-
PDF
- Split View
-
Views
-
Cite
Cite
Vaishali I Parekh, Lauren R Brinster, Bin Guan, William F Simonds, Lee S Weinstein, Sunita K Agarwal, A Knock-In Mouse Model of the Gcm2 Variant p.Y392S Develops Normal Parathyroid Glands, Journal of the Endocrine Society, Volume 7, Issue 11, November 2023, bvad126, https://doi.org/10.1210/jendso/bvad126
Close - Share Icon Share
Abstract
The glial cells missing 2 (GCM2) gene functions as a transcription factor that is essential for parathyroid gland development, and variants in this gene have been associated with 2 parathyroid diseases: isolated hypoparathyroidism in patients with homozygous germline inactivating variants and primary hyperparathyroidism in patients with heterozygous germline activating variants. A recurrent germline activating missense variant of GCM2, p.Y394S, has been reported in patients with familial primary hyperparathyroidism.
To determine whether the GCM2 p.Y394S missense variant causes overactive and enlarged parathyroid glands in a mouse model.
CRISPR/Cas9 gene editing technology was used to generate a mouse model with the germline heterozygous Gcm2 variant p.Y392S that corresponds to the human GCM2 p.Y394S variant. Wild-type (Gcm2+/+) and germline heterozygous (Gcm2+/Y392S) mice were evaluated for serum biochemistry and parathyroid gland morphology.
Gcm2+/Y392S mice did not show any change compared to Gcm2+/+ mice in serum calcium and parathyroid hormone levels, parathyroid gland histology, cell proliferation, or parathyroid gland size.
The mouse model of the p.Y392S variant of Gcm2 shows that this variant is tolerated in mice, as it does not increase parathyroid gland cell proliferation and circulating calcium or PTH levels. Further investigation of Gcm2+/Y392S mice to study the effect of this variant of Gcm2 on early events in parathyroid gland development will be of interest.
Parathyroid glands produce and secrete parathyroid hormone (PTH) to maintain calcium homeostasis. In response to low or high calcium levels, the calcium-sensing receptor (CASR) that is expressed on the surface of parathyroid gland cells (chief cells) stimulates or suppresses PTH secretion, respectively [1]. PTH positively regulates serum calcium concentration by increasing bone resorption and calcium reabsorption in the kidney, as well as by stimulating the production of active vitamin D in the kidney [2]. This essential regulation of PTH and calcium is disrupted in diseases of the parathyroid glands. In primary hyperparathyroidism (PHPT), 1 or more of the 4 parathyroid glands are enlarged due to tumor formation, leading to excessive or nonsuppressed secretion of PTH causing hypercalcemia, whereas in hypoparathyroidism, there is a deficiency of PTH secretion causing hypocalcemia [3, 4].
Glial cells missing 2 (GCM2, also known as GCMB) encodes a transcription factor that is specifically expressed in parathyroid gland cells [5, 6]. GCM2 is essential for parathyroid gland development, as mice with Gcm2 deletion lack parathyroid glands [7-9]. Homozygous germline inactivating variants in GCM2 have been reported in a subset of patients with isolated hypoparathyroidism (Phenotype MIM #618883) [10-14], whereas heterozygous germline activating variants in GCM2 have been reported in a subset of patients with PHPT (Phenotype MIM #617343) [14-24]. GCM2-inactivating variants include missense variants in the N-terminal DNA-binding domain that disrupt DNA binding and inhibit the transcriptional activity of GCM2, protein truncating variants that lack the transcription activation domains of GCM2, or gene deletion [10, 12, 13]. The transcriptional activity of GCM2 is intrinsically repressed by a 17–amino acid C-terminal conserved inhibitory domain (CCID) that is located between the 2 transcriptional activation domains [15]. GCM2 activating variants are gain-of-function missense variants that are mainly clustered in the CCID that de-repress (enhance) the transcriptional activity of GCM2 [14-16, 20].
Among the GCM2 missense variants associated with PHPT, a recurrent heterozygous germline missense variant of GCM2, p.Y394S, that also enhances the transcriptional activity of GCM2, has been reported in multiple patients [14-19, 21, 23, 24]. The direct contribution of this missense variant in parathyroid gland growth and function has not been determined. CRISPR/Cas9 gene editing in mice has facilitated the evaluation of missense and splice variants in endogenous loci [25]. In this study, we used the CRISPR/Cas9 system to generate a heterozygous germline knock-in of p.Y392S in the mouse Gcm2 gene (which corresponds to the human variant p.Y394S in GCM2) to mimic the human PHPT-associated germline heterozygous genotype of this variant. The phenotypes of wild-type (WT) and gene-edited mice were evaluated for serum biochemistry and parathyroid gland morphology.
