-
PDF
- Split View
-
Views
-
Cite
Cite
Taylor M Triolo, Laura Pyle, Sona Seligova, Liping Yu, Peter A Gottlieb, Andrea K Steck, Risk of Islet and Celiac Autoimmunity in Cotwins of Probands With Type 1 Diabetes, Journal of the Endocrine Society, Volume 4, Issue 6, June 2020, bvaa053, https://doi.org/10.1210/jendso/bvaa053
Close - Share Icon Share
Abstract
Concordance for persistent islet autoimmunity (IA) and type 1 diabetes in monozygotic twins after probands are diagnosed is variable (30%-70%). Risk for development of IA in dizygotic twins is thought to be similar to nontwin siblings. Little is known in regard to the development of celiac autoimmunity (CDA) in twins of subjects with type 1 diabetes.
Our aim was to investigate the development of IA and CDA in cotwins of probands with type 1 diabetes.
Since 1995, the Twin Family Study has followed 336 twins (168 twin probands with type 1 diabetes and 168 cotwins) for a median of 14 years (interquartile range:10-18 years). Cotwins were followed for the development of IA, type 1 diabetes, and CDA.
In monozygotic cotwins, cumulative incidence by age 20 was 14% for IA and 10% for CDA. Development of IA and CDA by age 20 was 9% and 12% in dizygotic cotwins, respectively. While the numbers are small, IA by age 30 years was 26% in monozygotic and 39% in dizygotic twins. In proportional hazards models, the proband’s younger age at diagnosis, but not sex or human leukocyte antigen were associated with time to IA and CDA in cotwins.
CDA risk by age 20 in cotwins was 10% to 12%. With long-term follow-up, cumulative incidence for IA is high in dizygotic twins, similar to monozygotic twins, suggesting a role of possible early environmental factors shared by type 1 diabetes discordant cotwins.
Clinical diagnosis, of type 1 diabetes is preceded by the development of islet autoantibodies, which can be present years before onset of type 1 diabetes [1]. Autoantibodies against insulin (IAA), glutamic acid decarboxylase (GAD), insulinoma-associated antigen-2 (IA-2), and zinc transporter (ZnT8) may be present prior to the development of clinically symptomatic dysglycemia [2-4]. Persistent islet autoimmunity (IA) is associated with a high risk of progression to type 1 diabetes [5,6].
Patients with type 1 diabetes are at risk for the development of non-IA and multiple autoimmune disorders [7-9]. The prevalence of celiac disease is increased in patients with type 1 diabetes [10,11] and in relatives of patients with celiac disease [12,13]. Celiac autoimmunity (CDA) can be detected by autoantibodies to tissue transglutaminase [14,15], and celiac disease is confirmed by biopsy [16]. Twin studies have shown that celiac disease is known to have a strong genetic component [17]. Less is known regarding the development of CDA in the cotwins of probands with type 1 diabetes.
Monozygotic twins are at risk to progress to type 1 diabetes after the proband twin is diagnosed. Reports on the prevalence of progression to type 1 diabetes in monozygotic twins have been variable (30%-70%) [18,19]. Cotwins are at an especially increased risk of development of type 1 diabetes if the proband is diagnosed at a young age [18,20]. With long-term follow-up, concordance rates of type 1 diabetes in the cotwin of monozygotic probands is >50% [21]. Risk for IA or type 1 diabetes in dizygotic twins has previously been thought to be similar to full siblings (6%-10% risk for type 1 diabetes) [22], but a recent study has shown that nonidentical twins with multiple islet autoantibodies have a risk similar to identical twins [19].
Since 1995, the Twin Family Study at the Barbara Davis Center has followed 168 twin pairs discordant for type 1 diabetes. Cotwins are followed for development of islet autoantibodies and/or the development of type 1 diabetes. In addition, monozygotic and dizygotic cotwins are also followed for development of CDA. The aim of this study was to compare the time to development of IA and CDA in cotwins and evaluate potential factors involved in progression to IA and CDA in monozygotic and dizygotic cotwins. We hypothesized that IA and CDA would be higher in monozygotic twins than dizygotic twins.
