-
PDF
- Split View
-
Views
-
Cite
Cite
Dorothy A. Machalek, Eric P. F. Chow, Suzanne M. Garland, Rebecca Wigan, Alyssa M. Cornall, Christopher K. Fairley, John M. Kaldor, Jane S. Hocking, Henrietta Williams, Anna McNulty, Charlotte Bell, Lewis Marshall, Catriona Ooi, Marcus Y. Chen, Sepehr N. Tabrizi, for the IMPACT and IMPRESS Study Groups, Human Papillomavirus Prevalence in Unvaccinated Heterosexual Men After a National Female Vaccination Program, The Journal of Infectious Diseases, Volume 215, Issue 2, 15 January 2017, Pages 202–208, https://doi.org/10.1093/infdis/jiw530
Close - Share Icon Share
In Australia, high uptake of the quadrivalent human papillomavirus (4vHPV) vaccine has led to reductions in the prevalence of human papillomavirus (HPV) genotypes 6, 11, 16, and 18 in women and girls aged ≤25 years. We evaluated the impact of the program impact on HPV prevalence in unvaccinated male subjects.
Sexually active heterosexual male subjects aged 16–35 years were recruited in 2014–2016. Participants provided a self-collected penile swab sample for HPV genotyping (Roche Linear Array) and completed a demographic and risk factor questionnaire.
The prevalence of 4vHPV genotypes among 511 unvaccinated male subjects was significantly lower in those aged ≤25 than in those aged >25 years: 3.1% (95% confidence interval, 1.5%–5.7%) versus 13.7% (8.9%–20.1%), respectively (P < .001); adjusted prevalence ratio, 0.22 (.09–.51; P < .001). By contrast, the prevalence of high-risk HPV genotypes other than 16 and 18 remained the same across age groups: 16.8% (95% confidence interval, 12.6%–21.9%) in men aged ≤25 years and 17.9% (12.4%–25.0%) in those aged >25 years (P = .76); adjusted prevalence ratio, 0.98, (.57–1.37; P = .58).
A 78% lower prevalence of 4vHPV genotypes was observed among younger male subjects. These data suggest that unvaccinated men may have benefited from herd protection as much as women from a female-only HPV vaccination program with high coverage.
Human papillomavirus (HPV) vaccination programs have been implemented among adolescent girls and young women in an increasing number of countries using the highly effective prophylactic quadrivalent and/or bivalent HPV vaccines [1]. Both vaccines protect against infection with HPV genotypes 16 and 18, which cause 70%–80% of cervical cancers globally [2, 3], as well as 70% of vaginal cancers, 43% of vulvar cancers, 50% of penile cancers, 88% of anal cancers, and 13%–56% of cancers of the base of tongue and oropharynx [4]. The quadrivalent HPV (4vHPV) vaccine also protects against infection with 2 additional HPV genotypes—6 and 11—that cause 85%–95% of anogenital warts [5].
In Australia, between 2007 and 2009, all girls and women aged 12–26 years were offered free vaccination using a 3-dose course of the 4vHPV vaccine. The program was delivered through schools for girls aged 12–18 years, and through community providers for women up to the age of 26 years. From 2010 the program continued for 12–13-year-old girls, and in 2013, it was extended to include 12–13-year-old boys with a 2-year catch-up for those aged 14–15 years [6, 7]. A National HPV Vaccination Program Register (NHVPR) was established to monitor vaccine coverage and to support the management and evaluation of the program [8]. In 2013, data from the NHVPR showed that 86% of adolescent girls and 78% of boys aged 12–13 years received ≥1 vaccine dose, with 77% and 67%, respectively, receiving all 3 doses [9, 10].
Surveillance data from several countries have provided growing evidence for the population-level benefits of HPV vaccination in young women, both direct, and through herd protection of those who remain unvaccinated. These benefits include rapid and substantial reductions in the prevalence of 4vHPV genotypes (HPV-6/11/16/18) and diagnoses of genital warts [7, 10–13]. The demonstrated benefit for young unvaccinated women is likely to be attributable to reductions in the risk of HPV acquisition and hence prevalence among their male sexual partners. Indeed, significant decreases in genital wart diagnoses have been observed in young Australian heterosexual men [9, 14].
A recent study from a single clinic of stored specimens (mostly [97%] urine specimens) collected between 2004 and 2015, from Australian-born men with chlamydia aged <25 years, reported a significant fall in 4vHPV genotypes after the introduction of female vaccination, suggesting herd protection [15]. To further investigate the evidence for male herd protection through the female vaccination program, we estimated the age-specific prevalence of penile HPV genotypes among 16–35-year-old unvaccinated heterosexual male subjects recruited through a network of clinic and community based settings across Australia.
METHODS
Study Population
Participants in this study were years recruited from 2 national cross-sectional HPV prevalence studies conducted between January 2014 and May 2016: the National HPV Monitoring Program (IMPACT) and the Impact of HPV Vaccination Research Study (IMPRESS). IMPACT is an ongoing HPV surveillance system that has established a national network of sentinel sites to monitor circulating HPV genotypes in the Australian population and is aimed at evaluating the effectiveness of the National HPV Vaccination Program. Heterosexual male subjects aged 16–35 years were recruited for HPV testing at 10 participating sites, including 7 sexual health services in Melbourne, Adelaide, Hobart, Cairns, Fremantle, and Sydney and 4 general practice clinics in Melbourne and regional Victoria. In addition, participants were recruited outside the clinic setting through Facebook, using strategies developed for the Vaccine Against Cervical Cancer Impact and Effectiveness (VACCINE) Study, as described elsewhere [16, 17].