Methods
Transcriptional Activity Assay
The luciferase reporter plasmid with 6 consecutive copies of a GCM2-binding site (pGL4-6xGBS-Luc) in the pGL4.23[Luc2/minP] vector (Promega) was previously described [15]. This is a commonly used GCM2-responsive luciferase reporter for analyzing the transcriptional activity of GCM2 [11, 12]. Cloning of the human WT GCM2 cDNA and the variant p.Y394S with a N-terminal triple FLAG-tag in the pcDNA6-3xFLAG vector was previously described [15]. The mouse WT Gcm2 cDNA clone with a C-terminal Myc-FLAG-tag in the pCMV6-Entry vector was purchased (Origene). The same plasmid was used for introducing the mouse variant p.Y392S (that corresponds to human GCM2 p.Y394S variant) with the QuikChange II Site-Directed Mutagenesis Kit (Agilent) using primers Gcm2-Y392S-F (5′- GTGGCTTACCAGGCCTcCCAGCACCCTGCTCTTAAA—3′) and Gcm2-Y392S-R (5′- TTTAAGAGCAGGGTGCTGGgAGGCCTGGTAAGCCAC—3′). The underlined triplet for p.Y392S (TAC > TCC) shows the mutated nucleotide in lower case. The plasmids were sequenced to confirm the presence of the variant (Quintarabio).
Human embryonic kidney cells, HEK293 (ATCC, Cat# CRL-1573), were cultured in a medium containing high glucose DMEM (GIBCO) supplemented with 10% fetal bovine serum (Gemini) and 1× antibiotic/antimycotic (GIBCO) in a humidified 5% CO2 incubator. Lipofectamine 2000 was used for plasmid transfection by the reverse transfection procedure (Invitrogen) in 12-well tissue culture plates with 250 ng of pGL4-6xGBS-Luc reporter plasmid together with 100 ng of the empty vector or GCM2 (WT or variant) expression plasmid, and 500 000 HEK293 cells. Cell lysates were prepared 24 hours post-transfection in 1× passive lysis buffer (Promega). Luciferase activity was measured in the cell lysates with a luminometer (Berthold Technologies, Lumat LB 9507). Luciferase assay experiments were performed with 3 independent plasmid preps. The mean ratio of luciferase activity counts of GCM2 (WT or variant) plasmid to empty vector from 3 independent experiments were plotted as fold transcriptional activity. The cell lysates were used in standard immunoblot assays to determine GCM2 (WT or variant) protein expression with anti-FLAG (Sigma-Aldrich Cat# F3165; RRID: AB_259529, http://antibodyregistry.org/AB_259529), loading control anti-p84 (GeneTex Cat# GTX70220; RRID:AB_372637, http://antibodyregistry.org/AB_372637), and secondary donkey anti-mouse IgG-HRP (Jackson ImmunoResearch Labs Cat# 715-035-151; RRID:AB_2340771, http://antibodyregistry.org/AB_2340771). The immunoblots were developed by ECL (Millipore) and imaged with a G-BOX imaging system (Syngene).
Germline Knock-In Mice
All animal experiments were performed according to the animal study protocols approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Institutional Animal Care and Use Committee (Animal Study Protocol number: K070-MDB-21). CRISPR/Cas9 gene editing was used to generate a mouse strain in the C57BL/6 background with a germline knock-in missense variant p.Y392S within the Gcm2 gene with the help of ingenious Targeting Laboratories. In addition to the targeted missense variant p.Y392S single nucleotide change (TAC > TCC), 2 silent nucleotide variations (CTG > CTT; AAA > AAG) were introduced at 15 and 18 nucleotides downstream of the TAC > TCC change to prevent a re-cut by the CRISPR/Cas9 enzyme. The 2 silent variants did not change the reading frame and encoded the same amino acids as the endogenous codons. The silent variants introduced the AflII (BspTI) restriction enzyme site that was used to establish a genotyping protocol for the Gcm2 gene-edited mice. For genotyping, genomic DNA from mouse tail snips was isolated and processed for PCR using the KAPA Hotstart Mouse Genotyping kit (Sigma-Aldrich) and primers Gcm2-geno-F (5′- GTGCCCACATTGCCTTATTACAAC—3′) and Gcm2-geno-R (5′- ATGGGCATTGGGAGGTTTTCCGAG—3′) to obtain a 210–base pair (bp) product. The PCR product was digested with the restriction enzyme AflII and run on a 3% agarose gel. The WT allele was detected as a single band at 210 bp, and the p.Y392S allele was detected as 2 bands at 130 bp and 80 bp from the AflII enzyme digest. Germline Gcm2 WT mice (Gcm2+/+) and Gcm2 p.Y392S heterozygous mice (Gcm2+/Y392S) were generated by crossing male and female germline WT and heterozygous mice.
