-
PDF
- Split View
-
Views
-
Cite
Cite
Walderez O Dutra, Daniela Faria Barbosa, Paulo Eduardo Alencar de Souza, Daniel Morgan, Shelene Poetker, Luiz Henrique Guimarães, Olívia Bacelar, Kenneth J Gollob, Edgar M Carvalho, A Th2-Type Response Is Associated With Exuberant Lesions in Pregnant Women Infected With Leishmania braziliensis, The Journal of Infectious Diseases, Volume 219, Issue 3, 1 February 2019, Pages 480–488, https://doi.org/10.1093/infdis/jiy510
Close - Share Icon Share
Abstract
Cutaneous leishmaniasis (CL) is characterized by an exaggerated inflammatory response. During pregnancy there is a decreased inflammatory response, and we have shown that pregnant women with CL develop exuberant lesions.
Cytokine production by peripheral blood mononuclear cells and the frequency of cells expressing cytokines in lesions from pregnant and nonpregnant women with CL were evaluated.
We observed that CL lesions from pregnant women displayed a more intense cellular infiltrate, associated with an increase in neutrophils and CD4+ cells. While no difference was observed regarding the number of interferon-gamma (IFN-γ)+ cells in lesions from pregnant compared to nonpregnant women with CL, interleukin-10 (IL-10) and IL-4 expression were approximately 3-times higher in lesions in pregnant women. Main sources of IL-4 and IL-10 were CD4+ and CD68+ cells, respectively. Expression of IL-4, but not IFN-γ or IL-10, was positively correlated with the intensity of inflammatory infiltrate in lesions from pregnant women.
These results provide evidence of an IL-4–mediated pathology in Leishmania braziliensis-infected pregnant women. These differences in lesion pathogenesis in pregnant and nonpregnant women may open possibilities for new therapies for CL treatment during pregnancy, which are currently lacking.
A great challenge in disease management in pregnant women is the restrictions imposed by the need for pregnancy-compatible therapeutic interventions, urging the search for alternatives that will cure the illnesses and not lead to consequences for the mother or baby. Because several medications cannot be administered during pregnancy, many diseases go untreated, causing a burden to the mother’s health, and potentially compromising pregnancy outcome.
Human cutaneous leishmaniasis (CL), caused by Leishmania (Viannia) braziliensis, leads to the appearance of 1 or more ulcerated skin lesions associated with an exuberant satellite lymphadenopathy [1]. Leishmaniasis is endemic in Brazil, as well as other tropical countries, and is mostly prevalent in young men, although it also affects women of reproductive age [2, 3]. Treatment of CL typically relies on the administration of pentavalent antimony during an extended period, which usually lasts for 21 days and, in refractory cases, may last for up to 6 months [4]. While relatively effective, this treatment regimen is not recommended for use in pregnant women due to its toxicity [5, 6]. There is no current therapy that can be employed in pregnant women to treat CL [7]. As a result, pregnant women who become infected with Leishmania display exuberant skin lesions, with typical but also atypical aspects, as shown previously by us [3]. An alarming association observed in our previous studies was that over 20% of pregnant women with CL reported preterm or stillbirths [3]. Thus, there is an urgent need to find alternative treatments that can be administered to pregnant women infected by Leishmania. Understanding the mechanisms that underlie the pathogenesis of CL during pregnancy will provide key information towards possible alternative targets and intervention strategies.
A number of studies have shown that the pathogenesis of CL is associated with the establishment of an exuberant inflammatory response, with local and systemic production of inflammatory cytokines such as interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) [8–12], as well as lower expression of IL-10 and its receptor [10]. Additionally, the recruitment of CD8+ cells expressing the cytotoxic molecule granzyme A to lesion sites has been implicated in the progression of CL [13–15]. Immune response during pregnancy is biased towards an antiinflammatory response [16, 17], which has been shown to be beneficial in some diseases [18] but also to exacerbate others [19].
Because Leishmania-infected pregnant women display exuberant CL lesions, despite the predominance of antiinflammatory immunity that usually accompanies pregnancy, we hypothesized that the pathogenic mechanism of CL lesion development was different in pregnant and nonpregnant women. We designed a study to determine the composition of the inflammatory infiltrate in CL lesions from pregnant and nonpregnant women, evaluating the frequencies of different cell populations, cytokine expression, and cytokine sources. We observed that lesions from pregnant women displayed a very intense inflammatory infiltrate, associated with the recruitment of CD4+ cells and high expression of IL-4, as opposed to the previously reported preferential recruitment of T cells expressing inflammatory cytokines in nonpregnant CL patients [10]. Our data showed that the composition of the inflammatory infiltrate is significantly different in CL lesions during pregnancy as compared to lesions from nonpregnant women. Importantly, our data suggest that distinct mechanisms are involved in the pathogenesis of CL during pregnancy. This finding may guide the search for alternative treatments for pregnant women affected by CL, which are currently not available.