IMPRESS is a study of 17–19-year-old heterosexual male subjects recruited from the 7 aforementioned sexual health clinics and through advertisements placed on notice boards at a university and family planning clinic in Melbourne. This study aimed to determine the impact of male HPV vaccination on the prevalence of penile HPV in teenage male subjects.
Subjects were eligible for either study if they reported sex with ≥1 woman during their lifetime. To obtain samples that reflected local prevalence and not that seen in international travelers or recent migrants, inclusion was limited to Australian citizens or men resident in Australia from age 12 years or younger. Participants were excluded if they were unable to provide informed consent or if they reported ever having sex with a man. Ethical approval for this study was granted from 8 human research and ethics committees governing recruitment sites and Facebook, and all participants provided written informed consent.
Procedures
Subjects completed a brief questionnaire on their sociodemographic characteristics, smoking status, age at first sexual intercourse, number of female sexual partners (lifetime and in the previous 12 months), and whether or not they had received HPV vaccination. They were instructed by the research assistant to self-collect a sample for HPV using a moistened flocked swab that was firmly rubbed over the entire surface of the penile shaft, coronal sulcus, glans penis, and if present, the retracted prepuce. Men were also provided with illustrated step-by-step instructions. Sampling of the penile shaft, the glans, and coronal sulcus has been reported to provide the highest detection rates for assessing HPV status because of direct contact with the female genital tract [18]. Furthermore, genital self-sampling methods have been shown to be comparable to sampling by clinicians with respect to specimen adequacy and HPV detection [19, 20].
Written consent was obtained to validate self-reported HPV vaccination status through the NHVPR, which is required to collect individual records on all HPV vaccination doses delivered through schools and primary care in Australia. Participants were classified as being unvaccinated if they reported not receiving the vaccine and if the NHVPR had no record of any HPV vaccine dose being administered. If participants reported receiving the vaccine but had no NHVPR record, registry staff contacted the healthcare provider identified by the participant to ascertain the number of doses and date of vaccination. Participants were excluded if the register was unable to confirm receipt of the vaccine with the provider.
HPV Testing
All samples underwent HPV DNA detection and genotyping at the HPV Regional Labnet, located at the Molecular Microbiology Department, Royal Women's Hospital, Melbourne, Australia. Swab samples were first vigorously agitated in 500 µL of phosphate-buffered saline to release the cellular material. Initially, as an in-house modification, a 100-µL aliquot was added to 1 mL of PreservCyt solution (Hologic) and screened for the presence of 14 high-risk HPV genotypes using the Cobas HPV test (Roche Diagnostics). Concurrently, DNA was extracted from a 200-µL aliquot of the original cell suspension (MagNA Pure 96 DNA and Viral Nucleic Acid Small Volume Kit, Pathogen Universal 200 protocol; Roche Molecular Diagnostics) and eluted in 100 µL.
Extracted DNA was assessed for adequacy by quantitative polymerase chain reaction (PCR) amplification of a 260–base pair segment of the human β-globin gene [21], and a 110–base pair segment to improve detection from keratinized cells, which may contain degraded DNA [22]. DNA extracts from samples negative with the Cobas test were screened for the presence of mucosal HPV DNA by PCR amplification using L1 consensus primer set PGMY09-PGMY11, followed by detection with enzyme-linked immunosorbent assay using a set of biotin-labeled probes [23, 24]. Samples positive for HPV by either screening test were subsequently genotyped using the Linear Array HPV genotyping test (Roche Molecular Diagnostics). Owing to possible cross-reactivity of the HPV-52 probe with types 33, 35, and 58 amplicons, samples positive for ≥1 of these 3 probes were further tested for HPV-52 using a type-specific PCR assay [25].
Statistical Analyses
For men who consented to NHVPR verification and had no evidence of vaccination, HPV prevalence estimates and 95% confidence intervals (CIs) in each of 4 age groups (16–20, 21–25, 26–30, and 31–35 years) were calculated according to the following: detection of any of the 37 HPV genotypes tested; 4vHPV genotypes (HPV-6/11/16/18); any high-risk HPV genotype (HPV-16/18/31/33/35/39/45/51/52/56/58/59/68); any high-risk HPV genotype other than vaccine-targeted genotypes 16 and 18; and the 5 additional HPV types covered by the HPV vaccine (9vHPV; HPV-31/33/45/52/58) [26]. To assess the potential herd effect of female vaccination on penile HPV detection, we compared HPV prevalence between male subjects aged 16–25 and those aged 26–35 years, because (1) population surveys have shown Australian men and their female sexual partners to be of similar age [27, 28] and (2) the low prevalence of vaccine-targeted HPV genotypes in Australian women aged ≤25 years reported in several studies [12, 14, 17], consistent with the higher vaccine coverage achieved in this age group compared with older women [29].
Crude and adjusted prevalence ratios and 95% CIs for each HPV genotype group were calculated to compare prevalence rates between men aged ≤25 years and those aged >25 years. Prevalence ratios were adjusted for source of recruitment; country of birth; education; socioeconomic status (upper or lower 50th centile, based on the Australian Bureau of Statistics Index of Relative Socioeconomic Disadvantage for each individual's residential postal code [12]), residential area (major city or other, based on the Accessibility/Remoteness Index of Australia classification [12]), smoking status, age at first vaginal sex, number of lifetime female partners, and female partners in the previous 12 months. We used χ2 tests to examine associations between age group and sociodemographic and behavioral characteristics. All estimates and 95% CIs were calculated using Poisson regression with robust variance [30]. Statistical significance was tested at the P < .05 level. Analyses were conducted using Stata software, version 14.1 (StataCorp).
