-
PDF
- Split View
-
Views
-
Cite
Cite
Venetia Qendri, Johannes A Bogaards, Johannes Berkhof, Health and Economic Impact of a Tender-Based, Sex-Neutral Human Papillomavirus 16/18 Vaccination Program in the Netherlands, The Journal of Infectious Diseases, Volume 216, Issue 2, 15 July 2017, Pages 210–219, https://doi.org/10.1093/infdis/jix272
Close - Share Icon Share
Abstract
Uptake of human papillomavirus (HPV) vaccine among girls in the Dutch immunization program has plateaued at around 60%. Vaccinating boys may be an appealing complementary strategy for the prevention of HPV-related diseases, especially since tender negotiations and reduced dosing schemes have driven down the cost of vaccination.
We expanded a previously published Bayesian synthesis framework to account for all vaccine type–related cancers and herd immunity effects from vaccinating girls and boys. We evaluated the efficiency of vaccinating boys relative to increasing vaccine uptake among girls and assessed the cost-effectiveness of a sex-neutral program.
Vaccinating 40% of boys along with 60% of girls yielded the same gain in life-years (LYs) as increasing the uptake in girls from 60% to 80%. The incremental cost-effectiveness ratio (ICER) of vaccinating boys was €9134/LY (95% credible interval [CrI], €7323/LY–€11231/LY) under 3% discounting. The ceiling vaccination costs at which the ICER remained below the per capita gross domestic product threshold was €240 (95% CrI, €200–€280) per vaccinated boy. If girls’ uptake increased to 90%, the ceiling costs decreased to €70 (95% CrI, €40–€100) per vaccinated boy.
Vaccinating boys along with girls is only modestly less efficient than increasing uptake among girls and highly likely to be cost-effective under current vaccine costs and uptake in the Netherlands.
Persistent infections with human papillomavirus (HPV) are etiologically linked to the development of cervical, vulvar, vaginal, anal, oropharyngeal, and penile cancers [1]. Cancers in the anus and oropharynx attributable to HPV have been found to increase over time in both sexes, but especially in males [2, 3], underlining the relevance of HPV prevention efforts for men [2].
Publicly funded HPV vaccination programs initially focused on girls’ immunization, owing to the high burden of cervical cancer around the world. Given that HPV is transmitted through sexual contacts, substantial uptake of HPV vaccine among girls is also expected to provide indirect protection to males and, hence, reduce the HPV-related burden in both sexes [4–6]. This prediction is supported by early postvaccination evidence from ecological studies in 9 high-income countries [7]. However, vaccine uptake associated with girls-only programs varies considerably. In the Netherlands, a girls-only vaccination program has been in place since 2010, and uptake among preadolescent girls has plateaued around 60%. At the achieved uptake level, this program is anticipated to reduce the total HPV-related burden in males by about one third, leaving ample room for improvement in reducing the HPV-related burden in both sexes [8]. Furthermore, men who have sex with men (MSM), although they face an excess risk for HPV-related diseases as compared to heterosexual men, may hardly receive indirect benefit from the girls-only program [9].
Several high-income countries have decided to vaccinate preadolescent boys in addition to girls at public expense. Australia was the first country to implement a sex-neutral vaccination program in 2012. Austria, the United States, Switzerland, Israel, several Canadian provinces, and the German region of Saxony followed with a recommendation to extend vaccine eligibility to boys, even though the cost-effectiveness of boys’ vaccination in formal health economic assessments of these countries was debatable or not made publicly available [10–15].
While the cost-effectiveness profile of boys’ vaccination is still strongly debated, it has been pointed out repeatedly that increasing uptake among girls is more efficient than vaccinating boys in reducing the overall burden of HPV [4, 5, 10, 16, 17]. Given this, it has been stated that the epidemiological and economic considerations about vaccinating boys should focus on the following issues [18]: the feasibility and incremental marginal costs of increasing uptake among girls versus introducing a sex-neutral program, the public health importance of achieving protection for MSM, and the possibility to reduce the price of HPV vaccines to a level at which boys’ vaccination can be considered cost-effective.
In this article, we consider these issues for the Netherlands. The article has the following structure. First, we reassess the cost-effectiveness of the current girls-only bivalent vaccination program, taking all HPV16/18-related cancers in men and women into account, as well as the recent decline in vaccine costs due to tender negotiations and reduced dosing schemes [19]. Next, we evaluate the efficiency of boys’ vaccination relative to increasing vaccination uptake among girls, expressed as the ratio of boys versus additional girls that need to be vaccinated to gain 1 life-year (LY) in the population. Finally, we assess the cost-effectiveness of boys’ vaccination, taking account of the concentrated HPV-related burden among MSM. Economic evaluations were performed from a public healthcare payer perspective, assuming a separate tender procedure for vaccination of boys as compared to girls-only vaccination.
METHODS
Model Structure
We expanded a previously published Bayesian data synthesis framework [8] to conduct a lifetime evaluation of a cohort of 200000 individuals (the approximate size of a Dutch birth cohort) who were HPV naive and aged 12 years. The Bayesian framework was developed to reflect the HPV-related health gain in men from a girls-only vaccination program, thereby explicitly distinguishing the HPV-related burden among MSM from that in the heterosexual population. In the present analysis, we also accounted for all HPV-related cancers among women and for the total costs ascribable to the incidence of and deaths due to the HPV-related cancers in both sexes (Supplementary Information 1). We considered cancers with the strong evidence of a causal relationship with HPV, according to the International Agency for Research on Cancer: cervical, vulvar, vaginal, anal, and oropharyngeal cancers in women and penile, anal, and oropharyngeal cancers in men. Outcomes were expressed in terms of LYs lost or gained and medical costs attributable to HPV-related cancers.
The Bayesian framework is informed by a heterosexual transmission model that has been fitted to Dutch data and predicts the sex-specific cumulative risk of infection with type- specific HPV before a particular age [20]. We used this model to obtain, for a nonvaccinated individual, the HPV16/18 infection risk reduction that corresponds to a vaccination uptake of 60% among girls only but also to calculate the incremental change in the risk reduction that occurs after an increase in the uptake among girls and/or boys. These infection risk reductions were estimated on the basis of the lifetime infection risk at the postvaccination equilibria of the different immunization scenarios. They were then projected on the HPV16/18-related burden in our Bayesian framework, to reflect how the different combinations of vaccination uptake benefit the nonvaccinated individuals. Indirect protection through girls-only vaccination was assumed not to affect the HPV-related burden attributable to homosexuality, which was measured by cancer-specific population attributable fractions (Supplementary Information 1).