METHODS
Patients
The 38 patients analyzed in this study, comprising 19 pregnant and 19 nonpregnant women, were from Corte de Pedra, an endemic area for L. braziliensis, located 280 km southeast of Salvador, Bahia, Brazil. Diagnosis of CL was performed by parasite isolation and/or histopathological analysis. For all cases, parasite species were typed and L. braziliensis was the causal agent. Blood collection for immunologic studies and biopsies were obtained previous to therapy. All nonpregnant CL patients were treated with meglumine antimoniate at time of the diagnosis, and pregnant women were treated after delivery. All patients were volunteers, and informed consent was obtained from all individuals prior to collection of blood and tissues from lesions. The Ethical Committee of Universidade Federal da Bahia approved all procedures involved in this study.
Immunologic Study in Peripheral Blood Mononuclear Cells
Peripheral blood mononuclear cells (PBMC) were separated from heparinized venous blood by Ficoll-Hypaque gradient centrifugation. The cells were cultured in RPMI 1640 (Life Technologies GibcoBRL, Grand Island, NY) containing 10% human AB serum (Sigma, St. Louis, MO), HEPES, and antibiotics at a concentration of 3 × 106 cells/mL. The cells were plated in 24-well flat-bottom microtiter plates (Falcon; Becton Dickinson, Lincoln Park, NJ) and stimulated with media alone (unstimulated) or 5 µg/mL of soluble Leishmania antigen (SLA). Cell cultures were incubated at 37°C with 5% CO2 for 72 hours and the levels of IFN-γ and TNF were determined in supernatants using the sandwich enzyme-linked immunosorbent assay (ELISA) technique (BD Bioscience Pharmingen, San Jose, CA). The results are expressed in pg/mL.
Biopsies
Skin biopsies were taken from the borders of active lesions, using a 4-mm diameter punch, after the application of local anesthetic. Lesions were maintained in a 30% sucrose solution for approximately 30 minutes at 4°C and then transferred to OCT Tissue Tek freezing media and immediately placed in dry ice. The material was stored at −70°C until analysis.
Histological and Immunofluorescence Staining
Individual 4–5 µm cryosections were placed in saline-precoated slides and fixed for 10 minutes with acetone. Slides were incubated with phosphate buffered saline (PBS) for 15 minutes and submitted to either hematoxilin-eosin (H&E) staining or to immunofluorescence using specific monoclonal antibodies. Standard H&E staining was performed to assure tissue integrity, as well as evaluation of the intensity, composition, and location of the inflammatory infiltrate. H&E stained sections were analyzed using a light microscopy (Axiovert, Zeiss). We acquired the data using a magnification of 400 ×, and the frequencies of neutrophils, eosinophils, and mononuclear cells were expressed as percentage of the total cell count. A total of 16 fields/sample were acquired for the histological analysis by 2 independent observers.
Immunofluorescence reactions involved incubation with fluorescein isothiocyanate (FITC) or phycoerythrin (PE)-labeled monoclonal antibodies directed to surface receptors (CD4 cloneS3.5, CD8 clone 3B5, and CD68 clone Ki-M7) or intracellular molecules (IFN-γ cloneB27, IL-10 clone 9D7, and IL-4 clone 11B11), respectively. Sections were incubated with antibody mixtures overnight at 4°C. After staining, preparations were extensively washed with PBS, counter-stained with 4′,6′diamidino-2-phenylindole (DAPI) and mounted using antifade mounting media (Molecular Probes, Eugene, OR). Slides were kept at 4°C, protected from light, until acquisition in a laser scanning confocal microscope (Zeiss, Thornwood, NY). Isotype controls were performed to confirm the lack of nonspecific staining. Monoclonal antibodies were purchased from Becton-Dickinson (San Jose, CA).
Confocal Analysis
Confocal analysis was performed using a Meta-510 Zeiss laser scanning confocal system running LSMix software coupled to a Zeiss microscope (Axiovert 100) with an oil immersion Plan-Apochromat objective (63×, 1.2 numerical aperture). A water-cooled argon UV laser (488 nm) or a krypton/argon laser was used to excite the preparation (through its 363, 488, or 568 nm line), and light emitted was selected with band pass filters (522/35 for FITC and DAPI, 598/40 for PE). For each section, the inflammatory infiltrate present in the connective tissue adjacent to the epithelia was located and an area presenting with a uniform infiltrate was selected for analysis. Within this inflammatory area, a minimum of 6 images (fields) were collected. Image analysis and processing were performed with the software Lasersharp (Bio-Rad), Confocal Assistant, Adobe Photoshop, and Image Tool, as previously [10,13]. Analyses were performed by counting the total number of cells in the 6 fields acquired and calculating the average of cells/section for each patient. This calculation was performed for each parameter analyzed, allowing for determination of the total number of inflammatory cells (total number of DAPI+ cells within the inflammatory infiltrate), the number of FITC or PE single-positive cells, as well as the number of double-positive cells. The counts were performed blindly and the results were expressed as the average of cells/field for each parameter for each patient, and then the values were averaged for each group.