RESULTS
From January 2014 to May 2016, a total of 653 men were recruited, including 449 (68.7%) recruited through the IMPACT study and 204 (31.3%) through IMPRESS. Data on 142 men were excluded from further analysis because of nonconsent to access the NHVPR (n = 33); documented receipt of ≥1 HPV vaccine dose (n = 16), or penile sample negative for human β-globin and therefore inadequate for HPV testing (n = 93).
The demographic and sexual behavioral characteristics among the remaining 511 unvaccinated heterosexual men are presented in Table 1. The median age of men included in this study was 23 years (interquartile range, 20–28 years). Overall, 68.7% of men were recruited from sexual health clinics, 42.1% were aged ≤16 years at first vaginal sex, and 66.9% reported ≥2 female sexual partners in the previous 12 months (Table 1).
Demographic and Behavioral Characteristics of 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| Variable . | Subjects, No. (%)a . |
|---|---|
| Age group | |
| 16–20 y | 185 (36.2) |
| 21–25 y | 136 (26.6) |
| 26–30 y | 108 (21.1) |
| 31–35 y | 82 (16.1) |
| Source of recruitment | |
| Sexual health clinics | 351 (68.7) |
| Otherb | 160 (31.3) |
| Country of birth | |
| Australia | 320 (62.6) |
| Other | 183 (35.8) |
| Educational level | |
| Secondary or less | 189 (37.0) |
| Tertiary | 310 (60.8) |
| Area of residence | |
| Major cities | 458 (89.6) |
| Other areas | 42 (8.2) |
| Socioeconomic status | |
| More disadvantaged | 86 (16.8) |
| Less disadvantaged | 414 (81.0) |
| Current smoker | |
| No | 362 (70.8) |
| Yes | 139 (27.2) |
| Age at first vaginal sex | |
| ≤16 y | 215 (42.1) |
| >16 y | 285 (55.8) |
| Lifetime No. of female partners | |
| <5 | 147 (28.8) |
| 5–10 | 122 (23.9) |
| 11–20 | 94 (18.4) |
| >20 | 135 (26.4) |
| No. of female partners in past 12 mo | |
| 0 | 13 (2.5) |
| 1 | 149 (29.2) |
| ≥2 | 342 (66.9) |
| Variable . | Subjects, No. (%)a . |
|---|---|
| Age group | |
| 16–20 y | 185 (36.2) |
| 21–25 y | 136 (26.6) |
| 26–30 y | 108 (21.1) |
| 31–35 y | 82 (16.1) |
| Source of recruitment | |
| Sexual health clinics | 351 (68.7) |
| Otherb | 160 (31.3) |
| Country of birth | |
| Australia | 320 (62.6) |
| Other | 183 (35.8) |
| Educational level | |
| Secondary or less | 189 (37.0) |
| Tertiary | 310 (60.8) |
| Area of residence | |
| Major cities | 458 (89.6) |
| Other areas | 42 (8.2) |
| Socioeconomic status | |
| More disadvantaged | 86 (16.8) |
| Less disadvantaged | 414 (81.0) |
| Current smoker | |
| No | 362 (70.8) |
| Yes | 139 (27.2) |
| Age at first vaginal sex | |
| ≤16 y | 215 (42.1) |
| >16 y | 285 (55.8) |
| Lifetime No. of female partners | |
| <5 | 147 (28.8) |
| 5–10 | 122 (23.9) |
| 11–20 | 94 (18.4) |
| >20 | 135 (26.4) |
| No. of female partners in past 12 mo | |
| 0 | 13 (2.5) |
| 1 | 149 (29.2) |
| ≥2 | 342 (66.9) |
a Totals do not always equal 511 because of a small amount of missing data. All men were confirmed to be unvaccinated with the National HPV Program Register.
b Other sources of recruitment included Facebook (19.0%), notice boards at a university (6.5%), and general practice (4.7%), and family planning (1.4%) clinics.
Demographic and Behavioral Characteristics of 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| Variable . | Subjects, No. (%)a . |
|---|---|
| Age group | |
| 16–20 y | 185 (36.2) |
| 21–25 y | 136 (26.6) |
| 26–30 y | 108 (21.1) |
| 31–35 y | 82 (16.1) |
| Source of recruitment | |
| Sexual health clinics | 351 (68.7) |
| Otherb | 160 (31.3) |
| Country of birth | |
| Australia | 320 (62.6) |
| Other | 183 (35.8) |
| Educational level | |
| Secondary or less | 189 (37.0) |
| Tertiary | 310 (60.8) |
| Area of residence | |
| Major cities | 458 (89.6) |
| Other areas | 42 (8.2) |
| Socioeconomic status | |
| More disadvantaged | 86 (16.8) |
| Less disadvantaged | 414 (81.0) |
| Current smoker | |
| No | 362 (70.8) |
| Yes | 139 (27.2) |
| Age at first vaginal sex | |
| ≤16 y | 215 (42.1) |
| >16 y | 285 (55.8) |
| Lifetime No. of female partners | |
| <5 | 147 (28.8) |
| 5–10 | 122 (23.9) |
| 11–20 | 94 (18.4) |
| >20 | 135 (26.4) |
| No. of female partners in past 12 mo | |
| 0 | 13 (2.5) |
| 1 | 149 (29.2) |
| ≥2 | 342 (66.9) |
| Variable . | Subjects, No. (%)a . |
|---|---|
| Age group | |
| 16–20 y | 185 (36.2) |
| 21–25 y | 136 (26.6) |
| 26–30 y | 108 (21.1) |
| 31–35 y | 82 (16.1) |
| Source of recruitment | |
| Sexual health clinics | 351 (68.7) |
| Otherb | 160 (31.3) |
| Country of birth | |
| Australia | 320 (62.6) |
| Other | 183 (35.8) |
| Educational level | |
| Secondary or less | 189 (37.0) |
| Tertiary | 310 (60.8) |
| Area of residence | |
| Major cities | 458 (89.6) |
| Other areas | 42 (8.2) |
| Socioeconomic status | |
| More disadvantaged | 86 (16.8) |
| Less disadvantaged | 414 (81.0) |
| Current smoker | |
| No | 362 (70.8) |
| Yes | 139 (27.2) |
| Age at first vaginal sex | |
| ≤16 y | 215 (42.1) |
| >16 y | 285 (55.8) |
| Lifetime No. of female partners | |
| <5 | 147 (28.8) |
| 5–10 | 122 (23.9) |
| 11–20 | 94 (18.4) |
| >20 | 135 (26.4) |
| No. of female partners in past 12 mo | |
| 0 | 13 (2.5) |
| 1 | 149 (29.2) |
| ≥2 | 342 (66.9) |
a Totals do not always equal 511 because of a small amount of missing data. All men were confirmed to be unvaccinated with the National HPV Program Register.