Model Inputs
Table 1 displays the median values with 95% credible intervals (CrIs) of the prior distributions of the inputs of our Bayesian framework. HPV-attributable fractions of penile and oropharyngeal cancers were estimated from local studies that used a validated algorithm for HPV DNA detection [3, 21]. Similar studies were not available for anal, vulvar, and vaginal cancers; alternatively, we used recent international studies comprising a high number of cancer samples and a validated protocol to retrospectively analyze the specimens [22–24]. In these studies, we focused on European estimates of the HPV-related cancer fractions to maximize representativeness for the Dutch setting. Regarding cervical cancer, we assumed 96% of the cases to be etiologically related to HPV; this value is slightly <100% since a small proportion of adenocarcinomas may not be caused by HPV [25, 26]. As type-specific attributable fractions show little variation across countries and studies, we obtained the proportion of cases due to HPV16/18 among HPV-positive cancers from worldwide [22–24, 27, 28] or European estimates [29].
| HPV-Related Cancer Site, Summary Measure . | Women, Value, Median (95% CrI) . | Men, Value, Median (95% CrI) . | Source . |
|---|---|---|---|
| Cervix | |||
| Cumulative risk of disease, ×10–5 | 706 (692–728) | … | … |
| Age at diagnosis, y, mean | 51 | … | … |
| 10-y relative survival | 0.60 (.58–.62) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 96 | … | … |
| HPV-16 | 60.6 (59.6–61.6) | … | [28] |
| HPV-18 | 10.2 (9.6–10.9) | … | [28] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death | 19 600 | … | [30] |
| Anus | |||
| Cumulative risk of disease, ×10–5 | 114 (103–112) | 87 (79–96) | … |
| Age at diagnosis, y, mean | 63 | 62 | … |
| 10-y relative survival | 0.55 (.49–.60) | 0.51 (.44–.57) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 87.5 (82.1–91.9) | 87.5 (82.1–91.9) | [22] |
| HPV-16 | 75.8 (71.6–79.6) | 75.8 (71.6–79.6) | [22] |
| HPV-18 | 3.5 (2.0–5.5) | 3.5 (2.0–5.5) | [22] |
| Cost, €a | |||
| Per incident case | 5000 | 5000 | [30] |
| Per death | 19500 | 19600 | [30] |
| Oropharynx | |||
| Cumulative risk of disease, ×10–5 | 196 (190–210) | 369 (354–384) | … |
| Age at diagnosis, y, mean | 62 | 61 | … |
| 10-y relative survival | 0.34 (.30–.38) | 0.28 (.25–.30) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 29.1 (18.9–41.0) | 29.1 (18.9–41.0) | [3] |
| HPV-16 | 86.5 (84.9–87.9) | 86.5 (84.9–87.9) | [29] |
| HPV-18 | 1.7 (1.2–2.4) | 1.7 (1.2–2.4) | [29] |
| OR for HPV positivityb | 0.29 (.12–.71) | 3.5 (1.4–8.6) | [3] |
| HR for HPV positivity | 0.47 (.35–.63) | 0.47 (.35–.63) | [31] |
| Cost, €a | |||
| Per incident case | 6000 | 6000 | [30] |
| Per death case | 19 500 | 19 600 | [30] |
| Vulva | |||
| Cumulative risk of disease, ×10–5 | 439 (422–457) | … | … |
| Age at diagnosis, y, mean | 69 | … | … |
| 10-y relative survival | 0.66 (.64–.69) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 18.3 (15.9–20.1) | … | [24] |
| HPV-16 | 72.8 (68.4–76.9) | … | [24] |
| HPV-18 | 4.7 (3.0–7.0) | … | [24] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Vagina | |||
| Cumulative risk of disease, ×10–5 | 59 (53–66) | … | … |
| Age at diagnosis, y, mean | 68 | … | … |
| 10-y relative survival | 0.34 (.27–.41) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 71.0 (63.5–77.8) | … | [23] |
| HPV-16 | 57.4 (51.8–62.9) | … | [23] |
| HPV-18 | 5.0 (2.9–7.8) | … | [23] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Penis | |||
| Cumulative risk of the disease, ×10–5 | … | 199 (184–217) | … |
| Age at diagnosis, y, mean | … | 68 | … |
| 10-y relative survival | … | 0.69 (.65–.74) | … |
| Attributable fraction, by HPV type, % | |||
| Any | … | 25.1 (19.5–31.2) | [21] |
| HPV-16 | … | 62.8 (57.6–67.9) | [27] |
| HPV-18 | … | 1.2 (.4–2.8) | [27] |
| HR for HPV positivity | … | 0.2 (.1–.9) | [21] |
| Cost, €a | |||
| Per incident case | … | 4000 | [30] |
| Per death case | … | 19 500 | [30] |
| HPV-Related Cancer Site, Summary Measure . | Women, Value, Median (95% CrI) . | Men, Value, Median (95% CrI) . | Source . |
|---|---|---|---|
| Cervix | |||
| Cumulative risk of disease, ×10–5 | 706 (692–728) | … | … |
| Age at diagnosis, y, mean | 51 | … | … |
| 10-y relative survival | 0.60 (.58–.62) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 96 | … | … |
| HPV-16 | 60.6 (59.6–61.6) | … | [28] |
| HPV-18 | 10.2 (9.6–10.9) | … | [28] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death | 19 600 | … | [30] |
| Anus | |||
| Cumulative risk of disease, ×10–5 | 114 (103–112) | 87 (79–96) | … |
| Age at diagnosis, y, mean | 63 | 62 | … |
| 10-y relative survival | 0.55 (.49–.60) | 0.51 (.44–.57) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 87.5 (82.1–91.9) | 87.5 (82.1–91.9) | [22] |
| HPV-16 | 75.8 (71.6–79.6) | 75.8 (71.6–79.6) | [22] |
| HPV-18 | 3.5 (2.0–5.5) | 3.5 (2.0–5.5) | [22] |
| Cost, €a | |||
| Per incident case | 5000 | 5000 | [30] |
| Per death | 19500 | 19600 | [30] |
| Oropharynx | |||
| Cumulative risk of disease, ×10–5 | 196 (190–210) | 369 (354–384) | … |
| Age at diagnosis, y, mean | 62 | 61 | … |
| 10-y relative survival | 0.34 (.30–.38) | 0.28 (.25–.30) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 29.1 (18.9–41.0) | 29.1 (18.9–41.0) | [3] |
| HPV-16 | 86.5 (84.9–87.9) | 86.5 (84.9–87.9) | [29] |
| HPV-18 | 1.7 (1.2–2.4) | 1.7 (1.2–2.4) | [29] |
| OR for HPV positivityb | 0.29 (.12–.71) | 3.5 (1.4–8.6) | [3] |
| HR for HPV positivity | 0.47 (.35–.63) | 0.47 (.35–.63) | [31] |
| Cost, €a | |||
| Per incident case | 6000 | 6000 | [30] |
| Per death case | 19 500 | 19 600 | [30] |
| Vulva | |||
| Cumulative risk of disease, ×10–5 | 439 (422–457) | … | … |
| Age at diagnosis, y, mean | 69 | … | … |
| 10-y relative survival | 0.66 (.64–.69) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 18.3 (15.9–20.1) | … | [24] |
| HPV-16 | 72.8 (68.4–76.9) | … | [24] |
| HPV-18 | 4.7 (3.0–7.0) | … | [24] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Vagina | |||
| Cumulative risk of disease, ×10–5 | 59 (53–66) | … | … |
| Age at diagnosis, y, mean | 68 | … | … |
| 10-y relative survival | 0.34 (.27–.41) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 71.0 (63.5–77.8) | … | [23] |
| HPV-16 | 57.4 (51.8–62.9) | … | [23] |
| HPV-18 | 5.0 (2.9–7.8) | … | [23] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Penis | |||
| Cumulative risk of the disease, ×10–5 | … | 199 (184–217) | … |
| Age at diagnosis, y, mean | … | 68 | … |
| 10-y relative survival | … | 0.69 (.65–.74) | … |
| Attributable fraction, by HPV type, % | |||
| Any | … | 25.1 (19.5–31.2) | [21] |
| HPV-16 | … | 62.8 (57.6–67.9) | [27] |
| HPV-18 | … | 1.2 (.4–2.8) | [27] |
| HR for HPV positivity | … | 0.2 (.1–.9) | [21] |
| Cost, €a | |||
| Per incident case | … | 4000 | [30] |
| Per death case | … | 19 500 | [30] |
Vaccine efficacy was set at 98.2% (95% CrI, 95%–99%) over sites and sexes.