Statistical Analysis
The comparisons for a given parameter were carried out using nonparametric Mann-Whitney test. Results were considered statistically different when the analysis returned a P < .05.
RESULTS
Clinic and Immunologic Features in Pregnant and Nonpregnant Women
The clinical features and cytokine production by PBMCs of the pregnant and nonpregnant women with CL who participated in the study are shown on Table 1. There was no difference regarding Montenegro skin test results, but the size and number of lesions, as well as illness duration, were greater in pregnant than nonpregnant women. Although there was no difference regarding the spontaneous or Leishmania antigen-stimulated TNF and IL-10 production by PBMC, IFN-γ production was significantly lower in pregnant women with CL as compared to nonpregnant women.
Demographic, Clinical, and Immunological Features of Pregnant Women and Nonpregnant Women with Leishmaniasis
| . | Pregnant CL n = 19 . | Nonpregnant CL n = 19 . |
|---|---|---|
| Age, y | 22 (17–27) | 23 (14–27) |
| Illness duration, moa | 2 (1–4) | 1 (1–2) |
| Lesion size, mma | 26 (16–40) | 15 (11–20) |
| Number of lesionsa | 2 (1–3) | 1(1–2) |
| MST, mm | 21 (16–26) | 16 (9–20) |
| TNF, medium, pg/mL | 8 (0–241) | 20 (0–53) |
| TNF, SLA, pg/mL | 1078 (472–2347) | 963 (35–2363) |
| IFN-γ, medium, pg/mL | 16 ± 50 | 79 ± 218 |
| IFN-γ, SLA, pg/mLa | 400 (165–1561) | 1950 (49–3555) |
| IL-10, medium, pg/mL | 19 (12–34) | 16 (5–153) |
| IL-10, SLA, pg/mL | 35 (20–54) | 43 (30–94) |
| . | Pregnant CL n = 19 . | Nonpregnant CL n = 19 . |
|---|---|---|
| Age, y | 22 (17–27) | 23 (14–27) |
| Illness duration, moa | 2 (1–4) | 1 (1–2) |
| Lesion size, mma | 26 (16–40) | 15 (11–20) |
| Number of lesionsa | 2 (1–3) | 1(1–2) |
| MST, mm | 21 (16–26) | 16 (9–20) |
| TNF, medium, pg/mL | 8 (0–241) | 20 (0–53) |
| TNF, SLA, pg/mL | 1078 (472–2347) | 963 (35–2363) |
| IFN-γ, medium, pg/mL | 16 ± 50 | 79 ± 218 |
| IFN-γ, SLA, pg/mLa | 400 (165–1561) | 1950 (49–3555) |
| IL-10, medium, pg/mL | 19 (12–34) | 16 (5–153) |
| IL-10, SLA, pg/mL | 35 (20–54) | 43 (30–94) |
Cytokines were measured in supernatants of peripheral blood mononuclear cells stimulated or not with soluble Leishmania antigen (SLA), as described in Methods.
The numbers are expressed in median (range).
Abbreviations: CL,cutaneous leishmaniasis; IFN-γ, interferon-gamma; IL, interleukin; MST, Montenegro skin test; TNF, tumor necrosis factor.
aStatistically significant comparisons, P < .05.
Demographic, Clinical, and Immunological Features of Pregnant Women and Nonpregnant Women with Leishmaniasis
| . | Pregnant CL n = 19 . | Nonpregnant CL n = 19 . |
|---|---|---|
| Age, y | 22 (17–27) | 23 (14–27) |
| Illness duration, moa | 2 (1–4) | 1 (1–2) |
| Lesion size, mma | 26 (16–40) | 15 (11–20) |
| Number of lesionsa | 2 (1–3) | 1(1–2) |
| MST, mm | 21 (16–26) | 16 (9–20) |
| TNF, medium, pg/mL | 8 (0–241) | 20 (0–53) |
| TNF, SLA, pg/mL | 1078 (472–2347) | 963 (35–2363) |
| IFN-γ, medium, pg/mL | 16 ± 50 | 79 ± 218 |
| IFN-γ, SLA, pg/mLa | 400 (165–1561) | 1950 (49–3555) |
| IL-10, medium, pg/mL | 19 (12–34) | 16 (5–153) |
| IL-10, SLA, pg/mL | 35 (20–54) | 43 (30–94) |
| . | Pregnant CL n = 19 . | Nonpregnant CL n = 19 . |
|---|---|---|
| Age, y | 22 (17–27) | 23 (14–27) |
| Illness duration, moa | 2 (1–4) | 1 (1–2) |
| Lesion size, mma | 26 (16–40) | 15 (11–20) |
| Number of lesionsa | 2 (1–3) | 1(1–2) |
| MST, mm | 21 (16–26) | 16 (9–20) |
| TNF, medium, pg/mL | 8 (0–241) | 20 (0–53) |
| TNF, SLA, pg/mL | 1078 (472–2347) | 963 (35–2363) |
| IFN-γ, medium, pg/mL | 16 ± 50 | 79 ± 218 |
| IFN-γ, SLA, pg/mLa | 400 (165–1561) | 1950 (49–3555) |
| IL-10, medium, pg/mL | 19 (12–34) | 16 (5–153) |
| IL-10, SLA, pg/mL | 35 (20–54) | 43 (30–94) |
Cytokines were measured in supernatants of peripheral blood mononuclear cells stimulated or not with soluble Leishmania antigen (SLA), as described in Methods.
