majority of patients was untreated (supposedly received supportive care), and the use of conventional regimens such as gemcitabine or S-1 was common among those undergoing chemotherapy.
Although chemotherapy has been the main treatment option for pancreatic cancer, the regimens of choice have increased significantly during the last decade. With accumulating evidence on combination chemotherapy, treatment options may further evolve in the future.
Introduction
Despite an improvement in overall outcome of cancer treatment, pancreatic cancer (PC) remains one of the most lethal malignancies, with a 5-year survival rate of <10%. For PC patients, surgical resection offers the only chance of cure. However, only ~20% of patients undergo surgery at initial diagnosis because of the difficulty in early detection and high tendency for metastasis (1). According to the Hospital-based Cancer Registry (HBCR) data from 27 728 patients in 2018 in Japan, ~11% underwent surgery, 17% received the combination of surgery and adjuvant chemotherapy, 39% received chemotherapy and ~28% were classified as untreated (2,3).
For unresectable PC patients, several chemotherapy regimens have been available over the last decade. The clinical practice guideline for PC, which was published by the Japan Pancreas Society (JPS), recommends the use of single agent or combination chemotherapy regimen, including FOLFIRINOX, gemcitabine + nab-paclitaxel, gemcitabine monotherapy, S-1 monotherapy and gemcitabine + Erlotinib (4), on the basis of age and performance status (PS). Among these regimens, FOLFIRINOX and gemcitabine + nab-paclitaxel are recommended as first-line therapy due to the efficacy that have been demonstrated in two previous randomized controlled trials (RCT) (5,6). Conversion surgery (neoadjuvant therapy) was performed for unresectable PC patients (7,8), but it was not recommended by the JPS guideline because of insufficient evidence (4).
Data available from the national database of HBCR showed that the majority of stage 0–2 PC patients underwent surgery, and ~60% of patients with stage 3 or 4 received chemotherapy only. This fact suggested that the treatment in cancer designated hospitals that contributed data to the HBCR was following the JPS guideline (4), which recommends surgery for resectable PC (up to stage 2) and chemotherapy for unresectable PC (stage 3 or higher). However, real-world treatment patterns for PC remain elusive in Japan. Furthermore, no data have been available for the trend in treatment patterns by age group. Therefore, the present study aimed to examine the trend in initial treatment patterns of PC based on a medical claims database from 2008 to 2018.
Method
Data source
This retrospective study was conducted using a hospital claims database provided by Medical Data Vision Co., Ltd (MDV; Tokyo, Japan). Available since April 2008, the MDV database contains medical and pharmacy claims from >26 million patients in 376 acute care hospitals (as of December 2018) that adopt Diagnosis Procedure Combination (DPC)/Per-Diem Payment System. The numbers of DPC hospitals covered by the MDV database have been increasing in recent years, and they represented 22% of all DPC hospitals in 2018. Inpatient or outpatient claims included age, gender, height, weight, part of laboratory tests, medical expense and cancer grade. All claims data were de-identified and new patient IDs were assigned according to each DPC hospital-specific IDs. Patient IDs in this database that are assigned by MDV are anonymized numbers that cannot be linked with data provided by DPC hospitals. The diagnoses were coded according to the 10th revision of the International Classification of Diseases and Related Health Problems (ICD-10) and Japanese Disease Name Codes. The MDV database has been used in various epidemiological studies (9–12); the PC patients included in this database had similar age and gender distributions to those reported by the HBCR (13).
Patient identification
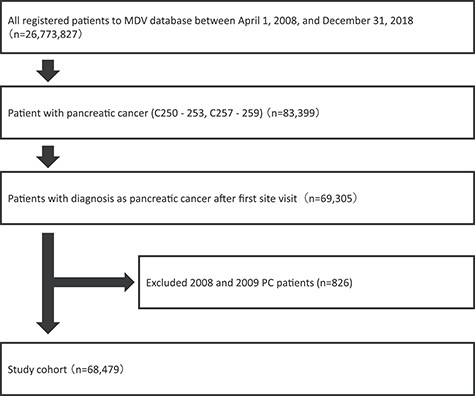
We extracted all available claims data collected and stored by the MDV from 1 April 2008 to 31 December 2018. Patients were included if they had a record of any of the following ICD-10 code diseases: C250–253 and C257–259. Tumour location included head, body, tail, duct and other parts. Malignant neoplasms of endocrine pancreas (C254) were excluded. Our analysis was restricted to the patients who received a definitive diagnosis of PC after the initial hospital visit. In addition, the data aggregated between 1 April 2008 and 31 December 2009 were excluded because the number of PC patients was <1000 each year during this period (Fig. 1).