Abbreviations: CrI, credible interval; HPV, human papillomavirus; HR, hazard ratio; OR, odds ratio.
aCosts are at 2011 prices.
bCalculated as the ratio of the odds of HPV positivity in the specified sex to the odds of HPV positivity in the other sex.
| HPV-Related Cancer Site, Summary Measure . | Women, Value, Median (95% CrI) . | Men, Value, Median (95% CrI) . | Source . |
|---|---|---|---|
| Cervix | |||
| Cumulative risk of disease, ×10–5 | 706 (692–728) | … | … |
| Age at diagnosis, y, mean | 51 | … | … |
| 10-y relative survival | 0.60 (.58–.62) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 96 | … | … |
| HPV-16 | 60.6 (59.6–61.6) | … | [28] |
| HPV-18 | 10.2 (9.6–10.9) | … | [28] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death | 19 600 | … | [30] |
| Anus | |||
| Cumulative risk of disease, ×10–5 | 114 (103–112) | 87 (79–96) | … |
| Age at diagnosis, y, mean | 63 | 62 | … |
| 10-y relative survival | 0.55 (.49–.60) | 0.51 (.44–.57) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 87.5 (82.1–91.9) | 87.5 (82.1–91.9) | [22] |
| HPV-16 | 75.8 (71.6–79.6) | 75.8 (71.6–79.6) | [22] |
| HPV-18 | 3.5 (2.0–5.5) | 3.5 (2.0–5.5) | [22] |
| Cost, €a | |||
| Per incident case | 5000 | 5000 | [30] |
| Per death | 19500 | 19600 | [30] |
| Oropharynx | |||
| Cumulative risk of disease, ×10–5 | 196 (190–210) | 369 (354–384) | … |
| Age at diagnosis, y, mean | 62 | 61 | … |
| 10-y relative survival | 0.34 (.30–.38) | 0.28 (.25–.30) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 29.1 (18.9–41.0) | 29.1 (18.9–41.0) | [3] |
| HPV-16 | 86.5 (84.9–87.9) | 86.5 (84.9–87.9) | [29] |
| HPV-18 | 1.7 (1.2–2.4) | 1.7 (1.2–2.4) | [29] |
| OR for HPV positivityb | 0.29 (.12–.71) | 3.5 (1.4–8.6) | [3] |
| HR for HPV positivity | 0.47 (.35–.63) | 0.47 (.35–.63) | [31] |
| Cost, €a | |||
| Per incident case | 6000 | 6000 | [30] |
| Per death case | 19 500 | 19 600 | [30] |
| Vulva | |||
| Cumulative risk of disease, ×10–5 | 439 (422–457) | … | … |
| Age at diagnosis, y, mean | 69 | … | … |
| 10-y relative survival | 0.66 (.64–.69) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 18.3 (15.9–20.1) | … | [24] |
| HPV-16 | 72.8 (68.4–76.9) | … | [24] |
| HPV-18 | 4.7 (3.0–7.0) | … | [24] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Vagina | |||
| Cumulative risk of disease, ×10–5 | 59 (53–66) | … | … |
| Age at diagnosis, y, mean | 68 | … | … |
| 10-y relative survival | 0.34 (.27–.41) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 71.0 (63.5–77.8) | … | [23] |
| HPV-16 | 57.4 (51.8–62.9) | … | [23] |
| HPV-18 | 5.0 (2.9–7.8) | … | [23] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Penis | |||
| Cumulative risk of the disease, ×10–5 | … | 199 (184–217) | … |
| Age at diagnosis, y, mean | … | 68 | … |
| 10-y relative survival | … | 0.69 (.65–.74) | … |
| Attributable fraction, by HPV type, % | |||
| Any | … | 25.1 (19.5–31.2) | [21] |
| HPV-16 | … | 62.8 (57.6–67.9) | [27] |
| HPV-18 | … | 1.2 (.4–2.8) | [27] |
| HR for HPV positivity | … | 0.2 (.1–.9) | [21] |
| Cost, €a | |||
| Per incident case | … | 4000 | [30] |
| Per death case | … | 19 500 | [30] |
| HPV-Related Cancer Site, Summary Measure . | Women, Value, Median (95% CrI) . | Men, Value, Median (95% CrI) . | Source . |
|---|---|---|---|
| Cervix | |||
| Cumulative risk of disease, ×10–5 | 706 (692–728) | … | … |
| Age at diagnosis, y, mean | 51 | … | … |
| 10-y relative survival | 0.60 (.58–.62) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 96 | … | … |
| HPV-16 | 60.6 (59.6–61.6) | … | [28] |
| HPV-18 | 10.2 (9.6–10.9) | … | [28] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death | 19 600 | … | [30] |
| Anus | |||
| Cumulative risk of disease, ×10–5 | 114 (103–112) | 87 (79–96) | … |
| Age at diagnosis, y, mean | 63 | 62 | … |
| 10-y relative survival | 0.55 (.49–.60) | 0.51 (.44–.57) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 87.5 (82.1–91.9) | 87.5 (82.1–91.9) | [22] |
| HPV-16 | 75.8 (71.6–79.6) | 75.8 (71.6–79.6) | [22] |
| HPV-18 | 3.5 (2.0–5.5) | 3.5 (2.0–5.5) | [22] |
| Cost, €a | |||
| Per incident case | 5000 | 5000 | [30] |
| Per death | 19500 | 19600 | [30] |
| Oropharynx | |||
| Cumulative risk of disease, ×10–5 | 196 (190–210) | 369 (354–384) | … |
| Age at diagnosis, y, mean | 62 | 61 | … |
| 10-y relative survival | 0.34 (.30–.38) | 0.28 (.25–.30) | … |
| Attributable fraction, by HPV type, % | |||
| Any | 29.1 (18.9–41.0) | 29.1 (18.9–41.0) | [3] |
| HPV-16 | 86.5 (84.9–87.9) | 86.5 (84.9–87.9) | [29] |
| HPV-18 | 1.7 (1.2–2.4) | 1.7 (1.2–2.4) | [29] |
| OR for HPV positivityb | 0.29 (.12–.71) | 3.5 (1.4–8.6) | [3] |
| HR for HPV positivity | 0.47 (.35–.63) | 0.47 (.35–.63) | [31] |
| Cost, €a | |||
| Per incident case | 6000 | 6000 | [30] |
| Per death case | 19 500 | 19 600 | [30] |
| Vulva | |||
| Cumulative risk of disease, ×10–5 | 439 (422–457) | … | … |
| Age at diagnosis, y, mean | 69 | … | … |
| 10-y relative survival | 0.66 (.64–.69) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 18.3 (15.9–20.1) | … | [24] |
| HPV-16 | 72.8 (68.4–76.9) | … | [24] |
| HPV-18 | 4.7 (3.0–7.0) | … | [24] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Vagina | |||
| Cumulative risk of disease, ×10–5 | 59 (53–66) | … | … |
| Age at diagnosis, y, mean | 68 | … | … |
| 10-y relative survival | 0.34 (.27–.41) | … | … |
| Attributable fraction, by HPV type, % | |||
| Any | 71.0 (63.5–77.8) | … | [23] |
| HPV-16 | 57.4 (51.8–62.9) | … | [23] |
| HPV-18 | 5.0 (2.9–7.8) | … | [23] |
| Cost, €a | |||
| Per incident case | 8000 | … | [30] |
| Per death case | 16 600 | … | [30] |
| Penis | |||
| Cumulative risk of the disease, ×10–5 | … | 199 (184–217) | … |
| Age at diagnosis, y, mean | … | 68 | … |
| 10-y relative survival | … | 0.69 (.65–.74) | … |
| Attributable fraction, by HPV type, % | |||
| Any | … | 25.1 (19.5–31.2) | [21] |
| HPV-16 | … | 62.8 (57.6–67.9) | [27] |
| HPV-18 | … | 1.2 (.4–2.8) | [27] |
| HR for HPV positivity | … | 0.2 (.1–.9) | [21] |
| Cost, €a | |||
| Per incident case | … | 4000 | [30] |
| Per death case | … | 19 500 | [30] |
Vaccine efficacy was set at 98.2% (95% CrI, 95%–99%) over sites and sexes.