The numbers are expressed in median (range).
Abbreviations: CL,cutaneous leishmaniasis; IFN-γ, interferon-gamma; IL, interleukin; MST, Montenegro skin test; TNF, tumor necrosis factor.
aStatistically significant comparisons, P < .05.
Inflammatory Infiltrate in CL Lesions of Pregnant and Nonpregnant Women
Histopathological analysis of lesion showed that the inflammatory infiltrates were predominantly composed of mononuclear cells in lesions from both groups of Leishmania-infected women (Table 2 and Figure 1A and 1C). We observed an increased frequency of neutrophils in lesions from pregnant as compared to nonpregnant women with CL (Table 1). A qualitative analysis showed that giant multinuclear cells were observed in lesions from Leishmania-infected pregnant women (Figure 1B). We also quantified the number of Leishmania-positive cells (Figure 1D) in the lesions from pregnant and nonpregnant women with CL and observed that the number of parasites was not statistically different (Figure 1E).
Percentage of Mononuclear and Polymorphonuclear (Neutrophil and Eosinophil) Cells in Lesions from Leishmania-Infected Pregnant and Nonpregnant Women
| Parameter . | Mean . | Median . | Minimum . | Maximum . |
|---|---|---|---|---|
| Pregnant CL (n = 7) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.35 | 0.31 | 0.00 | 1.03 |
| Neutrophilsa | 0.21 | 0.16 | 0.00 | 0.44 |
| Eosinophils | 0.14 | 0.02 | 0.00 | 0.59 |
| Nonpregnant CL (n = 6) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.47 | 0.44 | 0.08 | 1.08 |
| Neutrophilsa | 0.34 | 0.29 | 0.11 | 0.72 |
| Eosinophils | 0.13 | 0.02 | 0.00 | 0.54 |
| Parameter . | Mean . | Median . | Minimum . | Maximum . |
|---|---|---|---|---|
| Pregnant CL (n = 7) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.35 | 0.31 | 0.00 | 1.03 |
| Neutrophilsa | 0.21 | 0.16 | 0.00 | 0.44 |
| Eosinophils | 0.14 | 0.02 | 0.00 | 0.59 |
| Nonpregnant CL (n = 6) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.47 | 0.44 | 0.08 | 1.08 |
| Neutrophilsa | 0.34 | 0.29 | 0.11 | 0.72 |
| Eosinophils | 0.13 | 0.02 | 0.00 | 0.54 |
Hematoxilin-eosin stained sections were analyzed using light microscopy. All connective tissue from each lesion was evaluated in a power magnification of ×400. The comparisons of percentage for a given parameter between the groups were performed using the nonparametric Mann-Whitney test.
Abbreviation: CL, cutaneous leishmaniasis.
aStatistically significant comparison, P < .05.
Percentage of Mononuclear and Polymorphonuclear (Neutrophil and Eosinophil) Cells in Lesions from Leishmania-Infected Pregnant and Nonpregnant Women
| Parameter . | Mean . | Median . | Minimum . | Maximum . |
|---|---|---|---|---|
| Pregnant CL (n = 7) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.35 | 0.31 | 0.00 | 1.03 |
| Neutrophilsa | 0.21 | 0.16 | 0.00 | 0.44 |
| Eosinophils | 0.14 | 0.02 | 0.00 | 0.59 |
| Nonpregnant CL (n = 6) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.47 | 0.44 | 0.08 | 1.08 |
| Neutrophilsa | 0.34 | 0.29 | 0.11 | 0.72 |
| Eosinophils | 0.13 | 0.02 | 0.00 | 0.54 |
| Parameter . | Mean . | Median . | Minimum . | Maximum . |
|---|---|---|---|---|
| Pregnant CL (n = 7) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.35 | 0.31 | 0.00 | 1.03 |
| Neutrophilsa | 0.21 | 0.16 | 0.00 | 0.44 |
| Eosinophils | 0.14 | 0.02 | 0.00 | 0.59 |
| Nonpregnant CL (n = 6) | ||||
| % Mononuclear cells | 99 | 99 | 98 | 100 |
| % Polymorphonuclear cells | 0.47 | 0.44 | 0.08 | 1.08 |
| Neutrophilsa | 0.34 | 0.29 | 0.11 | 0.72 |
| Eosinophils | 0.13 | 0.02 | 0.00 | 0.54 |
Hematoxilin-eosin stained sections were analyzed using light microscopy. All connective tissue from each lesion was evaluated in a power magnification of ×400. The comparisons of percentage for a given parameter between the groups were performed using the nonparametric Mann-Whitney test.