Therapeutic modalities
According to the guidelines of PC, treatment options include surgery, chemotherapy, radiation and combinations of these modalities (4,14). These therapeutic modalities were reviewed by referring to the medication fee codes (Supplementary Table S1), which were available from the Health Insurance Claims Review & Reimbursement services database (15). Initiate treatments for each patient were extracted from the database. If a new treatment was added within 30 days after a prior treatment, we consider that this patient received a combination therapy. Furthermore, chemotherapy administered before surgery was classified as neoadjuvant therapy, and post-operative chemotherapy as adjuvant therapy. The ‘no active treatment’ group was defined as the group of patients for whom we could not identify the records of surgery, chemotherapy or radiotherapy from the database during the study period. We examined treatment modalities of PC patients by year (2010–18) and age group (<59, 60–69, 70–79, ≥80).
Chemotherapy regimen
We included six types of chemotherapy regimens that are recommended by the guidelines for PC (4,14). A combination regimen is defined based on the following criteria: (1) multiple medications prescribed on the same day, (2) a new agent added within 30 days after the previous prescription or (3) changes in multiple medication prescriptions. We further evaluated the chemotherapy regimen for PC patients by year (2010–18) and age group (<59, 60–69, 70–79, ≥80).
Ethics statement
The data for this observational study were extracted from a commercial, de-identified claims database. Informed consent is not required for de-identified data, as stated by the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. Exemption of ethics approval was granted by the Ethics Committee of Aichi Medical University School of Medicine.
Result
Of 26 773 827 patients in the database, we identified 68 479 patients who had a PC diagnosis code between 1 January 2010 and 31 December 2018. A higher percentage of males than females (male to female ratio: 1.17:1) were included in our analysis. The largest number of patients was seen in 2018 because of the expanding collection of claims data over the time. The age distributions of PC patients over 70 age groups are slightly increasing from 2010 to 2018, with the 70–79 age group showing the highest proportion (34.2%) during the study period (Table 1).
| Characteristics, n (%) . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 896 | 1356 | 1983 | 3045 | 4551 | 5237 | 5912 | 6923 | 6987 | 36 890 |
| 56.2% | 55.9% | 55.8% | 54.5% | 54.1% | 53.8% | 53.4% | 53.4% | 53.1% | 53.9% | |
| Female | 699 | 1069 | 1570 | 2539 | 3859 | 4490 | 5167 | 6037 | 6159 | 31 589 |
| 43.8% | 44.1% | 44.2% | 45.5% | 45.9% | 46.2% | 46.6% | 46.6% | 46.9% | 46.1% | |
| Age, categories | ||||||||||
| <60 | 247 | 357 | 543 | 796 | 1119 | 1267 | 1360 | 1588 | 1661 | 8938 |
| 15.5% | 14.7% | 15.3% | 14.3% | 13.3% | 13.0% | 12.3% | 12.3% | 12.6% | 13.1% | |
| 60–69 | 435 | 659 | 1030 | 1491 | 2278 | 2519 | 2876 | 3376 | 3073 | 17 737 |
| 27.3% | 27.2% | 29.0% | 26.7% | 27.1% | 25.9% | 26.0% | 26.0% | 23.4% | 25.9% | |
| 70–79 | 525 | 831 | 1171 | 1872 | 2812 | 3303 | 3756 | 4464 | 4655 | 23 389 |