Abbreviations: CrI, credible interval; HPV, human papillomavirus; HR, hazard ratio; OR, odds ratio.
aCosts are at 2011 prices.
bCalculated as the ratio of the odds of HPV positivity in the specified sex to the odds of HPV positivity in the other sex.
Vaccine efficacy against HPV16/18 in our analysis was set equal to a pooled estimate of 0.98 (95% CrI, .95–.99) in per-protocol populations of the bivalent and quadrivalent vaccine trials with end points of HPV16/18-associated cervical intraepithelial neoplasia grades 2 or 3 [8]. Efficacy was assumed to be lifelong and similar for both sexes. Although vaccine efficacy estimates for noncervical sites are less precise, we conjectured the same efficacy against HPV16/18-associated vaginal, vulvar, anal, penile, and oropharyngeal lesions. Medical costs related to the treatment and care of HPV16/18-related cancers are reported by de Kok et al [30].
Scenarios Investigated
First, we reevaluated the incremental cost-effectiveness ratio (ICER) of the girls-only vaccination program, considering the tender vaccine price, 2-dose schedule, and 60% vaccine uptake among 12-year-old girls. Next, we explored the impact of vaccinating boys as compared to an increase in the vaccine uptake among girls. In particular, we investigated how many boys need to get vaccinated to achieve a similar reduction in the burden of the HPV-related cancers as achieved when increasing uptake among girls. Finally, we assessed the cost-effectiveness of 40% uptake among boys conditional on 60% uptake among girls, assuming similar vaccine costs per vaccinated boy as those realized for girls. The assumption of 40% coverage among boys is based on the estimated uptake among boys in countries with sex-neutral programs [32].
ICERs were expressed as the net costs of vaccination, divided by the LYs gained. We assumed a vaccine cost of €65 per vaccinated girl, which is the sum of twice the last published tariff of €17.15 per dose, which was in effect from 2012 until 2014, and the total administrative costs of €13.81 per dose [33]. Costs were calculated from a healthcare payer perspective. Discount rates were set at 3% according to the guidelines from the World Health Organization, and ICERs were compared with a cost-effectiveness threshold of €40000/LY gained, the per capita Dutch gross domestic product.
Sensitivity Analyses
We determined the maximum cost per vaccinated girl or boy at which the ICER remained below the per capita Dutch gross domestic product. We recalculated ICERs by omitting savings related to cancer treatment and death, by excluding the benefit from the prevention of oropharyngeal cancers, and by focusing on cervical cancer prevention only. We also studied the influence of potential waning vaccine efficacy by assuming a constant efficacy for the first 13 years of vaccination and an annual loss of 20% for each subsequent year, as trials of the bivalent and quadrivalent vaccines have shown no evidence of waning efficacy for a decade after vaccine completion [34, 35]. In addition, we explored the ICERs of sex-neutral vaccination under improved uptake in the girls-only program up to 90% and under uptake in boys up to 60%. We further examined the influence of MSM in our calculations; we reevaluated the cost-effectiveness of boys’ vaccination by setting population attributable fractions equal to 0, an assumption that essentially treats the male population as completely heterosexual. We explored the temporal bias of using equilibrium risk reductions in the base-case scenario by computing the ICER for the first cohort of 12-year-olds eligible for sex-neutral vaccination (ie, birth cohort 2006 if sex-neutral vaccination is introduced in 2018; Supplementary Figure 2 in Supplementary Information 1). We also recalculated ICERs under 4% and 1.5% discount rates for costs and effects, respectively, combined with a threshold of €20000/LY gained, as recommended by the Dutch National Health Care Institute. Finally, to explore how the ICER changes in the context of the 9-valent HPV vaccine, we expanded our Bayesian framework with the 5 additional high-risk types that are included in the 9-valent vaccine: types 31, 33, 45, 52, and 58 (Supplementary Table 1 in Supplementary Information 2 for the respective site-specific fractions). We assumed the same vaccine efficacy of 98.2% for all high-risk HPV types. This scenario compares sex-neutral vaccination to girls-only vaccination against 7 high-risk types.
RESULTS
Cost-effectiveness of Girls-Only Vaccination Revisited
Our model predicts that the current girls-only vaccination program will prevent 471 undiscounted cancer cases/100000 women (95% CrI, 461–482 undiscounted cases/100000 women) and 93 undiscounted cancer cases/100000 men (95% CrI, 81–105 undiscounted cases/100000 men) when assuming a 60% uptake among preadolescent girls and lifelong vaccine immunity. These numbers correspond to a gain of 732 discounted LYs/100000 women (95% CrI, 679–788 discounted LYs/100000 women) and 103 discounted LYs/100000 men (95% CrI, 63–159 discounted LYs/100000 men) as compared to no vaccination (Table 2). Without vaccination, the medical costs of HPV16/18-related cancers amount to €3.27 million (95% CrI, €2.6 million–€3.9 million) per cohort of 100000 women and 100000 men followed from age 12 years to death (Table 2). These cancer care costs are reduced to €1.17 million (95% CrI, €0.9 million–€1.4 million) under the 60% girls-only program. The annual vaccination costs of this program amount to €3.8 million.