b Other sources of recruitment included Facebook (19.0%), notice boards at a university (6.5%), and general practice (4.7%), and family planning (1.4%) clinics.
Prevalence of Penile HPV Genotypes by Age Group Among 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| HPV Genotype . | Age 16–20 y (n = 185) . | Age 21–25 y (n = 136) . | Age 26–30 y (n = 108) . | Age 31–35 y (n = 82) . | Ptrend . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Any | 39 | 21.1 (15.0–28.8) | 42 | 30.1 (22.3–41.7) | 40 | 37.0 (26.5–50.4) | 22 | 26.8 (16.8–40.6) | .11 |
| Vaccine targeted (6, 11, 16, or 18) | 5 | 2.7 (.9–6.3) | 5 | 3.7 (1.2–8.6) | 18 | 16.7 (9.9–26.3) | 8 | 9.8 (4.2–19.2) | .001 |
| Any high risk | 30 | 16.2 (10.9–23.1) | 26 | 19.1 (12.5–28.0) | 28 | 25.9 (17.2–37.5) | 15 | 18.3 (10.2–30.2) | .30 |
| High risk, other than 16 and 18 | 28 | 15.1 (10.1–21.9) | 26 | 19.1 (12.5–28.0) | 22 | 20.4 (12.8–30.8) | 12 | 14.6 (7.6–25.6) | .77 |
| 6 or 11 | 2 | 1.1 (.1–3.9) | 2 | 1.5 (.2–5.3) | 10 | 9.3 (4.4–17.0) | 5 | 6.1 (2.0–14.2) | .003 |
| 16 or 18 | 3 | 1.6 (.3–4.7) | 3 | 2.2 (.5–6.4) | 10 | 9.3 (4.4–17.0) | 4 | 4.9 (1.3–12.5) | .02 |
| 31, 33, 45, 52, or 58 | 12 | 6.5 (3.4–11.3) | 16 | 11.8 (6.7–19.1) | 8 | 7.4 (3.2–14.6) | 3 | 3.7 (.8–10.7) | .50 |
| HPV Genotype . | Age 16–20 y (n = 185) . | Age 21–25 y (n = 136) . | Age 26–30 y (n = 108) . | Age 31–35 y (n = 82) . | Ptrend . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Any | 39 | 21.1 (15.0–28.8) | 42 | 30.1 (22.3–41.7) | 40 | 37.0 (26.5–50.4) | 22 | 26.8 (16.8–40.6) | .11 |
| Vaccine targeted (6, 11, 16, or 18) | 5 | 2.7 (.9–6.3) | 5 | 3.7 (1.2–8.6) | 18 | 16.7 (9.9–26.3) | 8 | 9.8 (4.2–19.2) | .001 |
| Any high risk | 30 | 16.2 (10.9–23.1) | 26 | 19.1 (12.5–28.0) | 28 | 25.9 (17.2–37.5) | 15 | 18.3 (10.2–30.2) | .30 |
| High risk, other than 16 and 18 | 28 | 15.1 (10.1–21.9) | 26 | 19.1 (12.5–28.0) | 22 | 20.4 (12.8–30.8) | 12 | 14.6 (7.6–25.6) | .77 |
| 6 or 11 | 2 | 1.1 (.1–3.9) | 2 | 1.5 (.2–5.3) | 10 | 9.3 (4.4–17.0) | 5 | 6.1 (2.0–14.2) | .003 |
| 16 or 18 | 3 | 1.6 (.3–4.7) | 3 | 2.2 (.5–6.4) | 10 | 9.3 (4.4–17.0) | 4 | 4.9 (1.3–12.5) | .02 |
| 31, 33, 45, 52, or 58 | 12 | 6.5 (3.4–11.3) | 16 | 11.8 (6.7–19.1) | 8 | 7.4 (3.2–14.6) | 3 | 3.7 (.8–10.7) | .50 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus.