Abbreviation: CL, cutaneous leishmaniasis.
aStatistically significant comparison, P < .05.

Histological analysis and parasite count in lesions from nonpregnant (n = 6) and pregnant (n = 7) patients with cutaneous leishmaniasis (CL). Hematoxylin-eosin–stained sections of lesions were analyzed under a light microscope. Sections obtained from pregnant (A) and nonpregnant (C) women with CL, showing the epithelia and connective layer containing the inflammatory infiltrate. B, Detail of multinucleate giant cells in lesions from pregnant women and (D) parasites inside cells from lesions in pregnant women. Bars indicate 25 μm. Number of parasite per section are represented in (E) as scatter plots with median and interquartile ranges from lesions in pregnant and nonpregnant women. Abbreviation: NS, nonsignificant.
Higher Intensity of Inflammatory Infiltrate Is Associated With Higher Number of CD4+ Cells in Lesions From Pregnant as Compared to Nonpregnant Women with CL
The intensity of the inflammatory infiltrate in lesions from pregnant and nonpregnant patients with CL was determined by counting the number of DAPI-positive cells per field, using confocal microscopy, as described in Methods. The average number of cells was significantly higher in lesions from CL pregnant, as compared to nonpregnant Leishmania-infected women, demonstrating the occurrence of a more intense inflammation related to CL during pregnancy (Figure 2A). The increase in the inflammatory infiltrate observed in lesions from Leishmania-infected pregnant women with CL was accompanied by an increase in the number of CD4+ cells (Figure 2B), but not of CD8+ (Figure 2C) or CD68+ (Figure 2D) cells, suggesting a preferential recruitment of CD4+ cells to the lesion sites in pregnant women (Figure 2B).

Determination of the intensity of the inflammatory infiltrate (A), and number of CD4+ (B), CD8+ (C), and CD68+ (D) cells in lesions from nonpregnant (n = 6) and pregnant (n = 7) patients with cutaneous leishmaniasis (CL). Tissue sections were stained with fluorescein isothiocyanate (FITC)-labeled monoclonal antibodies anti-CD4, anti-CD8, or anti-CD68 and counter stained with 4′,6′diamidino-2-phenylindole (DAPI). Six optical sections for each sample were obtained simultaneously with the 363 and 488 nm line of the argon/krypton laser and appropriate filters. Total DAPI+ cells were counted to determine the intensity of the inflammatory infiltrate for each patient. Total FITC+ (stained with anti-CD4, CD8, or CD68) cells on each slide were counted and for each patient to determine the number of cells positive for each marker. Values represent the scatter plots with median and interquartile ranges of each group following determination of the number of positive cells for the indicated molecule(s). P values comparing the different groups are presented. Abbreviation: NS, nonsignificant.
Lesions From Pregnant Women With CL Display a Higher Number of Cells Expressing IL-10 and IL-4, but Similar Numbers Expressing IFN-γ
The expression of the inflammatory cytokine IFN-γ was evaluated in lesions from pregnant and nonpregnant women with CL. Our analysis showed that the total numbers of cells expressing IFN-γ were statistically similar in lesions from pregnant and nonpregnant women with CL (Figure 3A). On the other hand, the number of IL-10 and IL-4–expressing cells were approximately 3-times higher in lesions from pregnant as compared to nonpregnant women with CL (Figure 3B and 3C). We also performed correlation analysis between the expression of the different cytokines and the intensity of the inflammatory infiltrate in lesions from pregnant women. We observed that the number of IFN-γ+ cells was not correlated with the intensity of the inflammatory infiltrate in lesions from pregnant women (Figure 3D). Despite the higher number of IL-10–expressing cells in lesions from CL pregnant women, the number of IL-10+ cells was not correlated with the intensity of the inflammatory infiltrate (Figure 3D). However, we observed that the higher the number of IL-4+ cells, the higher the intensity of the inflammatory infiltrate in pregnant women (Figure 3D). Thus, our data showed that the expression of the Th2 cytokine IL-4 was higher in lesions from pregnant patients with CL, as compared to nonpregnant women, and was associated with the intensity of the inflammatory infiltrate observed in leishmaniasis lesion during pregnancy.