Number of Life-Years (LYs) Lost and Total Costs Incurred Due to Human Papillomavirus (HPV) Types 16/18–Related Cancers in Scenarios of No Vaccination, 60% Uptake Among Girls Only, and 60% and 40% Uptake Among Girls and Boys, Respectively
| Sex, Cancer Site . | No Vaccination . | 60% Girls . | 60% Girls, 40% Boys . | |||
|---|---|---|---|---|---|---|
| LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | |
| Women | ||||||
| Cervix | 804 | 2084 | 225 | 608 | 121 | 341 |
| Vulva | 43 | 151 | 12 | 43 | 6 | 23 |
| Vagina | 38 | 84 | 11 | 24 | 6 | 13 |
| Anus | 101 | 215 | 29 | 60 | 16 | 33 |
| Oropharynx | 37 | 84 | 10 | 24 | 5 | 13 |
| Total | 1023 | 2594 | 287 | 747 | 154 | 412 |
| Men | ||||||
| Penis | 4 | 26 | 2 | 12 | 1 | 0.7 |
| Anus | 88 | 203 | 75 | 174 | 39 | 95 |
| Oropharynx | 189 | 432 | 102 | 230 | 48 | 105 |
| Total | 281 | 673 | 179 | 423 | 88 | 204 |
| Both sexes, total | 1304 | 3267 | 466 | 1170 | 242 | 615 |
| Sex, Cancer Site . | No Vaccination . | 60% Girls . | 60% Girls, 40% Boys . | |||
|---|---|---|---|---|---|---|
| LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | |
| Women | ||||||
| Cervix | 804 | 2084 | 225 | 608 | 121 | 341 |
| Vulva | 43 | 151 | 12 | 43 | 6 | 23 |
| Vagina | 38 | 84 | 11 | 24 | 6 | 13 |
| Anus | 101 | 215 | 29 | 60 | 16 | 33 |
| Oropharynx | 37 | 84 | 10 | 24 | 5 | 13 |
| Total | 1023 | 2594 | 287 | 747 | 154 | 412 |
| Men | ||||||
| Penis | 4 | 26 | 2 | 12 | 1 | 0.7 |
| Anus | 88 | 203 | 75 | 174 | 39 | 95 |
| Oropharynx | 189 | 432 | 102 | 230 | 48 | 105 |
| Total | 281 | 673 | 179 | 423 | 88 | 204 |
| Both sexes, total | 1304 | 3267 | 466 | 1170 | 242 | 615 |
Data are median values per 100000 individuals. The discount rate is 3%/y for both treatment costs and effects. Undiscounted LYs and numbers of cancer cases are reported in Supplementary Tables 2 and 3 in Supplementary Information 2.
aData are costs of treatment of and deaths due to HPV-related cancers.
Number of Life-Years (LYs) Lost and Total Costs Incurred Due to Human Papillomavirus (HPV) Types 16/18–Related Cancers in Scenarios of No Vaccination, 60% Uptake Among Girls Only, and 60% and 40% Uptake Among Girls and Boys, Respectively
| Sex, Cancer Site . | No Vaccination . | 60% Girls . | 60% Girls, 40% Boys . | |||
|---|---|---|---|---|---|---|
| LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | |
| Women | ||||||
| Cervix | 804 | 2084 | 225 | 608 | 121 | 341 |
| Vulva | 43 | 151 | 12 | 43 | 6 | 23 |
| Vagina | 38 | 84 | 11 | 24 | 6 | 13 |
| Anus | 101 | 215 | 29 | 60 | 16 | 33 |
| Oropharynx | 37 | 84 | 10 | 24 | 5 | 13 |
| Total | 1023 | 2594 | 287 | 747 | 154 | 412 |
| Men | ||||||
| Penis | 4 | 26 | 2 | 12 | 1 | 0.7 |
| Anus | 88 | 203 | 75 | 174 | 39 | 95 |
| Oropharynx | 189 | 432 | 102 | 230 | 48 | 105 |
| Total | 281 | 673 | 179 | 423 | 88 | 204 |
| Both sexes, total | 1304 | 3267 | 466 | 1170 | 242 | 615 |
| Sex, Cancer Site . | No Vaccination . | 60% Girls . | 60% Girls, 40% Boys . | |||
|---|---|---|---|---|---|---|
| LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | LYs . | Cost, €, ×1000a . | |
| Women | ||||||
| Cervix | 804 | 2084 | 225 | 608 | 121 | 341 |
| Vulva | 43 | 151 | 12 | 43 | 6 | 23 |
| Vagina | 38 | 84 | 11 | 24 | 6 | 13 |
| Anus | 101 | 215 | 29 | 60 | 16 | 33 |
| Oropharynx | 37 | 84 | 10 | 24 | 5 | 13 |
| Total | 1023 | 2594 | 287 | 747 | 154 | 412 |
| Men | ||||||
| Penis | 4 | 26 | 2 | 12 | 1 | 0.7 |
| Anus | 88 | 203 | 75 | 174 | 39 | 95 |
| Oropharynx | 189 | 432 | 102 | 230 | 48 | 105 |
| Total | 281 | 673 | 179 | 423 | 88 | 204 |
| Both sexes, total | 1304 | 3267 | 466 | 1170 | 242 | 615 |
Data are median values per 100000 individuals. The discount rate is 3%/y for both treatment costs and effects. Undiscounted LYs and numbers of cancer cases are reported in Supplementary Tables 2 and 3 in Supplementary Information 2.
aData are costs of treatment of and deaths due to HPV-related cancers.
The current girls-only program is projected to be very cost-effective, evidenced by an ICER of €2146/LY gained (95% CrI, €1522–€2803 per LY gained), compared with no vaccination, when all HPV16/18-related cancers are considered. If only cervical cancer prevention is considered, the ICER is €4214/LY gained (95% CrI, €3376–€5086 per LY gained). Figure 1 shows that irrespective of the noncervical health outcomes considered in calculations, girls-only vaccination would remain cost-effective even at high market prices of €300–€400 per fully vaccinated girl, which were used in previous health economic assessments [30, 36–39]. The ICER of the girls-only program under the Dutch guidelines for discounting is reported in Supplementary Figure 4 in Supplementary Information 2.
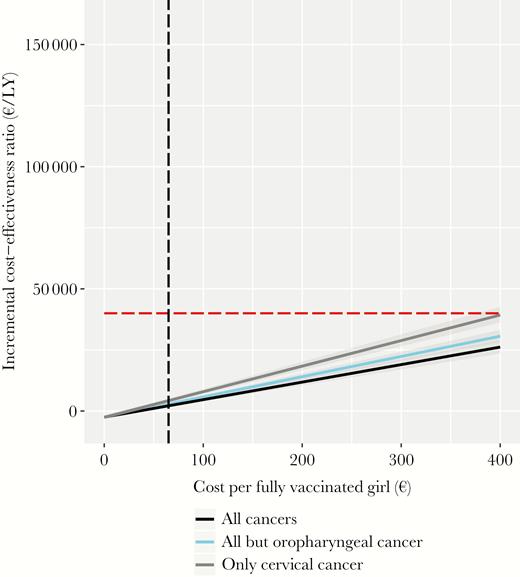
Incremental cost-effectiveness of vaccinating 60% of girls only, compared with that of a scenario of no vaccination, over a wide range of costs per fully vaccinated girl and different health outcomes. The discount rate was set at 3%. The red horizontal line represents the cost-effectiveness ratio threshold of €40000/life-year (LY) gained. The vertical black line represents the costs of €65/vaccinated girl.