Parathyroid Gland Tissue Procurement and Histology
Mouse necropsy was performed to procure intact tissues from the tongue to the stomach as one piece that was immediately placed in 16% buffered formalin (Fisher Scientific). The neck region of the mouse was then trimmed to collect the tiny parathyroid glands (∼0.4 × 0.2 mm, see below) still on the thyroid lobes attached to the trachea, to generate formalin fixed and paraffin embedded (FFPE) tissue blocks for obtaining cross sections containing the esophagus, trachea, thyroid and parathyroid together (tracheal block). FFPE tracheal blocks were prepared from similar numbers of male and female mice at age 18 to 24 months (10-15 mice each, Gcm2+/+ and Gcm2+/Y392S), and 5-µm sections were used for hematoxylin and eosin (H&E) staining to observe tissue histology, to measure parathyroid gland area, and for immunohistochemistry (IHC). We only examined parathyroid gland morphology in older mice because genetically engineered mouse models of parathyroid-related genes (Ccnd1, Men1, Cdc73, and Rgs5) have shown atypical parathyroid glands or parathyroid tumors at age >12 to 18 months [26-29], and because serum calcium and PTH in the Gcm2+/Y392S mice at the 6-month and 12- to 15-month time points was unaffected (please see below).
Parathyroid Gland Tissue RNA and Gcm2 Expression
Parathyroid gland tissue was obtained by manual microdissection of FFPE tissue sections of tracheal blocks. Regions corresponding to parathyroid glands were marked on H&E-stained slides and used as a template to mark the same regions on slides of unstained serial sections. The parathyroid gland areas were scrapped from the unstained slides using a sterile needle or the edge of a razor blade and processed immediately for RNA isolation with the CELLDATA RNAstorm FFPE RNA Extraction kit (Biotium). DNase-treated total RNA (25 ng) was used for RNA amplification and first strand cDNA synthesis with the Ovation FFPE WTA system (Tecan Genomics). The purified first strand cDNA (20 ng) was used for PCR to amplify a 226-bp region of Gcm2 containing p.Y392S with primers Gcm2-rt-F (5′- CCCACATTGCCTTATTACAACCTA—3′) and Gcm2-rt-R (5′- GGTCTAAGGCTGGTGGATAGATG—3′). The PCR products were purified and sequenced with the forward primer and the sequences were inspected manually to observe WT and variant in the sequence.
Serum Biochemistry
Whole blood was drawn from the tail vein and collected in gold top serum separator tubes (Becton Dickinson) and processed for serum isolation. Total calcium was measured with a colorimetric assay kit (Stanbio) and PTH was measured with a mouse PTH ELISA kit (Quidel Cat# 60-2305, RRID_ AB_2941776, http://antibodyregistry.org/AB_2941776).
Immunohistochemistry
The IHC to detect CASR, Ki-67, and PTH in FFPE tissue sections of tracheal blocks was performed using EnVision FLEX IHC kit (Agilent Cat# K8000, RRID:AB_2890017, http://antibodyregistry.org/AB_2890017). Antibodies used were: anti-CASR (1:300 dilution) (Abcam Cat# ab19347; RRID:AB_444867, http://antibodyregistry.org/AB_444867), anti-Ki-67 (1:300 dilution) (Abcam Cat# ab15580; RRID:AB_443209, http://antibodyregistry.org/AB_443209), and anti-PTH (1:300 dilution) (Bio-Rad Cat# 7170-6216; RRID:AB_620616, http://antibodyregistry.org/AB_620616). IHC was also performed on FFPE tissue sections of tracheal blocks with the secondary antibody alone (rabbit or mouse, from the EnVision IHC kit above) as a negative control. Two different anti-GCM2 antibodies detected GCM2 on immunoblots but did not show specific nuclear GCM2 staining in IHC experiments (Santa Cruz Biotechnology Cat# sc-79496; RRID:AB_2108126, http://antibodyregistry.org/AB_2108126 and Cat# sc-390603; RRID:AB_2943628, http://antibodyregistry.org/AB_2943628).
Microscopy and Imaging
Tissue sections processed for H&E staining and IHC were imaged on the Keyence BZ-X700 microscope (Keyence), or by digital slide scanning (Histoserv).
Parathyroid Gland Measurement
Images of H&E-stained FFPE tissue sections of tracheal blocks containing a whole cross-section of the parathyroid gland were observed in the Histoserv digital software (Histoserv). Using the built-in scale, 2 dimensions (length and breadth) were measured in µm at right angles and the product of the 2 dimensions was used to calculate parathyroid gland size in µm2 (converted to mm2, 1 µm2 = 10−6 mm2).
Statistics
All statistical analyses were performed with GraphPad Prism software (version 9.4) to calculate mean and SD, to create graphs, Student t test, and P < .05 was considered significant.