Determination of the number of interferon-gamma (IFN-γ)+ cells (A), interleukin-10 (IL-10)+ (B), and IL-4+ (C) cells in lesions from nonpregnant (n = 6) and pregnant (n = 7) patients with cutaneous leishmaniasis (CL). Tissue sections were stained with phycoerythrin (PE)-labeled monoclonal antibodies anti-IFN-γ, anti-IL-10, or anti-IL-4 and counter stained with 4′,6′diamidino-2-phenylindole (DAPI). Six optical sections for each sample were obtained simultaneously with the 363 and 488 nm line of the argon/krypton laser and the appropriate set of filters. Total PE+ (stained with anti-IFN-γ, IL-10, or IL-4) cells on each slide were counted and for each patient to determine the number of cells positive for each marker. Values represent the scatter plots with median and interquartile ranges of each group following determination of the number of positive cells for the indicated molecule(s). P values comparing the different groups are presented. Analyses of correlation between the intensity of the inflammatory infiltrate, as determined by the number of DAPI+ cells, and the total number of (D) IFN-γ+, (E) IL-10+, and (F) IL4+ cells in lesions from pregnant women with CL are shown, with R2 and P values for each correlation.
CD4+ Cells Account for Over 60% of the Expression of IL-4 in Lesions from Pregnant Women With CL, While Most IL-10 Comes From CD68+ Cells
In order to gain insight into the cellular sources of the immunoregulatory cytokines, we determined the number of CD4+ T cells expressing IFN-γ and IL-4, as well as the number of CD68+ cells expressing IL-10. Our results showed that while the number of CD4+ cells expressing IFN-γ was not different between groups (Figure 4A), the number of IL-4–expressing CD4+ cells was significantly higher in lesions from pregnant as compared to nonpregnant women (Figure 4B), confirming a bias towards a Th2 response. CD4+ cells were responsible for over 60% of IL-4 expression in lesions from both pregnant and nonpregnant women with CL (Table 3). We also determined that the number of CD68+IL-10+ cells was higher in lesions from pregnant as compared to nonpregnant women with CL (Figure 4C). Interestingly, while CD68+ cells are responsible for approximately 40% of IL-10 expression in lesions from nonpregnant women with CL, they contribute to over 60% of IL-10 expression in lesions from pregnant women with CL (Table 3).
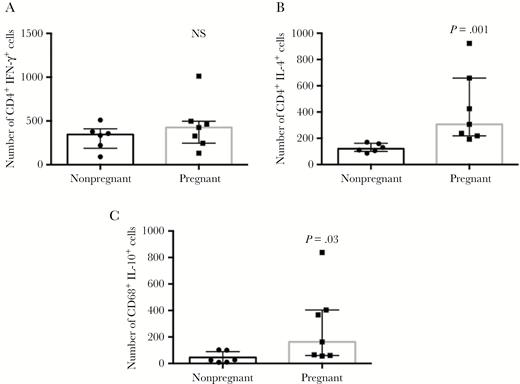
Determination of the number of interferon-gamma (IFN-γ)+CD4+ cells (A), interleukin-4 (IL-4)+CD4+ cells (B), and IL-10+CD68+ (C) cells in lesions from nonpregnant (n = 6) and pregnant (n = 7) patients with cutaneous leishmaniasis (CL). Tissue sections were stained with phycoerythrin-labeled monoclonal antibodies anti-IFN-γ, anti-IL-10, or anti-IL-4, in combination with fluorescein isothiocyanate-labeled anti-CD4 or anti-CD68. Six optical sections for each sample were obtained simultaneously with the 488 nm line of the argon/krypton laser and the appropriate set of filters. The numbers of double-positive cells (IFN-γ+CD4+, IL-4+CD4+, and IL-10+CD68+) were counted in each slide and for each patient. Values represent the scatter plots with median and interquartile ranges of each group following determination of the number of positive cells for the indicated molecules. P values comparing the different groups are presented.