Relative Efficiency of Boys’ Vaccination Versus Increased Uptake in Girls
The 60% girls-only program will confer a 64% reduction (95% CrI, 60%–68%) in the overall LYs lost due to HPV16/18 infections, with a 71% reduction (95% CrI, 67%–75%) occurring in women and a 37% reduction (95% CrI, 27%–48%) in men. Figure 2 shows the overall reduction in the vaccine-preventable burden under different combinations of improved vaccine uptake starting from 60% coverage among girls. This contour plot indicates that vaccinating 41% of boys along with 60% of girls confers a similar gain as increasing the girls-only uptake from 60% to 80%. Thus, approximately, vaccinating 2 boys will lead to a similar reduction in the overall HPV16/18-related cancer burden as vaccinating 1 additional girl (Supplementary Figure 1 in Supplementary Information 2 for sex- and type-specific reductions).
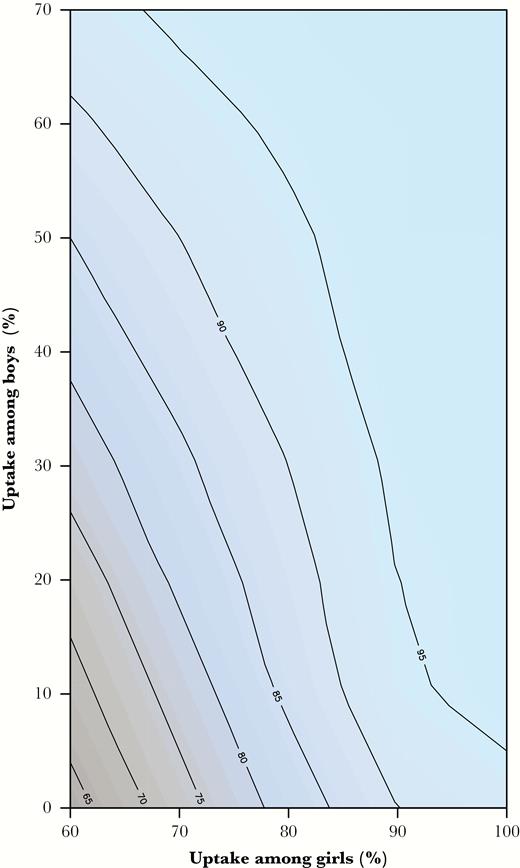
Projected total impact of vaccinating preadolescent girls and/or boys at different combinations of uptake, starting at 60% of uptake among girls only.
Cost-effectiveness of Sex-Neutral Vaccination
Vaccinating 40% of boys in addition to 60% of girls saves 224 LYs (95% CrI, 190–265 LYs) and reduces the cancer care costs to €615 000 (95% CrI, €484 000–€753 000), whereas the incremental costs of adding boys to the immunization program are €2.6 million/year. The ICER of extending the girls-only program to a sex-neutral program with 40% uptake in boys is €9134 (95% CrI, €7323–€11231).
Sex-neutral vaccination remains cost-effective if the costs per vaccinated boy are below €240 (95% CrI, €200–€280), €170 (95% CrI, €140–€200), or €110 (95% CrI, €90–€130), depending on the included health outcomes (Figure 3). Table 3 illustrates that the sex-neutral program maintains an attractive cost-effectiveness profile in all scenarios of our sensitivity analysis. Boys’ vaccination exceeds the cost-effectiveness threshold only when uptake among girls reaches 80%–90% and prevention of cervical cancer is the sole outcome of interest (Supplementary Figure 2A–C in Supplementary Information 2). Otherwise, as shown in Figure 4 boys vaccination remains cost-effective at improved uptake among girls of 70%, 80%, and 90%, with respective maximum acceptable costs of €180 (95% CrI, €150–€220), €120 (95% CrI, €90–€150), and €70 (95% CrI, €40–€100) per vaccinated boy.
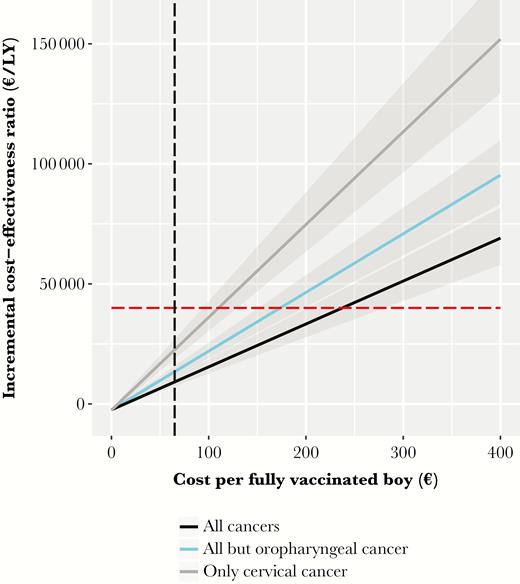
Incremental cost-effectiveness of vaccinating 40% of preadolescent boys and 60% of girls over a wide range of cost per fully vaccinated boy and different health outcomes. The discount rate was set at 3%. The red horizontal line represents the threshold of €40000/life-year (LY) gained. The vertical black line represents the costs of €65/vaccinated girl.
Impact of Different Assumptions on the Incremental Cost-effectiveness of the Sex-Neutral Human Papillomavirus (HPV) Vaccination Program as Compared to the Current Girls-Only Vaccination Program
| Assumption . | ICER, €/LY Gained (95% CrI) . | |
|---|---|---|
| Base case | 9134 | (7323–11231) |
| Discard all cancer care savings | 11586 | (9789–13704) |
| Discard oropharyngeal cancer prevention | 13409 | (11372–15749) |
| Discard all noncervical cancer prevention | 22486 | (18762–26907) |
| Waning vaccine efficacy by 20% after 13th y of vaccine administration | 19106 | (14316–25056) |
| Higher uptake among girls | ||
| 70% among girls, 40% among boys | 13083 | (10023–16380) |
| 80% among girls, 40% among boys | 20631 | (15881–26950) |
| 90% among girls, 40% among boys | 36363 | (23667–64867) |
| Higher uptake among boys | ||
| 60% among girls, 50% among boys | 9935 | (7562–11412) |
| 60% among girls, 60% among boys | 9412 | (7663–11442) |
| PAFs equal to 0 | 10020 | (8017–12280) |
| Cost-effectiveness for first cohort eligible for sex-neutral vaccination | 20720 | (15746–27096) |
| Discount rates of 4% and 1.5%, threshold €20000/LY gaineda | 4390 | (3617–5253) |
| Account for additional health gain from prevention of HPV31/33/45/52/58 infection | 7627 | (6199–9250) |
| Assumption . | ICER, €/LY Gained (95% CrI) . | |
|---|---|---|
| Base case | 9134 | (7323–11231) |
| Discard all cancer care savings | 11586 | (9789–13704) |
| Discard oropharyngeal cancer prevention | 13409 | (11372–15749) |
| Discard all noncervical cancer prevention | 22486 | (18762–26907) |
| Waning vaccine efficacy by 20% after 13th y of vaccine administration | 19106 | (14316–25056) |
| Higher uptake among girls | ||
| 70% among girls, 40% among boys | 13083 | (10023–16380) |
| 80% among girls, 40% among boys | 20631 | (15881–26950) |
| 90% among girls, 40% among boys | 36363 | (23667–64867) |
| Higher uptake among boys | ||
| 60% among girls, 50% among boys | 9935 | (7562–11412) |
| 60% among girls, 60% among boys | 9412 | (7663–11442) |
| PAFs equal to 0 | 10020 | (8017–12280) |
| Cost-effectiveness for first cohort eligible for sex-neutral vaccination | 20720 | (15746–27096) |
| Discount rates of 4% and 1.5%, threshold €20000/LY gaineda | 4390 | (3617–5253) |
| Account for additional health gain from prevention of HPV31/33/45/52/58 infection | 7627 | (6199–9250) |
Abbreviations: CrI, credible interval; ICER, incremental cost-effectiveness ratio; LY, life-year; PAF, population attributable fraction.