Results
Mouse Ortholog of the Human GCM2 Variant p.Y394S Enhances Transcriptional Activity
Homology of the mouse and human GCM2 protein in the 17 amino acid CCID sequence showed that they were mostly identical except for the absence of 2 residues in the mouse sequence (Fig. 1A). Therefore, the PHPT-associated human GCM2 variant p.Y394S corresponded to p.Y392S in the mouse Gcm2 sequence. To determine whether the absence of 2 amino acids in the mouse Gcm2 CCID compared to the human GCM2 CCID would show any functional difference, the transcriptional activity of mouse Gcm2 (WT and p.Y392S) was compared to the transcriptional activity of human GCM2 (WT and p.Y394S). Luciferase reporter assays showed that the mouse WT Gcm2 and the p.Y392S variant activated transcription 16-fold and 37-fold over empty vector, respectively, and the human WT GCM2 and the p.Y394S variant activated transcription 6-fold and 18-fold over empty vector, respectively (Fig. 1B). Compared to the corresponding WT, there was a 2.3-fold increase in the transcriptional activity of the mouse Gcm2 p.Y392S variant and 3-fold increase in the transcriptional activity of the human GCM2 p.Y394S variant. Immunoblotting showed similar expression levels of WT GCM2/Gcm2 and variants in these experiments (Fig. 1C). Therefore, the mouse ortholog of the human GCM2 variant p.Y394S also shows a gain-of-function effect on transcriptional activity.
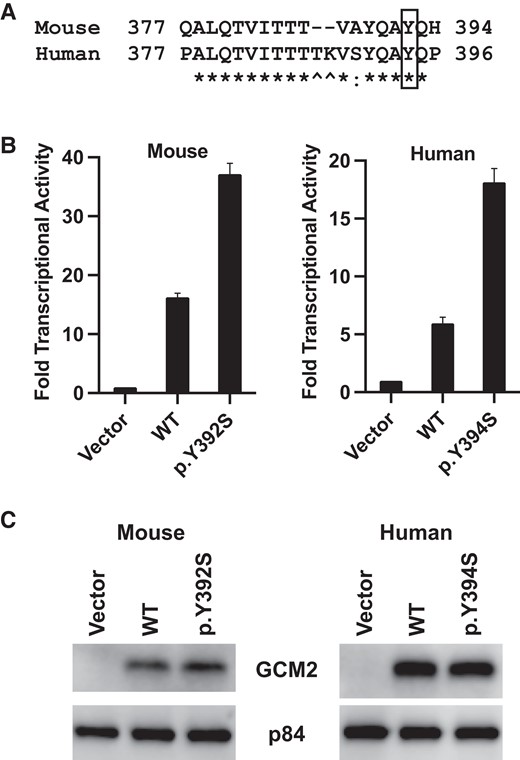
Mouse Gcm2 variant p.Y392S shows enhanced transcriptional activity. (A) Homology of mouse and human GCM2. Protein sequence alignment of mouse and human GCM2 was retrieved from NCBI HomoloGene. Protein homology is shown of a 20 amino acid region containing the C-terminal conserved inhibitory domain (CCID). The variant p.Y394S in human GCM2 corresponds to p.Y392S in mouse Gcm2, marked with a box; * indicates identical residue; a colon (:) indicates conserved residue; and ^ marks the 2 residues that are absent in the mouse sequence. (B) Transcriptional activity of mouse and human GCM2 protein and variants. HEK293 cells were transfected with the pGL4-6xGBS-Luc luciferase reporter plasmid together with the indicated plasmids (empty vector, FLAG-tagged WT GCM2, or FLAG-tagged variant). Cell lysates were analyzed for luciferase activity counts using a Luminometer. The ratio of luciferase activity counts of GCM2 plasmids (WT or variant) over empty vector was plotted as mean and SD from 3 independent experiments, representing fold increase of transcriptional activity. (C) Representative immunoblots from luciferase assay data shown in B. Equal amounts of cell lysates from each transfection were analyzed with anti-FLAG to detect the transfected GCM2 (WT or variant). Anti-p84 was used as the loading control.
Generation of Knock-In Mice With the Gcm2 Variant p.Y392S
A germline knock-in of the Gcm2 variant p.Y392S (TAC > TCC) was generated with the CRISPR/Cas9 gene editing technology to obtain Gcm2+/Y392S mice that would mimic the germline heterozygous condition of this variant (GCM2 p.Y394S) in PHPT patients (Fig. 2A and 2B). The FFPE tissue blocks of the tracheal region of Gcm2+/+ and Gcm2+/Y392S mice were processed as sequential sections for H&E staining to find the parathyroid glands (Fig. 2C and 2D). Serial FFPE tissue sections from the same tracheal blocks were used for anti-PTH IHC to confirm parathyroid gland tissue (Fig. 2C and 2D). To determine whether the variant was expressed in mouse parathyroid gland cells, RNA isolated from parathyroid tissue in FFPE tracheal blocks of Gcm2+/+ and Gcm2+/Y392S mice was used for RT-PCR followed by PCR product sequencing (Fig. 2E). The sequence traces showed that the heterozygous p.Y392S was expressed at the RNA level in the parathyroid gland cells of Gcm2+/Y392S mice. The litter size, gender ratio, body weight, and survival of Gcm2+/Y392S mice was comparable to WT mice. Equal numbers of male and female Gcm2+/+ and Gcm2+/Y392S mice were analyzed to study serum biochemistry and parathyroid gland morphology.