Prevalence of Penile HPV Genotypes by Age Group Among 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| HPV Genotype . | Age 16–20 y (n = 185) . | Age 21–25 y (n = 136) . | Age 26–30 y (n = 108) . | Age 31–35 y (n = 82) . | Ptrend . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Any | 39 | 21.1 (15.0–28.8) | 42 | 30.1 (22.3–41.7) | 40 | 37.0 (26.5–50.4) | 22 | 26.8 (16.8–40.6) | .11 |
| Vaccine targeted (6, 11, 16, or 18) | 5 | 2.7 (.9–6.3) | 5 | 3.7 (1.2–8.6) | 18 | 16.7 (9.9–26.3) | 8 | 9.8 (4.2–19.2) | .001 |
| Any high risk | 30 | 16.2 (10.9–23.1) | 26 | 19.1 (12.5–28.0) | 28 | 25.9 (17.2–37.5) | 15 | 18.3 (10.2–30.2) | .30 |
| High risk, other than 16 and 18 | 28 | 15.1 (10.1–21.9) | 26 | 19.1 (12.5–28.0) | 22 | 20.4 (12.8–30.8) | 12 | 14.6 (7.6–25.6) | .77 |
| 6 or 11 | 2 | 1.1 (.1–3.9) | 2 | 1.5 (.2–5.3) | 10 | 9.3 (4.4–17.0) | 5 | 6.1 (2.0–14.2) | .003 |
| 16 or 18 | 3 | 1.6 (.3–4.7) | 3 | 2.2 (.5–6.4) | 10 | 9.3 (4.4–17.0) | 4 | 4.9 (1.3–12.5) | .02 |
| 31, 33, 45, 52, or 58 | 12 | 6.5 (3.4–11.3) | 16 | 11.8 (6.7–19.1) | 8 | 7.4 (3.2–14.6) | 3 | 3.7 (.8–10.7) | .50 |
| HPV Genotype . | Age 16–20 y (n = 185) . | Age 21–25 y (n = 136) . | Age 26–30 y (n = 108) . | Age 31–35 y (n = 82) . | Ptrend . | ||||
|---|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | No. . | % (95% CI) . | ||
| Any | 39 | 21.1 (15.0–28.8) | 42 | 30.1 (22.3–41.7) | 40 | 37.0 (26.5–50.4) | 22 | 26.8 (16.8–40.6) | .11 |
| Vaccine targeted (6, 11, 16, or 18) | 5 | 2.7 (.9–6.3) | 5 | 3.7 (1.2–8.6) | 18 | 16.7 (9.9–26.3) | 8 | 9.8 (4.2–19.2) | .001 |
| Any high risk | 30 | 16.2 (10.9–23.1) | 26 | 19.1 (12.5–28.0) | 28 | 25.9 (17.2–37.5) | 15 | 18.3 (10.2–30.2) | .30 |
| High risk, other than 16 and 18 | 28 | 15.1 (10.1–21.9) | 26 | 19.1 (12.5–28.0) | 22 | 20.4 (12.8–30.8) | 12 | 14.6 (7.6–25.6) | .77 |
| 6 or 11 | 2 | 1.1 (.1–3.9) | 2 | 1.5 (.2–5.3) | 10 | 9.3 (4.4–17.0) | 5 | 6.1 (2.0–14.2) | .003 |
| 16 or 18 | 3 | 1.6 (.3–4.7) | 3 | 2.2 (.5–6.4) | 10 | 9.3 (4.4–17.0) | 4 | 4.9 (1.3–12.5) | .02 |
| 31, 33, 45, 52, or 58 | 12 | 6.5 (3.4–11.3) | 16 | 11.8 (6.7–19.1) | 8 | 7.4 (3.2–14.6) | 3 | 3.7 (.8–10.7) | .50 |
Abbreviations: CI, confidence interval; HPV, human papillomavirus.
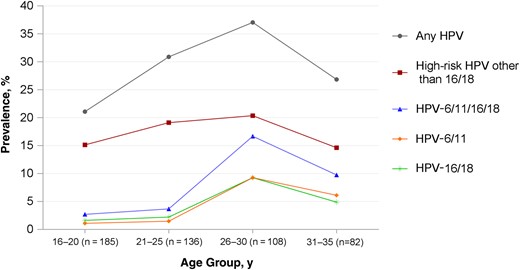
Prevalence of penile human papillomavirus (HPV) genotypes by age group among 511 register-confirmed unvaccinated Australian heterosexual male subjects.
Compared with men aged >25 years, those aged ≤25 years were less likely to be Australian born (P < .001), less likely to be tertiary educated (P < .001), more likely to report younger age at first vaginal sex (P = .048), and reported fewer female sexual partners in their lifetime (P < .001). Men in each age group were similar with respect to source of recruitment, area of residency, socioeconomic status, smoking, and number of female partners in the previous 12 months.
Crude and adjusted prevalence ratios for penile HPV genotypes, adjusted for source of recruitment, country of birth, education, lifetime female partners, and age at first vaginal sex, are presented in Table 3. The combined prevalence of 4vHPV-targeted genotypes was significantly lower in men aged ≤25 years than in those aged >25 years (3.1% vs 13.7%, respectively; P < .001), with an adjusted prevalence ratio of 0.22 (95% CI, .09–.51; P < .001). The adjusted prevalence ratios were 0.14 (95% CI, .04–.62; P = .009) for low-risk HPV genotypes 6 and 11, and 0.29 (95% CI, .11–.74; P = .009) for high-risk HPV genotypes 16 and 18 when these categories were examined separately. By contrast, the prevalence of high risk-HPV genotypes other than HPV-16/18 did not change with increasing age (P = .76), with an adjusted prevalence ratio of 0.98 (95% CI, .57–1.37; P = .58). Finally, the adjusted prevalence ratios for any HPV and for additional HPV genotypes that the 9vHPV vaccine would provide protection against were 0.79 (95% CI, .57–1.10; P = .16) and 2.09 (95% CI, .99–4.45; P = .054), respectively.