aThe ICER of the girls-only program under the Dutch guidelines for discounting is reported in Supplementary Figure 4 in Supplementary Information 2.
Impact of Different Assumptions on the Incremental Cost-effectiveness of the Sex-Neutral Human Papillomavirus (HPV) Vaccination Program as Compared to the Current Girls-Only Vaccination Program
| Assumption . | ICER, €/LY Gained (95% CrI) . | |
|---|---|---|
| Base case | 9134 | (7323–11231) |
| Discard all cancer care savings | 11586 | (9789–13704) |
| Discard oropharyngeal cancer prevention | 13409 | (11372–15749) |
| Discard all noncervical cancer prevention | 22486 | (18762–26907) |
| Waning vaccine efficacy by 20% after 13th y of vaccine administration | 19106 | (14316–25056) |
| Higher uptake among girls | ||
| 70% among girls, 40% among boys | 13083 | (10023–16380) |
| 80% among girls, 40% among boys | 20631 | (15881–26950) |
| 90% among girls, 40% among boys | 36363 | (23667–64867) |
| Higher uptake among boys | ||
| 60% among girls, 50% among boys | 9935 | (7562–11412) |
| 60% among girls, 60% among boys | 9412 | (7663–11442) |
| PAFs equal to 0 | 10020 | (8017–12280) |
| Cost-effectiveness for first cohort eligible for sex-neutral vaccination | 20720 | (15746–27096) |
| Discount rates of 4% and 1.5%, threshold €20000/LY gaineda | 4390 | (3617–5253) |
| Account for additional health gain from prevention of HPV31/33/45/52/58 infection | 7627 | (6199–9250) |
| Assumption . | ICER, €/LY Gained (95% CrI) . | |
|---|---|---|
| Base case | 9134 | (7323–11231) |
| Discard all cancer care savings | 11586 | (9789–13704) |
| Discard oropharyngeal cancer prevention | 13409 | (11372–15749) |
| Discard all noncervical cancer prevention | 22486 | (18762–26907) |
| Waning vaccine efficacy by 20% after 13th y of vaccine administration | 19106 | (14316–25056) |
| Higher uptake among girls | ||
| 70% among girls, 40% among boys | 13083 | (10023–16380) |
| 80% among girls, 40% among boys | 20631 | (15881–26950) |
| 90% among girls, 40% among boys | 36363 | (23667–64867) |
| Higher uptake among boys | ||
| 60% among girls, 50% among boys | 9935 | (7562–11412) |
| 60% among girls, 60% among boys | 9412 | (7663–11442) |
| PAFs equal to 0 | 10020 | (8017–12280) |
| Cost-effectiveness for first cohort eligible for sex-neutral vaccination | 20720 | (15746–27096) |
| Discount rates of 4% and 1.5%, threshold €20000/LY gaineda | 4390 | (3617–5253) |
| Account for additional health gain from prevention of HPV31/33/45/52/58 infection | 7627 | (6199–9250) |
Abbreviations: CrI, credible interval; ICER, incremental cost-effectiveness ratio; LY, life-year; PAF, population attributable fraction.
aThe ICER of the girls-only program under the Dutch guidelines for discounting is reported in Supplementary Figure 4 in Supplementary Information 2.
Boys’ vaccination remains cost-effective even when incremental indirect health gains are restricted to the first birth cohort eligible for sex-neutral vaccination. However, the ICER for the first cohort of vaccinated boys will be more than double that of future cohorts, when postvaccination dynamics have reached equilibrium. Notably, the inflation of health gains only lasts for about 10 cohorts of vaccinated preadolescents (Supplementary Figure 2 in Supplementary Information 1). On the other hand, the ICER drops to almost half of its base-case value when the Dutch guidelines for discounting are considered and remains cost-effective at the corresponding threshold of €20000/LY gained. Finally, the ICER of adding boys to a girls-only vaccination program decreases by 16% if the vaccine also prevents infection due to HPV31, 33, 45, 52, and 58 (Table 3). Site-specific discounted and undiscounted health gains in the context of vaccination against HPV16/18/31/33/45/52/58 are provided in Supplementary Tables 4 and 5 in Supplementary Information 2.
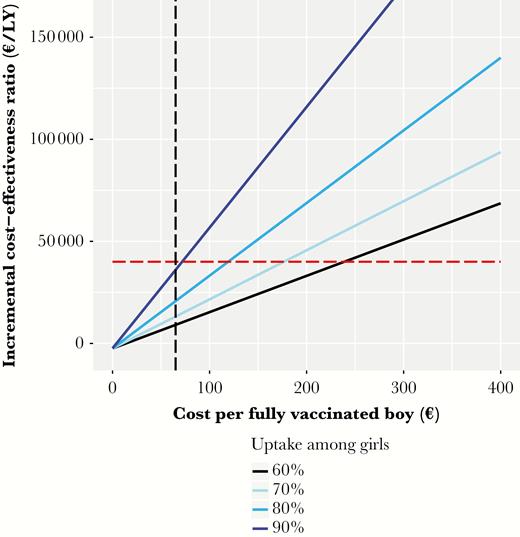
Incremental cost-effectiveness ratio of vaccinating 40% of boys and 60%, 70%, 80%, or 90% of girls over a wide range of cost per fully vaccinated boy. The red horizontal line represents the threshold of €40000/life-year (LY) gained. The vertical black line represents the costs of €65/vaccinated girl.
DISCUSSION
Our study reevaluated the health and economic impact of the current girls-only HPV immunization program in the Netherlands and assessed the relative efficiency and incremental cost-effectiveness of expanding HPV vaccine eligibility to boys. Our results indicate that vaccinating preadolescent boys in addition to girls is highly likely to be good value for money in health economic terms.