| 32.9% | 34.3% | 33.0% | 33.5% | 33.4% | 34.0% | 33.9% | 34.4% | 35.4% | 34.2% | |
| ≥80 | 388 | 578 | 809 | 1425 | 2201 | 2638 | 3087 | 3532 | 3757 | 18 415 |
| 24.3% | 23.8% | 22.8% | 25.5% | 26.2% | 27.1% | 27.9% | 27.3% | 28.6% | 26.9% | |
| Total | 1595 | 2425 | 3553 | 5584 | 8410 | 9727 | 11 079 | 12 960 | 13 146 | 68 479 |
| Characteristics, n (%) . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 896 | 1356 | 1983 | 3045 | 4551 | 5237 | 5912 | 6923 | 6987 | 36 890 |
| 56.2% | 55.9% | 55.8% | 54.5% | 54.1% | 53.8% | 53.4% | 53.4% | 53.1% | 53.9% | |
| Female | 699 | 1069 | 1570 | 2539 | 3859 | 4490 | 5167 | 6037 | 6159 | 31 589 |
| 43.8% | 44.1% | 44.2% | 45.5% | 45.9% | 46.2% | 46.6% | 46.6% | 46.9% | 46.1% | |
| Age, categories | ||||||||||
| <60 | 247 | 357 | 543 | 796 | 1119 | 1267 | 1360 | 1588 | 1661 | 8938 |
| 15.5% | 14.7% | 15.3% | 14.3% | 13.3% | 13.0% | 12.3% | 12.3% | 12.6% | 13.1% | |
| 60–69 | 435 | 659 | 1030 | 1491 | 2278 | 2519 | 2876 | 3376 | 3073 | 17 737 |
| 27.3% | 27.2% | 29.0% | 26.7% | 27.1% | 25.9% | 26.0% | 26.0% | 23.4% | 25.9% | |
| 70–79 | 525 | 831 | 1171 | 1872 | 2812 | 3303 | 3756 | 4464 | 4655 | 23 389 |
| 32.9% | 34.3% | 33.0% | 33.5% | 33.4% | 34.0% | 33.9% | 34.4% | 35.4% | 34.2% | |
| ≥80 | 388 | 578 | 809 | 1425 | 2201 | 2638 | 3087 | 3532 | 3757 | 18 415 |
| 24.3% | 23.8% | 22.8% | 25.5% | 26.2% | 27.1% | 27.9% | 27.3% | 28.6% | 26.9% | |
| Total | 1595 | 2425 | 3553 | 5584 | 8410 | 9727 | 11 079 | 12 960 | 13 146 | 68 479 |
| Characteristics, n (%) . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 896 | 1356 | 1983 | 3045 | 4551 | 5237 | 5912 | 6923 | 6987 | 36 890 |
| 56.2% | 55.9% | 55.8% | 54.5% | 54.1% | 53.8% | 53.4% | 53.4% | 53.1% | 53.9% | |
| Female | 699 | 1069 | 1570 | 2539 | 3859 | 4490 | 5167 | 6037 | 6159 | 31 589 |
| 43.8% | 44.1% | 44.2% | 45.5% | 45.9% | 46.2% | 46.6% | 46.6% | 46.9% | 46.1% | |
| Age, categories | ||||||||||
| <60 | 247 | 357 | 543 | 796 | 1119 | 1267 | 1360 | 1588 | 1661 | 8938 |
| 15.5% | 14.7% | 15.3% | 14.3% | 13.3% | 13.0% | 12.3% | 12.3% | 12.6% | 13.1% | |
| 60–69 | 435 | 659 | 1030 | 1491 | 2278 | 2519 | 2876 | 3376 | 3073 | 17 737 |
| 27.3% | 27.2% | 29.0% | 26.7% | 27.1% | 25.9% | 26.0% | 26.0% | 23.4% | 25.9% | |
| 70–79 | 525 | 831 | 1171 | 1872 | 2812 | 3303 | 3756 | 4464 | 4655 | 23 389 |
| 32.9% | 34.3% | 33.0% | 33.5% | 33.4% | 34.0% | 33.9% | 34.4% | 35.4% | 34.2% | |
| ≥80 | 388 | 578 | 809 | 1425 | 2201 | 2638 | 3087 | 3532 | 3757 | 18 415 |
| 24.3% | 23.8% | 22.8% | 25.5% | 26.2% | 27.1% | 27.9% | 27.3% | 28.6% | 26.9% | |
| Total | 1595 | 2425 | 3553 | 5584 | 8410 | 9727 | 11 079 | 12 960 | 13 146 | 68 479 |
| Characteristics, n (%) . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . | 2018 . | Total . |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Male | 896 | 1356 | 1983 | 3045 | 4551 | 5237 | 5912 | 6923 | 6987 | 36 890 |
| 56.2% | 55.9% | 55.8% | 54.5% | 54.1% | 53.8% | 53.4% | 53.4% | 53.1% | 53.9% | |
| Female | 699 | 1069 | 1570 | 2539 | 3859 | 4490 | 5167 | 6037 | 6159 | 31 589 |
| 43.8% | 44.1% | 44.2% | 45.5% | 45.9% | 46.2% | 46.6% | 46.6% | 46.9% | 46.1% | |
| Age, categories | ||||||||||
| <60 | 247 | 357 | 543 | 796 | 1119 | 1267 | 1360 | 1588 | 1661 | 8938 |