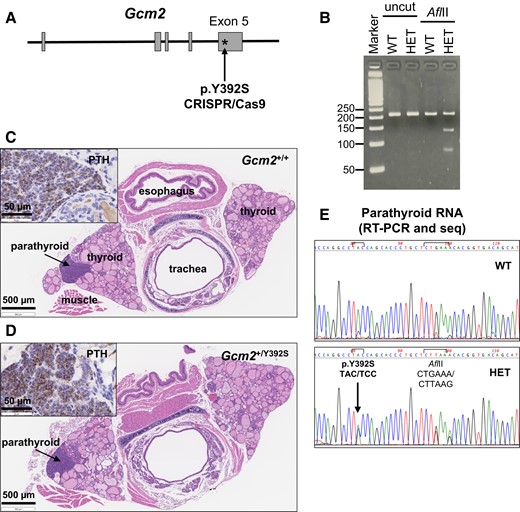
Knock-in mouse model of the Gcm2 variant p.Y392S. (A) Strategy for variant knock-in. CRISPR/Cas9 gene editing technology was used to generate mice with the missense variant p.Y392S (TAC > TCC) within exon 5 of the mouse Gcm2 gene (Ensembl Transcript: ENSMUST00000021791). (B) Agarose gel images of representative mouse genotyping analysis. PCR amplification of tail DNA from Gcm2+/+ (WT) and Gcm2+/Y392S (HET) mice was performed using primers flanking the region where the p.Y392S variant and the downstream AflII site was introduced. Uncut and AflII digested PCR products were analyzed by agarose gel electrophoresis. The PCR product of WT mice shows a band at 210 bp in both uncut and AflII cut lanes. The PCR product of HET mice shows the 210 bp WT band and 2 bands at 130 and 80 bp generated by the AflII digest. (C and D) Brightfield microscopy images of tracheal region tissue sections to detect parathyroid glands. FFPE tissue sections of tracheal blocks of Gcm2+/+ (C) and Gcm2+/Y392S (D) mice were processed for hematoxylin and eosin (H&E) staining to locate parathyroid glands. Inset shows parathyroid hormone (PTH) staining by anti-PTH immunohistochemistry of the parathyroid glands in a serial section. (E) Gcm2 expression in parathyroid gland RNA. Expression of WT Gcm2 and p.Y392S variant was assessed in parathyroid gland RNA by RT-PCR and PCR product sequencing. Compared to WT, the Gcm2 nucleotide sequence of Gcm2+/Y392S (HET) shows the presence of the heterozygous p.Y392S variant and the downstream AflII restriction enzyme site.
Knock-In Mice With the Gcm2 Variant p.Y392S Show Normal Levels of Calcium and PTH
The impact of the Gcm2 p.Y392S variant on parathyroid gland associated features was evaluated by measuring serum calcium and PTH levels. At 6 months and at 12 to 15 months, circulating calcium and PTH levels were similar in Gcm2+/+ (n = 7) and Gcm2+/Y392S (n = 9) mice (Fig. 3A-D). Also, circulating calcium and PTH levels were similar in Gcm2+/+ (n = 8) and Gcm2+/Y392S (n = 9) mice at age 24 months, the study endpoint (Fig. 4A and 4B). Further analysis of parathyroid tissue was performed with anti-PTH and anti-CASR IHC of FFPE tissue sections of 24-month-old mice. PTH staining in the cytoplasm and CASR staining in the cell membrane was similar in the parathyroid glands of Gcm2+/+ and Gcm2+/Y392S mice (Fig. 4C-H). These data show that the Gcm2 variant p.Y392S did not affect parathyroid gland associated features.
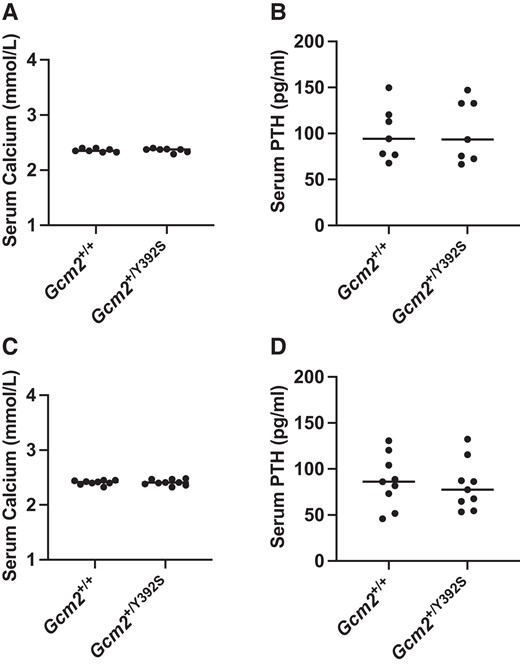
Knock-in of the Gcm2 variant p.Y392S shows normal serum calcium and PTH at age 6 months and 12-15 months. Calcium and parathyroid hormone (PTH) assay. At age 6 months, in n = 7 mice (A and B) and age 12-15 months, in n = 9 mice (C and D), the circulating calcium and PTH levels are shown in Gcm2+/+ and Gcm2+/Y392S mice. Both Gcm2+/+ and Gcm2+/Y392S mice show normal levels of calcium and PTH.