Cost-effectiveness studies around the time of vaccine introduction estimated that the girls-only program would only be marginally cost-effective in the Netherlands [36, 39]. However, these studies focused only on cervical disease, assumed high costs of about €350 per vaccinated girl, and omitted herd effects. Later estimates were more beneficial, owing to inclusion of cross-protection and inclusion of noncervical HPV-associated diseases, but still omitted herd effects [37, 38]. The revised ICER in our analysis was about 20 times lower than the conventional cost-effectiveness threshold. This figure increased 1.96 times if we excluded noncervical sites, an additional 1.35 times if we also excluded indirect effects on cervical cancer, and another 7.35 times if we increased the cost per vaccinee from €65 to €350. This simple calculation illustrates that the 20-fold drop in ICER is mainly driven by reduced vaccine costs.
Multiple studies showed a substantial decrease in ICER in the girls-only program by incorporation of noncervical outcomes [40], but ICER improvements due to vaccine costs reductions resulting from tender negotiations and 2-dose schedules have not been previously considered. A growing number of countries have currently adopted 2-dose schedules, and national tender procedures in many of these countries have contributed to cuts in vaccine dose prices of ≥50% [16, 19]. Similar to the Netherlands, a drop of about 80% has been reported for the quadrivalent vaccine in Sweden and the bivalent vaccine in Belgium [41, 42]. In regions of Spain and Italy, per-dose tender prices of the vaccines are currently at about 30% of the list prices and are similar for the bivalent and the quadrivalent vaccines [43–47]. These facts expand the relevance of the revised cost-effectiveness calculations of the girls-only program to other settings.
We found that enhancing uptake among girls is more efficient for reducing the total burden than switching to a sex-neutral program, which accords with previous predictions [4, 5, 10, 16, 17]. However, girls-only programs have already been in place for some time, and uptake in many countries has stabilized below expected levels. Measures to improve coverage among girls merit careful assessment in terms of feasibility and incremental program costs [48]. However, the magnitude of the asymmetry in overall efficiency from vaccinating boys versus more girls is limited and supports the implementation of a sex-neutral program, in the sense that it may be more feasible to reach 40% of boys than to increase coverage among girls from 60% to 80% [48]. A sex-neutral program may itself constitute a measure to boost vaccine uptake among girls and also deals with ethical considerations around exclusion of boys from national immunization programs. These arguments aside, the novelty of our results is that the sex-neutral program has a favorable cost- effective profile even if coverage among girls improves.
Various models have estimated the impact of a sex-neutral national immunization program [5, 6, 10, 11, 13–17, 49], with results that mainly advised against implementation [5, 6, 10, 12–15, 17]. Only 3 of those studies [13, 16, 17] addressed the burden of the disease in the homosexual population, and about half of the studies restricted HPV-related outcomes to cervical disease [6, 14, 15, 49], strongly suggesting that the health impact of boys’ vaccination was previously underestimated. Furthermore, almost all evaluations assumed 3-dose schedules and market price of the vaccines (€85–€150), neglecting the impact of tender negotiations and 2-dose schedules [50]. An exception is the study by Burger et al [16] in Norway, which accounted for expected tender costs of the vaccine. This study reported that at 70% coverage among girls, boys’ vaccination is cost-effective if the total cost per male vaccinee is lower than about $150 (threshold $30000/quality-adjusted LY). Finally, it is worth mentioning that costs of vaccine administration in the Netherlands are low, in line with costs in several other countries [6, 15, 16, 33], which lowers the impediment to implementing a sex-neutral program. However, other countries reported administration costs of €75–€84 [17], playing a decisive negative role in cost-effectiveness calculations [17].
Modeling choices in this analysis were mostly conservative. First, by using LYs as outcome measure instead of quality-adjusted LYs, we neglected the gain in quality of life owing to the prevention of cancer. The burden of the HPV-related cancers could increase by 15%–20% if quality-adjusted LYs were used instead of LYs [8, 38, 39], which would have a positive impact on the ICER. Second, we disregarded the increasing incidence trend in oropharyngeal and anal cancers [2, 3]. Continuation of this trend would indicate even higher health gains from boys’ vaccination than currently anticipated. Third, in the base case, we considered bivalent vaccination because the Netherlands currently uses the bivalent vaccine and our primary aim was to quantify the impact of switching from the current girls-only program to a sex-neutral immunization program. In countries with a quadrivalent HPV6/11/16/18 vaccine, sex-neutral vaccination may lead to additional health effects and savings, in particular because of a reduction in the occurrence of genital warts. Of note, given that herd effects achieved by girls-only vaccination are likely higher for HPV6/11 than for HPV16/18 [18], the incremental benefit from the prevention of infection due to these types would probably be limited under a sex-neutral program. In countries with a 9-valent HPV vaccination program, including boys will lead to a larger health gain than in the context of a 2-valent or 4-valent vaccination program, mainly because boys’ vaccination will enhance the herd effects against cervical cancer caused by HPV types other than HPV16/18 (Supplementary Tables 4 and 5 in Supplementary Information 2). We predicted the extra gain in LYs from sex-neutral vaccination in the context of 9-valent vaccination as compared to 2-valent vaccination to be about 15%.
Our results hold under equilibrium herd effects from sex-neutral vaccination. In contrast to the aforementioned modeling choices, this assumption is nonconservative and leads to inflated health gains during the period until the new equilibrium has been attained. However, we showed that even for the birth cohort that benefited the least from herd protection (ie, the first cohort of 12-year-old individuals eligible for sex-neutral vaccination), vaccinating boys remained a cost-effective intervention. The inflation in health gains is predicted to last for about 10 cohorts of vaccinated preadolescents (Supplementary Figure 2 in Supplementary Information 1).
In summary, we estimated that a sex-neutral national HPV vaccination program in the Dutch setting is highly likely to be good value for money, based on total costs and coverage in the current girls-only program. Our conclusions are stable under different tender prices and improved coverage among girls, suggesting that timely cost-effectiveness evaluation of boys’ vaccination seems warranted in high-income countries with similarly organized immunization programs and tender procedures as the Netherlands.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. V. Q. expanded the initial Bayesian model, conducted the analysis, drafted and revised the manuscript, and is the guarantor of the article’s findings. J. A. B. advised on the design of the analysis and the expansion of the model and drafted and revised the manuscript. J. B. oversaw the design of the model, advised on the design of the analysis, and drafted and revised the manuscript.
Disclaimer. The funder had no role in the identification, design, conduct, reporting, and interpretation of the analysis.
Financial support. This work was supported by the Comparing Health Services Interventions for the Prevention of HPV-Related Cancer project, under European Commission FP7 Framework Health 2013 Innovation 1 (grant 603019).
Potential conflicts of interest. J. B. has received speaker fees from Qiagen and consultancy fees from Roche, GlaxoSmithKline, and Merck/SPMSD; these fees were collected by his employer. V. Q. and J. B. received travel support from DDL Diagnostic Laboratory for visiting International Papillomavirus Conferences (IPVC). J. A. B. certifies no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: EUROGIN 2016 Congress, Salzburg, Austria, 15–18 June 2016.
Correspondence: V. Qendri, MSc, Department of Epidemiology and Biostatistics, VU University Medical Center, PO Box 7057 MF F-wing ST, 1007 MB, Amsterdam, the Netherlands ([email protected]).