Relative Contribution of CD4+ and CD68+ Cells to the Expression of IL-4 and IL-10, Respectively, in Lesions From Pregnant and Nonpregnant Women With Cutaneous Leishmaniasis
| Parameter . | Nonpregnant CL (n = 6) . | Pregnant CL (n = 7) . |
|---|---|---|
| Number of IL-4+ cells | 265 ± 230a | 629 ± 377a |
| Number of CD4+IL-4+ cells | 126 ± 25a | 405 ± 258a |
| Relative contribution of CD4+ cells to IL-4 expression | 67 ± 28 | 68 ± 18 |
| Number of IL-10+ cells | 160 ± 130a | 558 ± 382a |
| Number of CD68+IL-10+ cells | 56 ± 50 | 302 ± 253 |
| Relative contribution of CD68+ cells to IL-10 expression | 38 ± 34 | 62 ± 23 |
| Parameter . | Nonpregnant CL (n = 6) . | Pregnant CL (n = 7) . |
|---|---|---|
| Number of IL-4+ cells | 265 ± 230a | 629 ± 377a |
| Number of CD4+IL-4+ cells | 126 ± 25a | 405 ± 258a |
| Relative contribution of CD4+ cells to IL-4 expression | 67 ± 28 | 68 ± 18 |
| Number of IL-10+ cells | 160 ± 130a | 558 ± 382a |
| Number of CD68+IL-10+ cells | 56 ± 50 | 302 ± 253 |
| Relative contribution of CD68+ cells to IL-10 expression | 38 ± 34 | 62 ± 23 |
Numbers of cells were counted based on the expression of specific fluorescent markers for either IL-4 or IL-10 alone, a combination of IL-4 and CD4 or IL-10 and CD68, as indicated and described in Methods. The relative contributions of CD4+ and CD68+ cells to IL-4 and IL-10 expression, respectively, were calculated as the percentage of CD4+ or CD68+ cells expressing each cytokine in relation to the total expression of that given cytokine.
The numbers are expressed in mean ± SD. The Relative contribution is expressed in percentage.
Abbreviation: CL, cutaneous leishmaniasis; IL, interleukin.
aStatistically significant differences between pregnant and nonpregnant CL groups, P < .05.
Relative Contribution of CD4+ and CD68+ Cells to the Expression of IL-4 and IL-10, Respectively, in Lesions From Pregnant and Nonpregnant Women With Cutaneous Leishmaniasis
| Parameter . | Nonpregnant CL (n = 6) . | Pregnant CL (n = 7) . |
|---|---|---|
| Number of IL-4+ cells | 265 ± 230a | 629 ± 377a |
| Number of CD4+IL-4+ cells | 126 ± 25a | 405 ± 258a |
| Relative contribution of CD4+ cells to IL-4 expression | 67 ± 28 | 68 ± 18 |
| Number of IL-10+ cells | 160 ± 130a | 558 ± 382a |
| Number of CD68+IL-10+ cells | 56 ± 50 | 302 ± 253 |
| Relative contribution of CD68+ cells to IL-10 expression | 38 ± 34 | 62 ± 23 |
| Parameter . | Nonpregnant CL (n = 6) . | Pregnant CL (n = 7) . |
|---|---|---|
| Number of IL-4+ cells | 265 ± 230a | 629 ± 377a |
| Number of CD4+IL-4+ cells | 126 ± 25a | 405 ± 258a |
| Relative contribution of CD4+ cells to IL-4 expression | 67 ± 28 | 68 ± 18 |
| Number of IL-10+ cells | 160 ± 130a | 558 ± 382a |
| Number of CD68+IL-10+ cells | 56 ± 50 | 302 ± 253 |
| Relative contribution of CD68+ cells to IL-10 expression | 38 ± 34 | 62 ± 23 |
Numbers of cells were counted based on the expression of specific fluorescent markers for either IL-4 or IL-10 alone, a combination of IL-4 and CD4 or IL-10 and CD68, as indicated and described in Methods. The relative contributions of CD4+ and CD68+ cells to IL-4 and IL-10 expression, respectively, were calculated as the percentage of CD4+ or CD68+ cells expressing each cytokine in relation to the total expression of that given cytokine.
The numbers are expressed in mean ± SD. The Relative contribution is expressed in percentage.
Abbreviation: CL, cutaneous leishmaniasis; IL, interleukin.
aStatistically significant differences between pregnant and nonpregnant CL groups, P < .05.
DISCUSSION
The pathology associated with CL is characterized by the appearance of skin lesions that heal upon specific treatment or, in some cases, spontaneously. Moreover, upon cure, recurrence of leishmaniasis is rare, indicating the establishment of a protective immune response [20, 21]. The immune response of individuals with active CL is characterized by the production of inflammatory cytokines, which likely contribute to parasite clearance but also tissue destruction [21].
It is well documented that pregnancy is associated with dramatic changes in the immune response, shifting the immunoregulatory profile towards a noninflammatory, nonactivating one [16]. A previous study by our group has shown that pregnant women who are infected with L. braziliensis develop larger lesions, usually with atypical macroscopic aspect [3]. However, despite the worse clinical presentation, patients respond well to treatment and even spontaneous cure can be observed after delivery [3]. Approximately 20% of pregnant women infected with L. braziliensis reported premature or stillbirths [3]. Previous reports have shown a high occurrence of infectious diseases, such as brucellosis, toxoplasmosis, or fascioliasis, in Egyptian women who underwent miscarriages [22]. In a murine model, L. major infection was also associated with implantation failure and fetal resorption, which was related to the presence of a somewhat strong Leishmania-induced Th1 response [23]. Not only Leishmania infection but also its treatment has been associated with severe consequences to the fetuses. A 57% frequency of spontaneous abortions was observed in women with visceral leishmaniasis treated with stibogluconate [24]. Other therapies, such as amphotericin B, have shown good results in the treatment of pregnant women with visceral leishmaniasis [25], which is important to avoid vertical transmission [26]. However, there is no report of the use of amphotericin B to treat pregnant women with CL, and no other therapy is currently available.