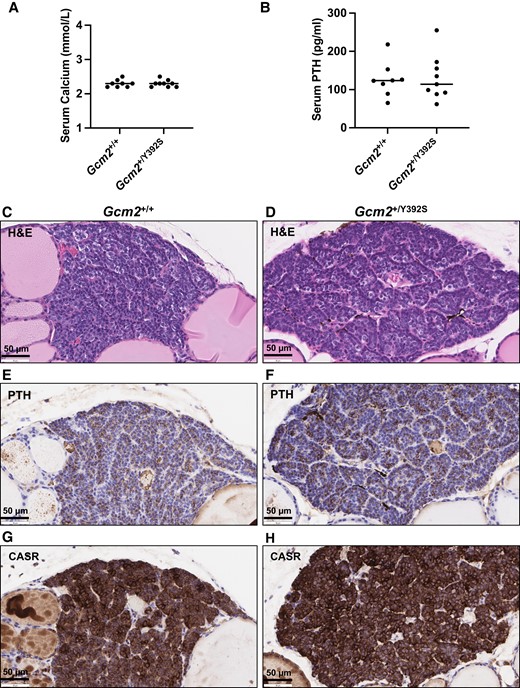
Knock-in mice with the Gcm2 variant p.Y392S show normal parathyroid gland associated features at age 24 months. (A and B) Calcium and parathyroid hormone (PTH) assay. Serum from Gcm2+/+ (n = 8) and Gcm2+/Y392S (n = 9) mice at age 24 months was used for measuring the circulating calcium and PTH levels. Both Gcm2+/+ and Gcm2+/Y392S mice show normal levels of calcium and PTH. (C-H) Representative brightfield microscopy images of parathyroid gland morphology. Serial sections of Gcm2+/+ and Gcm2+/Y392S parathyroid glands at age 24 months showing hematoxylin and eosin (H&E) staining, and immunohistochemistry with anti-PTH and anti-CASR. H&E staining showed similar histology of Gcm2+/+ and Gcm2+/Y392S (C and D), PTH staining was similar in the cytoplasm (brown) of Gcm2+/+ and Gcm2+/Y392S (E and F), and CASR staining was similar in the cell membrane (brown) of Gcm2+/+ and Gcm2+/Y392S (G and H). Blue color is from nuclear hematoxylin counter stain.
Knock-In Mice With the Gcm2 Variant p.Y392S Show Normal Parathyroid Glands
To determine whether the Gcm2 p.Y392S variant caused parathyroid neoplasia, parathyroid gland histology was observed in FFPE tissue sections from Gcm2+/+ (n = 14) and Gcm2+/Y392S (n = 11) 18- to 24-month-old mice that were processed for H&E staining (Fig. 5A and 5B). The parathyroid gland cells were arranged in clusters with a fibrovascular stroma, and mainly consisted of polygonal chief cells with eosinophilic or clear cytoplasm and a darkly stained nucleus. No obvious differences were noted in the images of total 17 parathyroid glands each from Gcm2+/+ and Gcm2+/Y392S mice.