Crude PRs and aPRs for Penile HPV Genotypes Among 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| HPV Genotype . | Age ≤25 y (n = 321) . | Age >25 y (n = 190) . | PR (95% CI) . | P Value . | aPRa (95% CI) . | P Value . | ||
|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | |||||
| Any | 81 | 25.2 (20.0–31.4) | 62 | 32.6 (25.0–41.8) | 0.77 (.59–1.02) | .07 | 0.79 (.57–1.10) | .16 |
| Vaccine targeted (6, 11, 16, and 18) | 10 | 3.1 (1.5–5.7) | 26 | 13.7 (8.9–20.1) | 0.23 (.11–.46) | <.001 | 0.22 (.09–.51) | <.001 |
| Any high risk | 56 | 17.5 (13.2–22.7) | 43 | 22.6 (16.4–30.5) | 0.77 (.54–1.10) | .15 | 0.77 (.51–1.16) | .21 |
| High risk, other than 16 and 18 | 54 | 16.8 (12.6–21.9) | 34 | 17.9 (12.4–25.0) | 0.94 (.64–1.39) | .76 | 0.98 (.57–1.37) | .58 |
| 6 or 11 | 4 | 1.2 (0.3–3.2) | 15 | 7.9 (4.4–13.0) | 0.16 (.05–.47) | .001 | 0.14 (.04–.62) | .009 |
| 16 or 18 | 6 | 1.9 (0.06–4.1) | 14 | 7.4 (4.0–12.4) | 0.25 (.10–.65) | .004 | 0.29 (.11–.74) | .009 |
| 31, 33, 45, 52, or 58 | 28 | 8.7 (5.8–12.6) | 11 | 5.8 (2.9–10.4) | 1.51 (.77–2.96) | .23 | 2.09 (.99–4.45) | .054 |
| HPV Genotype . | Age ≤25 y (n = 321) . | Age >25 y (n = 190) . | PR (95% CI) . | P Value . | aPRa (95% CI) . | P Value . | ||
|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | |||||
| Any | 81 | 25.2 (20.0–31.4) | 62 | 32.6 (25.0–41.8) | 0.77 (.59–1.02) | .07 | 0.79 (.57–1.10) | .16 |
| Vaccine targeted (6, 11, 16, and 18) | 10 | 3.1 (1.5–5.7) | 26 | 13.7 (8.9–20.1) | 0.23 (.11–.46) | <.001 | 0.22 (.09–.51) | <.001 |
| Any high risk | 56 | 17.5 (13.2–22.7) | 43 | 22.6 (16.4–30.5) | 0.77 (.54–1.10) | .15 | 0.77 (.51–1.16) | .21 |
| High risk, other than 16 and 18 | 54 | 16.8 (12.6–21.9) | 34 | 17.9 (12.4–25.0) | 0.94 (.64–1.39) | .76 | 0.98 (.57–1.37) | .58 |
| 6 or 11 | 4 | 1.2 (0.3–3.2) | 15 | 7.9 (4.4–13.0) | 0.16 (.05–.47) | .001 | 0.14 (.04–.62) | .009 |
| 16 or 18 | 6 | 1.9 (0.06–4.1) | 14 | 7.4 (4.0–12.4) | 0.25 (.10–.65) | .004 | 0.29 (.11–.74) | .009 |
| 31, 33, 45, 52, or 58 | 28 | 8.7 (5.8–12.6) | 11 | 5.8 (2.9–10.4) | 1.51 (.77–2.96) | .23 | 2.09 (.99–4.45) | .054 |
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence intervals; HPV, human papillomavirus; PR, prevalence ratio.
a Adjusted for source of recruitment, country of birth, highest level of education, lifetime number of female partners, and age at first vaginal sex. The older group was coded as the reference category.
Crude PRs and aPRs for Penile HPV Genotypes Among 511 Register-Confirmed Unvaccinated Australian Heterosexual Male Subjects
| HPV Genotype . | Age ≤25 y (n = 321) . | Age >25 y (n = 190) . | PR (95% CI) . | P Value . | aPRa (95% CI) . | P Value . | ||
|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | |||||
| Any | 81 | 25.2 (20.0–31.4) | 62 | 32.6 (25.0–41.8) | 0.77 (.59–1.02) | .07 | 0.79 (.57–1.10) | .16 |
| Vaccine targeted (6, 11, 16, and 18) | 10 | 3.1 (1.5–5.7) | 26 | 13.7 (8.9–20.1) | 0.23 (.11–.46) | <.001 | 0.22 (.09–.51) | <.001 |
| Any high risk | 56 | 17.5 (13.2–22.7) | 43 | 22.6 (16.4–30.5) | 0.77 (.54–1.10) | .15 | 0.77 (.51–1.16) | .21 |
| High risk, other than 16 and 18 | 54 | 16.8 (12.6–21.9) | 34 | 17.9 (12.4–25.0) | 0.94 (.64–1.39) | .76 | 0.98 (.57–1.37) | .58 |
| 6 or 11 | 4 | 1.2 (0.3–3.2) | 15 | 7.9 (4.4–13.0) | 0.16 (.05–.47) | .001 | 0.14 (.04–.62) | .009 |
| 16 or 18 | 6 | 1.9 (0.06–4.1) | 14 | 7.4 (4.0–12.4) | 0.25 (.10–.65) | .004 | 0.29 (.11–.74) | .009 |
| 31, 33, 45, 52, or 58 | 28 | 8.7 (5.8–12.6) | 11 | 5.8 (2.9–10.4) | 1.51 (.77–2.96) | .23 | 2.09 (.99–4.45) | .054 |
| HPV Genotype . | Age ≤25 y (n = 321) . | Age >25 y (n = 190) . | PR (95% CI) . | P Value . | aPRa (95% CI) . | P Value . | ||
|---|---|---|---|---|---|---|---|---|
| No. . | % (95% CI) . | No. . | % (95% CI) . | |||||
| Any | 81 | 25.2 (20.0–31.4) | 62 | 32.6 (25.0–41.8) | 0.77 (.59–1.02) | .07 | 0.79 (.57–1.10) | .16 |
| Vaccine targeted (6, 11, 16, and 18) | 10 | 3.1 (1.5–5.7) | 26 | 13.7 (8.9–20.1) | 0.23 (.11–.46) | <.001 | 0.22 (.09–.51) | <.001 |
| Any high risk | 56 | 17.5 (13.2–22.7) | 43 | 22.6 (16.4–30.5) | 0.77 (.54–1.10) | .15 | 0.77 (.51–1.16) | .21 |
| High risk, other than 16 and 18 | 54 | 16.8 (12.6–21.9) | 34 | 17.9 (12.4–25.0) | 0.94 (.64–1.39) | .76 | 0.98 (.57–1.37) | .58 |
| 6 or 11 | 4 | 1.2 (0.3–3.2) | 15 | 7.9 (4.4–13.0) | 0.16 (.05–.47) | .001 | 0.14 (.04–.62) | .009 |