| 15.5% | 14.7% | 15.3% | 14.3% | 13.3% | 13.0% | 12.3% | 12.3% | 12.6% | 13.1% | |
| 60–69 | 435 | 659 | 1030 | 1491 | 2278 | 2519 | 2876 | 3376 | 3073 | 17 737 |
| 27.3% | 27.2% | 29.0% | 26.7% | 27.1% | 25.9% | 26.0% | 26.0% | 23.4% | 25.9% | |
| 70–79 | 525 | 831 | 1171 | 1872 | 2812 | 3303 | 3756 | 4464 | 4655 | 23 389 |
| 32.9% | 34.3% | 33.0% | 33.5% | 33.4% | 34.0% | 33.9% | 34.4% | 35.4% | 34.2% | |
| ≥80 | 388 | 578 | 809 | 1425 | 2201 | 2638 | 3087 | 3532 | 3757 | 18 415 |
| 24.3% | 23.8% | 22.8% | 25.5% | 26.2% | 27.1% | 27.9% | 27.3% | 28.6% | 26.9% | |
| Total | 1595 | 2425 | 3553 | 5584 | 8410 | 9727 | 11 079 | 12 960 | 13 146 | 68 479 |
Chemotherapy, surgery and adjuvant therapy are the main options for initial PC treatment (Fig. 2). Chemotherapy was the most frequently selected treatment, its number was twice that of surgery, including adjuvant therapy. In 2018, 26% of the patients are treated with chemotherapy only, and 13% of the patients underwent surgery and half of the patients received chemotherapy followed by surgery. Although radiation therapy is listed as an option in the JPS guideline for PC, few patients underwent it. On the other hand, 6% of patients underwent surgery followed by adjuvant chemotherapy in 2018. However, the number of patients undergoing chemotherapy continued to decrease during the study period. On the contrary, over half of the patients were classified as ‘untreated’ [no active treatment, presumably received best supportive care (BSC)] over the study period, which may be an overestimate due to the limitations of the MDV database.
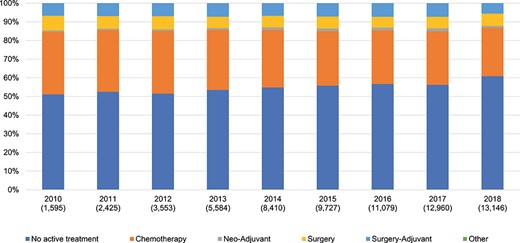
PC treatment option for all ages. The proportion of no active treatment may be an overestimate because of the double count of patients and underrepresentation of designated hospitals in the database. PC, pancreatic cancer.
Treatment patterns were similar among the three age groups ≤79 years (Fig. 3). The majority of patients in the ≥80 age group received BSC, with the proportion increasing gradually over the year. The proportion of patients who underwent chemotherapy has decreased among all age groups over the last 9 years.

PC treatment option by age. The proportion of no active treatment may be an overestimate because of the double count of patients and underrepresentation of designated hospitals in the database.
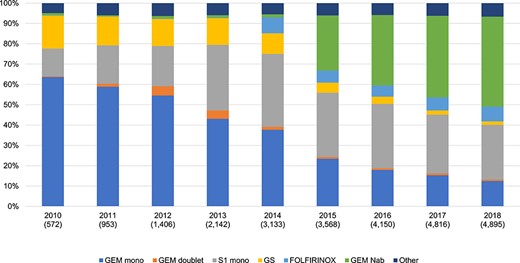
Chemotherapy pattern for all age groups. Gem mono, gemcitabine monotherapy; Gem doublet, combination therapy containing gemcitabine regimen except for gemcitabine + S-1 and gemcitabine + nab-paclitaxel; GS, gemcitabine + S-1; GEM Nab, gemcitabine + nab-paclitaxel.
Chemotherapy
The percentage of patients receiving gemcitabine monotherapy decreased from 62% in 2010 to 12% in 2018. The proportion of patients receiving S-1 (containing tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate) monotherapy has increased since 2010, reaching 27% in 2018. After gemcitabine + nab-paclitaxel was reimbursed in 2014, the use of gemcitabine and nab-paclitaxel has been increasing from 2015 and over 40% of the patients received it in 2018. On the other hand, the trend in the use of FOLFIRINOX remained stable from 2014 to 2018 after its reimbursement in 2013 (Fig. 4).