One puzzling question was, if pregnant women have a less inflammatory response, why would they present such exuberant leishmaniasis lesions? We hypothesized that the inflammatory infiltrate associated with lesions was different in pregnant compared to nonpregnant women. Our results show that CL lesions from pregnant women indeed display a quantitatively and qualitatively distinct cellular composition as compared to lesions from nonpregnant women, characterized by an increase in the number of IL-4 and IL-10–producing cells.
The role of IL-10 in down-modulating the immune response in human leishmaniasis is well documented [27]. In mice, IL-4 plays an important role in parasite dissemination and pathology [28]. However, there is a lack of evidence that IL-4 plays a role in human leishmaniasis. Here we show that the intensity of the inflammatory infiltrate was greater in lesions from pregnant as compared to nonpregnant women with CL and associated with an increase in neutrophils and CD4+ cells. Both these cell types are able to produce cytokines, including IL-4, which increased in lesions from pregnant women. In fact, we determined that around 70% of the IL-4 produced by lymphocytes in the lesions came from CD4+ cells. While we did not perform specific staining for IL-4 and neutrophils or other lymphocyte populations, it is possible that these cells can also produce IL-4, as previously shown [29], contributing for the establishment of a Th2-biased environment, although neutrophils do comprise a small percentage of cells present in lesions in pregnant women with CL. An interesting qualitative observation was the presence of giant multinucleated cells in lesions from pregnant women. Previous studies have also shown that IL-4 is able to induce the fusion of monocytes and macrophages into multinucleated giant cells (MGC) [30] and that these giant cells display enhanced macrophage activities such as phagocytosis and production of cytokines [31]. Thus, it is possible that the IL-4–rich environment in lesions from pregnant women stimulated the formation of MGC. Given that previous studies have shown that MGC display intense candidacidal activity [32], it is possible that these cells play a role in parasite control. Further studies concerning the functional potential of these cells need to be performed to clarify their role in leishmaniasis.
Despite the predominance of a Th2 environment, we did not observe an increase in parasite numbers in lesions from pregnant women with CL. However, studying pregnant hamsters infected with L. panamensis, which causes cutaneous lesions, Osorio et al showed that the estrogen produced during pregnancy increased the expression of inducible nitric oxide synthase, which enhances the ability of macrophages and neutrophils to kill Leishmania [33]. Thus, it is possible that this mechanism contributed to parasite control, even in an apparently unfavorable environment for macrophage activation.
It is noteworthy that IFN-γ expression was observed in lesions from pregnant women, which also may help macrophage activation and cellular recruitment to lesion sites. While IFN-γ levels were similar in lesions from pregnant and nonpregnant women, IL-10 increased in lesions from pregnant women, adding to the establishment of a Th2-like environment, consistent with pregnancy. IL-10 expression has been strongly associated with success of pregnancy, due to its immunomodulatory activity [16]. While the number of IL-10+ cells was elevated in lesions from pregnant women, similar to IL-4, it was not correlated with the intensity of the inflammatory infiltrate, as was IL-4. The correlation of IL-4 expression and the intensity of the inflammatory infiltrate may be associated with the ability of IL-4 to induce adhesion molecule expression and increase cell recruitment [34]. This suggests a prominent role for IL-4 in the establishment of CL lesions during pregnancy, implicating IL-4 in the pathogenesis of human leishmaniasis.
Previous studies have shown that the use of conventional antimony treatment with immunotherapeutic-based interventions allows for the use of lower doses of drug, decreasing toxicity [35]. In addition, other immune-based therapies have been successful in the treatment of resistant forms of leishmaniasis [36, 37]. Taken together, our data show marked differences in comparing the inflammatory infiltrate of Leishmania lesions from pregnant to nonpregnant women, suggesting, for the first time, a role for IL-4 in the pathogenesis of CL during pregnancy. These data may form the basis for much needed immune-based alternative therapies directed at treating CL during pregnancy.
Notes
Acknowledgment. We thank Ednaldo Lago and the team at Corte de Pedra for outstanding assistance in work with patients.
Financial support. This work was supported by Instituto Nacional de Ciência e Tecnologia, Fundação de Amparo `a Pesquisa do Estado de Minas Gerais, and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Potential conflicts of interest. K. J. G. is the founder of BRISA Diagnostics. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