| 16 or 18 | 6 | 1.9 (0.06–4.1) | 14 | 7.4 (4.0–12.4) | 0.25 (.10–.65) | .004 | 0.29 (.11–.74) | .009 |
| 31, 33, 45, 52, or 58 | 28 | 8.7 (5.8–12.6) | 11 | 5.8 (2.9–10.4) | 1.51 (.77–2.96) | .23 | 2.09 (.99–4.45) | .054 |
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence intervals; HPV, human papillomavirus; PR, prevalence ratio.
a Adjusted for source of recruitment, country of birth, highest level of education, lifetime number of female partners, and age at first vaginal sex. The older group was coded as the reference category.
Finally, we examined the distribution of genotypes among HPV-positive men only, under the assumption that even if the absolute prevalence changed by age group owing to changes in sexual activity, the distribution of types among HPV-positive men should not. Similar patterns to that seen in the overall study population were observed (Supplementary Figure 1 and Table 1).
DISCUSSION
This is the first study to our knowledge to investigate herd protection by comparing prevalence of HPV in younger unvaccinated men, who are likely to have received the greatest benefit from vaccination of women in their age cohort, with that of older men, whose female partners are less likely to have been vaccinated. We found the prevalence of penile HPV genotypes targeted by the 4vHPV vaccine (HPV-6/11/16/18) was very low (3%) among male subjects aged ≤25 years, 78% lower than that among those aged >25 years (14%). By contrast, there was no difference in the prevalence of other high-risk HPV genotypes not targeted by the vaccine.
These differences in HPV prevalence could not be explained by differences in other characteristics that might influence rates of HPV infection. Given the absence of a difference in the prevalence for nonvaccine genotypes, the most likely explanation is the herd effect of HPV vaccination of girls and women. Our study is the first to provide evidence from multiple sites, suggesting that herd protection of men by female HPV vaccination has occurred across Australia. With the introduction of male vaccination, and with cohorts of vaccinated women now covering an increased age range, our study also provides baseline data for ongoing monitoring of the impact of the National HPV Vaccination Program on HPV infection in men.
Australia has relatively high HPV vaccine coverage of women, with 74% coverage for 1 dose and 55% for 3 doses among 18–26-year-old women in 2011 [31]. Previous Australian sentinel surveillance data have shown that this vaccine coverage was sufficient to reduce the combined prevalence of 4vHPV-targeted genotypes by 78% in the overall study population and 95% among fully vaccinated women [12]. In the same study, reductions in prevalence of 75% and 35% were found in partially vaccinated and unvaccinated women, respectively [12]. The 78% lower combined prevalence of the vaccine-targeted HPV genotypes among younger men in this study suggests that reductions in HPV prevalence in unvaccinated men may be of a similar magnitude to the reductions achieved in women.
No comparison data are available on the age-specific prevalence of HPV among men in Australia before the implementation of the female vaccination program. One hypothetical explanation for the higher prevalence of vaccine-targeted HPV genotypes in older men observed in our study is that HPV clears more slowly with increasing age. However, data from a large prospective study of genital HPV natural history suggest this is unlikely to be the case [32]. Moreover, the relatively flat age-specific prevalence curves observed in our study for HPV genotypes not targeted by the vaccine are consistent with patterns of HPV by age, reported in multiple studies of the epidemiology of genital HPV infection in heterosexual men [33, 34].
A number of factors should be considered when interpreting the results from this study. First, we excluded 15% of participants with penile swab samples that were inadequate for HPV testing [19, 20]. This rate of unassessable samples was higher than previously reported [19] and probably represents inadequate self-sampling by some participants. Nevertheless, these men did not differ from those included in this study with respect to demographic or HPV risk factors. Furthermore, the proportions of younger versus older men with penile samples inadequate for HPV testing did not differ (15% vs 16%, respectively; P = .78).