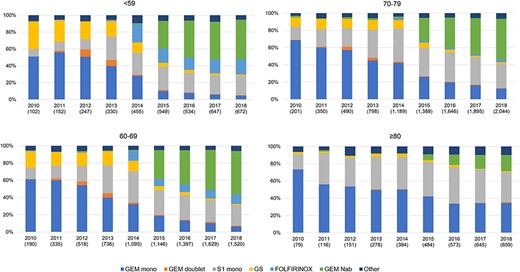
Across all age groups, the proportion of patients receiving gemcitabine + nab-paclitaxel has increased from 2015 (Fig. 5). In 2018, although gemcitabine + nab-paclitaxel was the most commonly prescribed regimen in the patients aged ≤79 years, gemcitabine or S-1 monotherapy was mainly used in the ≥80 age group. Approximately, 14% of the patients aged ≤59 years received FOLFIRINOX, and the proportion decreased with increasing age. Among the ≥80 age group, gemcitabine or S-1 monotherapy remained the most common treatment option during the study period.
Discussion
On the basis of a hospital claims database constructed by the MDV, we examined the real-world treatment patterns of PC from 1 January 2010 to 31 December 2018 in Japan. We found that chemotherapy is the first-line treatment option for PC patients of all ages. The proportion of patients undergoing surgery is about half of those receiving chemotherapy. These trends persisted throughout the study period.
The JPS guideline recommends that surgery should be considered in the treatment of resectable PC, and chemotherapy should be used for unresectable PC (4). However, diagnosis of PC at early, curable stage remains difficult due to the lack of specific symptoms and validated biomarkers. The national database of HBCR showed that 62% of the patients with stage III or higher were categorized as unresectable (3). Although we did not analyse stage at diagnosis, the low proportion of patients who underwent surgery in our database suggested that the majority of patients had unresectable tumours at diagnosis and such trend remained unchanged from 2010.
In this study, ~50–60% of the patients were categorized into the ‘no active treatment’ group. This proportion should be interpreted cautiously because it was apparently higher than that shown in the HBCR database or in the previous study using real-world treatment data (3). The major reason is that the database could not capture patient transfer information between hospitals. Patients may be hospitalized at a DPC hospital for the purpose of only supportive care, such as biliary drainage and pain control, while they were receiving chemotherapy at other DPC hospitals. This limitation resulted in the double count of some patients in the databases, causing seemingly higher proportions of no active treatment. Another possible reason is the underrepresentation of designated cancer hospitals in the database. The database covered only 50% (188 out of 376) of cancer treatment facilities, suggesting that many patients may receive only diagnosis or supportive care at non-designated cancer hospitals, whereas they may receive active treatments at other designated cancer hospitals that are not covered by the database. As active systemic treatment of PC tends to be concentrated in designated cancer hospitals in Japan, as evidence by the HRBC database, the underrepresentation of designated cancer hospitals may partly account for the higher proportion of no active treatment.
Despite limitations inherent in medical claims database, our results showed that the proportion of patients receiving BSC differed between ≤79 and ≥80 age groups. This finding drawn from real-world data suggests that in addition to cancer stage, other factors such as patient background should also be considered in determining treatment modalities.
Our data indicate that several changes had occurred in chemotherapy patterns during 2010 and 2018. First, gemcitabine, S-1 and gemcitabine/S-1 (GS) combination used to be standard chemotherapy by 2012, but S-1 has been increasingly administered and use of gemcitabine monotherapy and GS combination has been decreased after 2013. In 2013, the gemcitabine and S-1 trial (GEST) study demonstrated non-inferiority of S-1 monotherapy to gemcitabine in terms of overall survival among locally advanced or metastatic PC patients (16). In addition, the interim analysis of the Japan adjuvant study group of pancreatic cancer (JASPAC) 01 trial indicated that adjuvant chemotherapy with S-1 improves overall survival in resected PC patients (17). On the other hand, GS combination therapy did not show statistically significant improvement in overall survival compared with gemcitabine monotherapy in the GEST study (16). Second, FORFIRINOX was introduced to clinical practice in 2014, but the proportion of patients receiving FORFIRINOX had not exceeded 10% by 2018. In 2014, the results of FOLFIRINOX phase II study for Japanese patients were published (18). Although a favourable treatment outcome was observed for metastatic PC patients, the proportion of patients experiencing grade 3 or 4 adverse events, such as neutropenia and febrile neutropenia, was higher in this trial than in the original FOLFIRINOX II/III study (5). Third, gemcitabine + nab-paclitaxel has been increasingly prescribed since 2015, and it became the most common regimen in 2018. A phase I/II study of gemcitabine + nab-paclitaxel in Japanese patients showed similar efficacy and safety profiles compared with the original phase II/III study of gemcitabine + nab-paclitaxel (19). Of note, the frequency of febrile neutropenia was similar between these two studies. Although strength of recommendation levels was similar for use of FOLFIRINOX or gemcitabine + nab-paclitaxel in the JPS guideline, our data suggest that Japanese physicians prefer regimens with fewer adverse events.