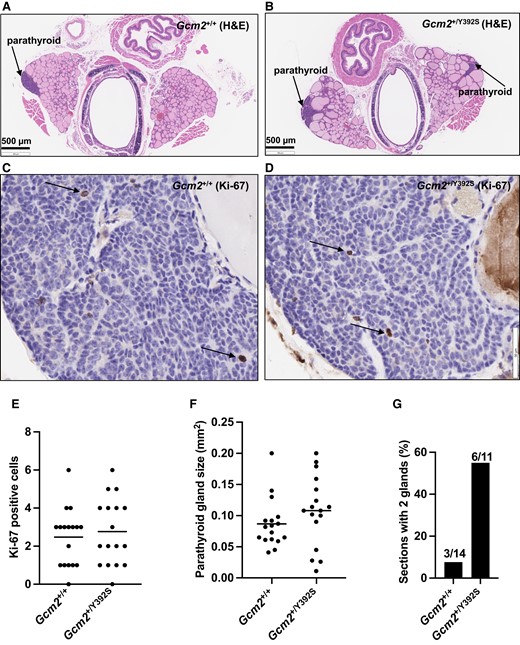
Knock-in mice with the Gcm2 variant p.Y392S show normal parathyroid cell proliferation. (A-D) Representative brightfield microscopy images of H&E and Ki-67 staining. Serial sections of Gcm2+/+ and Gcm2+/Y392S parathyroid glands at age 24 months showing hematoxylin and eosin (H&E) staining (low power), and immunohistochemistry with anti-Ki-67 (high power). Gcm2+/+ (A and C) and Gcm2+/Y392S (B and D) show similar parathyroid glands and similar Ki-67 staining (arrows pointing to Ki-67 positive cells with a brown nucleus). Blue color in C and D is from nuclear hematoxylin counter stain. (E) Parathyroid gland cell proliferation. To assess the level of cell proliferation, Ki-67 positive cells were counted from parathyroid tissue sections processed for anti-Ki-67 IHC as shown in C and D using 17 parathyroid glands each of Gcm2+/+ and Gcm2+/Y392S mice at age 18-24 months. All Ki-67 positive cells were counted in the entire parathyroid gland in the section. Similar number of Ki-67 positive cells were observed in Gcm2+/+ and Gcm2+/Y392S parathyroid glands indicating that the Gcm2+/Y392S parathyroid glands did not show increased cell proliferation. (F) Parathyroid gland area. Parathyroid gland size was measured from digital images of H&E-stained sections of 17 parathyroid glands from 14 Gcm2+/+ mice and 17 parathyroid glands from 11 Gcm2+/Y392S mice at age 18-24 months. Using the built-in scale of the Histoserv digital software, the length and breadth of the parathyroid glands were recorded in µm, and the area was calculated (length × breadth) in µm2 (converted to mm2, 1 µm2 = 10−6 mm2). Overall, Gcm2+/+ and Gcm2+/Y392S mice showed parathyroid glands of similar size. (G) Number of parathyroid glands per section. The number of parathyroid glands in H&E-stained sections of tracheal blocks from 14 Gcm2+/+ mice and 11 Gcm2+/Y392S mice at age 18-24 months was recorded. The occurrence of 2 glands per section for Gcm2+/Y392S mice was higher (55%) compared to Gcm2+/+ mice (21%).
To assess whether cell proliferation was affected in the parathyroid glands of Gcm2+/Y392S mice, Ki-67 positive cells were counted from FFPE tissue sections that were processed for anti-Ki-67 IHC from Gcm2+/+ (n = 14) and Gcm2+/Y392S (n = 11) mice at age 18 to 24 months (Fig. 5C and 5D). Both Gcm2+/+ and Gcm2+/Y392S parathyroid gland sections showed 0 to 6 Ki-67-positive cells (Gcm2+/+ 2.5 ± 1.5 and Gcm2+/Y392S 2.8 ± 1.8). Therefore, there was no significant difference in the proliferation of parathyroid gland cells in mice with the Gcm2 p.Y392S variant (P value = .6) (Fig. 5E).
Parathyroid gland carcinomas show increased gland size and high Ki-67 proliferative index, whereas benign hyperplasia and adenomas show increased gland size but without a high Ki-67 proliferative index [30]. Therefore, despite no difference in Ki-67 positive parathyroid cells between Gcm2+/+ and Gcm2+/Y392S mice, the parathyroid gland size was evaluated. The length, breadth, and area of the parathyroid glands was measured in H&E-stained tissue sections from Gcm2+/+ (n = 14) and Gcm2+/Y392S (n = 11) mice at age 18 to 24 months. The Gcm2+/+ parathyroid gland length was 266 to 706 µm (418 ± 125 µm), breadth 116 to 434 µm (211 ± 78 µm), and area 0.04 to 0.2 mm2 (0.09 ± 0.04 mm2). The Gcm2+/Y392S parathyroid gland length was 108 to 567 µm (389 ± 105 µm), breadth 74 to 422 µm (263 ± 110 µm), and area 0.01 to 0.2 mm2 (0.11 ± 0.06 mm2). There was no significant difference in the size of the parathyroid glands in mice with the Gcm2 p.Y392S variant compared with WT mice (P value = .2) (Fig. 5F). One parameter that showed a difference between the Gcm2+/+ and Gcm2+/Y392S tracheal block tissue sections was the number of parathyroids glands per section (Fig. 5G). The Gcm2+/+ mice mostly showed a single parathyroid gland in the tracheal block tissue section, and only 3 of 14 showed 2 parathyroid glands. The Gcm2+/Y392S mice mostly showed 2 parathyroid glands in the tracheal block tissue section (6 of 11).
Therefore, the parathyroid gland tissue histology, cell proliferation rate, and parathyroid gland size in mice with the Gcm2 p.Y392S variant was similar compared with the WT mice. The significance of frequently finding 2 parathyroid glands in the tracheal block tissue sections of Gcm2+/Y392S mice vs a single gland in the Gcm2+/+ mice remains to be determined.