Second, our estimate of the extent of herd protection, which is based on a cross-sectional comparison of HPV prevalence across age groups, is likely to underestimate the true magnitude of benefit to men from herd effects [35]. This is because men in the older group are also likely to have already benefited from herd protection. An estimated 55% of 18–26-year-old women, corresponding to those aged 26–34 years in 2015, received ≥1 dose of the HPV vaccine in Australia's 2007–2009 catch up program, with an estimated 32% receiving all 3 doses [29]. Third, whereas men were recruited from diverse sources, most were sourced from sexual health clinics, which may have biased sampling toward higher-risk men and therefore a higher prevalence of HPV infection. Nevertheless, even if absolute prevalence rates are not generalizable to all Australian heterosexual men, the comparison between age-specific prevalence may well be. Finally, the results may not be generalizable to countries where female vaccine uptake is lower or among subgroups such as men who have sex with men [36, 37].
There is ongoing global debate as to whether boys should be included in national HPV vaccination programs. At the time of this study only a handful of countries (Australia, Austria, Israel, the United States, and 6 provinces of Canada) had implemented male HPV vaccination programs in addition to existing female vaccination programs. Cost-effectiveness studies have been conducted to help determine whether any further health benefits would be obtained from the addition of male HPV vaccination [38]. However, these have been based on mathematical modeling without actual male prevalence data such as those presented in this study. The World Health Organization recommends the establishment of sentinel surveillance to monitor the impact of HPV vaccination programs on HPV prevalence [39]. With the introduction of male vaccination in Australia, ongoing surveillance of HPV prevalence with linkage to the national vaccine register will allow monitoring of the impact of HPV vaccination over time, informing whether routine vaccination of boys will result in any benefits over and above those that have already been achieved through the existing female vaccination program.
Notes
Acknowledgments. The National HPV Monitoring Program (IMPACT) is led by the World Health Organization Reference Laboratory for HPV detection and genotyping, located at the Royal Women's Hospital in collaboration with a consortium of member organizations. The member organizations include the Kirby Institute at the University of New South Wales, the University of Melbourne, Melbourne Sexual Health Centre Alfred Health, Family Planning Victoria, Family Planning New South Wales, and the University of Sydney, Department of Child Health. The IMPACT study team includes Deborah Bateson, Catriona Bradshaw, M. Y. C., A. M. C., Basil Donovan, C. K. F., S. M. G., Andrew Grulich, Richard Hillman, J. S. H., Fengyi Jin, J. M. K., Marlene Kong, Matthew Law, D. A. M., Skye McGregor, Kathleen McNamee, Robert Monaghan, Samuel Phillips, Marin Poljak, Mary Poynten, David Regan, S. Rachel Skinner, Mary Stewart, H. W., R. W., S. N. T. (principal investigator for IMPACT), and the external advisory group: Linda Selvey, Vicky Sheppeard, David Smith, Kaushi Kogar, and Marilyn Jodie Clarke. The IMPRESS Study is led by the Melbourne Sexual Health Centre, Alfred Health. The study team includes R. W., Catriona Bradshaw, Julia Brotherton, C. K. F., S. M. G., J. M. K., J. S. H., David Regan, S. N. T., and M. Y. C. (principal investigator for IMPRESS). We would like to thank all the site investigators, C. O., L. M., C. B., A. M., Louise Owen, Darren Russell, and the clinic staff: Melissa Power, Jane Gilbert, Carol Pavitt, Emma Clements, El Thompson, Barb Lennox, Fiona Anderson, Collette Cashman, Michelle Andrews, Alison Beverley, and Mandy Johnson. We also thank Edmund Molesworth, Belinda Hengel, Kathy Rowed, Samantha Sukkel, and Jessica Nia for their assistance in recruitment; and Jennifer Brosi, Lisette Bicknell, and Chantal Kim at the National HPV Vaccination Program Register for assistance with validating vaccination status. Finally, we thank all the IMPACT and IMPRESS participants.
Author contribution. All authors contributed to the study design. D. A. M., E. P. F. C., M. Y. C., and S. N. T. contributed to data analysis. D. A. M., E. P. F. C., J. M. K., M. Y. C., and S. N. T. contributed to data interpretation, and writing of the report. S. M. G., R. W., A. M. C., C. K. F., J. S. H., H. W., A. M., C. B., L. M., and C. O. contributed to site and participant enrollment and reviewed the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Disclaimer. The views expressed in this publication are those of the authors and do not necessarily represent the position of the Australian Government nor those of Merck Sharp & Dohme.
Financial support. The IMPRESS Study is supported by a Merck Investigator Initiated Studies Program (protocol 50939). The National HPV Monitoring Program is supported by the Australian Government Department of Health (HPV Surveillance Fund H1314G010). E. P. F. C. is supported by the Australian National Health and Medical Research Council (Early Career Fellowship 1091226).
Potential conflicts of interest. D. A. M. and E. P. F. C. have each received educational grants from Seqirus (formerly bioCSL) to assist with education, training, and academic purposes in the area of HPV. S. M. G. reports grants from Merck, GlaxoSmithKline, CSL, and the Commonwealth Department of Health outside the submitted work; has received nonfinancial support from Merck; and has delivered lectures and received speaking fees from Merck Sharp & Dohme and Sanofi Pasteur MSD for work performed in her personal time. C. K. F. has received research funding from Merck outside the submitted work, and owns shares in CSL Biotherapies, the manufacturer of Gardasil. M. Y. C. has received grants from Merck Sharp & Dohme, during the conduct of the study. S. N. T. has received grants from bioCSL, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
Author notes
Presented in part: European Research Organisation on Genital Infection and Neoplasia (EUROGIN) Conference, Salzburg, Austria, 15–18 July 2016.
D. A. M. and E. P. F. C. contributed equally to this work.
M. Y. C. and S. N. T. contributed equally to this work.