Chemotherapy patterns vary across countries. Similar to the USA (20), the proportion of patients receiving gemcitabine monotherapy decreased significantly in recent years, whereas that of FOLFIRINOX and gemcitabine + nab-paclitaxel increased in Japan. On the other hand, other chemotherapy regimens differ between the two countries. First, S-1 is used for PC in Japan but not in the USA. In Japan, S-1 has been one of the major chemotherapy regimens since 2010, and the prescriptions remained steady even after the introduction of gemcitabine + nab-paclitaxel. Several clinical trials in Japan demonstrated the efficacy of S-1 monotherapy for PC (16,21) in both the initial chemotherapy and the post-operative adjuvant chemotherapy. However, S-1 is not recommended in the US guidelines because its efficacy has not been proved in a non-Japanese population (22). Second, the proportion of patients receiving FOLFIRINOX was higher in the USA than in Japan. One possible reason is that FOLIFIRINOX was recommended as the first-line regimen in the 2018 American Society of Clinical Oncology guideline (23).
One notable finding is that chemotherapy patterns differed by age group. Although the proportion of patients ≤79 years receiving FORFIRINOX or gemcitabine + nab-paclitaxel has increased after their reimbursement, the use of these new regimens in patients ≥80 was limited and traditional regimens such as gemcitabine and S-1 were mainly used. In addition, the administration of FOLFIRINOX decreased with increasing age, it was not used in patients ≥80 years. Although recent RCTs have shown superior therapeutic efficacy of FORFIRINOX over conventional chemotherapy, concerns remain over its haematologic and gastrointestinal toxicity. This fact indicates that choosing an optimal chemotherapy regimen should be individualized to each patient, based on risk and benefit analysis. We did not analyse the correlation between grade, complications and PS with use of regimens. However, our finding that chemotherapy patterns vary by age suggests that patient background needs to be factored in the selection of optimal chemotherapy regimens.
Our study has several limitations. First, as with most studies relying on hospital claims data, the completeness of data related to PC treatment is a concern. We could not exclude the possibility of errors, omissions or variations in the database. Second, information on PS, treatment outcome and prognosis were not available. The lack of these data did not allow for a detailed analysis of the associations of PS or stage with treatment option. Third, claims data included in the MDV database were limited to DPC hospitals. It is possible that patients treated in small clinics may have been overlooked; however, PC patients usually visit larger hospitals included in MDV database even if they first consulted with physicians in a small clinic. Fourth, the chemotherapy group included those patients who received neoadjuvant therapy, but did not undergo surgery due to disease progression or other reasons. These patients could not be excluded because it is impossible to differentiate these patients from other patients who received routine chemotherapy.
In summary, our hospital claims-based study showed that chemotherapy is the major treatment option for PC patients in Japan. Components in the first-line regimen has changed since 2010, with combination regimens (gemcitabine + nab-paclitaxel) accounting for 44% in 2018. Such changes have been reflected in the updated treatment guidelines. More clinical trials are warranted to test the efficacy of combination chemotherapy regimens in order to improve the prognosis of PC.
Funding
This study did not receive any funding.
Conflict of Interest
Toshiaki Suzuki is an employee of Pfizer R&D Japan G.K. This study is a collaboration with the commercial database provider Medical Data Vision Co., Ltd. (MDV; Tokyo, Japan). The authors were not financially compensated for the collaboration. All other authors report no conflict of interest in this work.
References
Margaret A. Tempero, Mokenge P. Malafa, Mahmoud Al-Hawary, et al. Pancreatic Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines. https://www.nccn.org/professionals/physician_gls/default.aspx (2 October 2020, date last accessed).