Discussion
PHPT is characterized by elevated serum calcium and PTH, and tumors of one or multiple parathyroid glands [31]. Heterozygous germline missense variants (p.L379Q, p.V382M, p.I383M, p.T386S, p.K388E, and p.Y394S) located within the CCID of GCM2 have been reported in a subset of patients with familial PHPT [14-24]. In functional assays, these missense variants enhance the transcriptional activity of GCM2, suggesting that GCM2 could act as a proto-oncogene in parathyroid neoplasia. The GCM2 p.Y394S missense variant has been reported in various publications, however, with a reduced penetrance for primary hyperparathyroidism [18, 23, 24]. To study the direct contribution of this missense variant in parathyroid neoplasia, we generated a germline heterozygous knock-in mouse model of the Gcm2 variant p.Y392S (that corresponds to human GCM2 p.Y394S). The mouse Gcm2 variant p.Y392S exhibited the transcription enhancing effect that was observed for the human GCM2 p.Y394S variant. However, mice with this variant (Gcm2+/Y392S) showed normal serum levels of calcium and PTH and normal parathyroid gland morphology indicating that the p.Y392S variant did not cause parathyroid neoplasia.
Further analysis of tracheal block sections of Gcm2+/+ and Gcm2+/Y392S mice showed that the occurrence of 2 glands per section was higher in mice expressing the p.Y392S variant compared with WT mice. Humans have 4 parathyroid glands (2 pairs) that develop from the embryonic endodermal third and fourth pharyngeal pouches between 5 and 12 weeks of gestation, whereas mice have only 2 parathyroid glands that develop from the third pharyngeal pouch between embryonic day E8 and E12 [32]. After embryonic development and migration together with the thymus, the parathyroids are finally located in the neck region, behind the thyroid lobes [33]. The frequent detection of 2 parathyroid glands per section of tracheal blocks in Gcm2+/Y392S mice suggests that the variant may contribute to tissue migration and bilateral symmetry of parathyroid gland location. Early developmental studies in this mouse model may help to determine whether the Gcm2 p.Y392S variant could modify parathyroid tissue migration during organogenesis.
One of the limitations of our study is that we did not investigate mice that were germline homozygous for the Gcm2 p.Y392S variant because the study was designed to mimic the human PHPT genotype as a germline heterozygous variant. Therefore, to assess the effect of this variant on parathyroid gland function, it will be important to perform serum biochemistry and parathyroid gland analysis of mice homozygous for the Gcm2 p.Y392S variant. Given the influence of strain background on the phenotype of some genetically engineered mouse models [34], future studies to assess PHPT in Gcm2+/Y392S mice could be conducted in different mouse backgrounds. We only analyzed the parathyroid glands of mice at age 18 to 24 months because most of the prior genetically engineered mouse models of parathyroid neoplasia associated genes (Ccnd1, Men1, Cdc73, and Rgs5) showed parathyroid tumors after age 12 to 18 months [26-29], and because serum calcium and PTH in the Gcm2+/Y392S mice at the 6-month and 12- to 15-month time points were unaffected.
The onset of PHPT in patients with heterozygous germline inactivating variants in MEN1 occurs at age 20 to 25 years [3], whereas patients with heterozygous germline activating variants in GCM2 develop PHPT at a later age (median, 57 years) [15]. Also, patients with the GCM2 p.Y394S variant have shown a reduced penetrance for PHPT [18, 23, 24]. It is possible that Gcm2+/Y392S mice may develop tumors at a later age, after 24 months, and with a reduced penetrance. Therefore, further studies to evaluate parathyroid tumor development could include older Gcm2+/Y392S mice and could increase the number of mice analyzed, and such experiments may be technically challenging due to potential age-related diseases in older mice (more than 24 months). Additionally, it remains to be determined whether any co-regulatory mechanisms that are expressed in older age may enhance the function of GCM2 to affect parathyroid gland proliferation and function.
Generating mouse models of human disease-associated missense variants using the CRISPR/Cas9 system can help to study the contribution of these variants in development and disease. We have attempted to do the same with a recurrent missense variant of human GCM2 p.Y394S that is associated with PHPT. Our results with the Gcm2+/Y392S mouse model show that the Gcm2 gain-of-function missense variant p.Y392S is tolerated in mice and does not affect the growth of parathyroid glands and associated features of serum calcium and PTH levels.
Acknowledgments
The authors are grateful to the animal care staff of the NIH animal facilities and Dr. Martha Quezado of NCI/NIH for advice on parathyroid pathology.
Funding
This work was supported by the Intramural Research Program of the NIH: National Institute of Diabetes and Digestive and Kidney Diseases (grant number 1ZIADK075035).
Disclosures
The authors have nothing to disclose.
Data Availability
All the data generated or analyzed during this study are included in this article.
References
Abbreviations
- bp
base pair
- CASR
calcium-sensing receptor
- CCID
C-terminal conserved inhibitory domain
- FFPE
formalin fixed, paraffin embedded
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- PHPT
primary hyperparathyroidism
- PTH
parathyroid hormone
- WT
wild-type