1. Material and Methods
A. Study population
Since 1995 the Twin Family Study of Islet Cell Autoimmunity at the Barbara Davis Center for Diabetes (BDC) has followed 336 twins (168 twin probands diagnosed with type 1 diabetes and 168 cotwins). This study has been recruiting twins locally at the BDC as well as nationally through TrialNet, previous Diabetes Prevention Trial (DPT-1), and Joslin study cohorts. Cotwin subjects were enrolled after the initial proband was diagnosed with type 1 diabetes and followed for development of IA and CDA and/or the development of type 1 diabetes. Proband twins complete an initial visit but are not followed over time in this study. Islet autoantibody negative cotwins are followed for islet autoantibody development every 1 to 2 years, while islet autoantibody positive cotwins are asked to come for monitoring visits every 6 to 12 months. Follow-up metabolic testing (hemoglobin A1c, blood glucose, and/or oral glucose tolerance test) for early detection of type 1 diabetes is recommended for all islet autoantibody-positive subjects. Race/ethnicity was self-reported. Subjects were enrolled with informed consent, and the study was approved by the University of Colorado Institutional Review Board.
B. Islet autoantibodies and genetic testing
The development of IA and type 1 diabetes were the primary outcomes for the cotwins enrolled in this study. Cotwin participants were screened for autoantibodies to IAA, GAD, IA-2, and ZnT8. Autoantibodies were measured by radioimmunoassay in the Immunogenetic Laboratory at the BDC (Aurora, CO, US), as previously described [2-4]. In the 2018 Islet Autoantibody Standardization Program Workshop, sensitivities and specificities were 66% and 99%, respectively, for IAA; 82% and 99%, respectively, for GADA; 62% and 100%, respectively, for IA-2A; and 68% and 100%, respectively, for ZnT8A. While IA was the primary outcome, autoantibodies for CDA were also collected. CDA was measured with tissue transglutaminase autoantibodies as previously described [23]. CDA was defined as a once positive tissue transglutaminase autoantibody level ≥0.05 U/mL. Subjects with positive CDA were referred to pediatric gastroenterology for additional evaluation and follow-up.
In same-sex twins, zygosity was confirmed by deoxyribonucleic acid testing of a standard panel of 15 genetic loci and a sex marker using the AmpFLSTR Identifiler PCR Amplification kit (Applied Biosystems, Foster City, CA, US). Human leukocyte antigen (HLA) Class II genotyping at the BDC was done using Luminex xMAP technology (Luminex, Austin, TX, US). Sequence-specific oligonucleotide (SSO) microspheres and polymerase chain reaction reagents used in the assay were obtained from One Lambda, Inc (Canoga Park, CA, US). Briefly, the specific HLA region to be typed was amplified labeling the amplicon with Biotin. Amplicon was applied to SSO-coupled microspheres. After incubation, streptavidin-phycoerythrin was added to bead/amplicon mixture. Microspheres were visualized using Luminex 200 or LabScan3D instrument. The HLA Alleles were determined by HLA Fusion software (One Lambda).
C. Statistical analysis
If a participant was single islet autoantibody positive, another single islet autoantibody positive visit or a multiple islet autoantibody positive visit was required to confirm positive islet autoantibody status. We compared the distribution of continuous variables using the Mann-Whitney U test and compared proportions using a χ2 test unless 20% of the cells had an expected value of <5, in which case we used Fisher’s exact test. The age of development of type 1 diabetes in the proband, age of IA in the cotwin and age of CDA in the cotwin were analyzed by Kaplan-Meier curves. For the Kaplan-Meier curves, follow-up time was defined as the time from birth to development of IA/type 1 diabetes or the most recent visit. While some subjects have been followed past 30 years of age, data was analyzed at 20 years of age as there are limited number of subjects older than 20 years in this cohort at this time. Cox proportional hazards models were used to test whether the proband’s age at diagnosis, HLA status, or sex was associated with time to IA or CDA in the cotwins. Results were considered statistically significant with P-value < 0.05. We conducted statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC, US).
2. Results
In total 168 probands were seen for an initial visit at diagnosis of type 1 diabetes and 168 cotwins were followed for a median of 14 years (interquartile range: 10-18 years). The age range of the probands at initial visit was 1 to 46 years; the age range of the cotwins was 0 to 54 years at initial visit and 1 to 63 years at last visit. Characteristics of these 80 monozygotic twin pairs and 88 dizygotic twin pairs are shown in Table 1. There were no differences in sex or race/ethnicity between the monozygotic and dizygotic twins with or without type 1 diabetes. There was no difference between the twin groups in the presence of the high-risk HLA genotype DRB1*03 DQA1*05:01 DQB1*02:01 (abbreviated DR3)/DRB1*04 DQA1*03:01 DQB1*0302 (abbreviated DR4) nor in the presence of the high-risk HLA haplotype DR3. However, dizygotic probands had a higher prevalence (69.8%) of the high-risk HLA haplotype DR4, compared to 57.0% of monozygotic probands, 56.4% of the monozygotic cotwins, and 48.3% of the dizygotic cotwins (P = 0.04). In total, 12/80 (15%) of monozygotic cotwins and 5/88 (5.7%) of dizygotic cotwins developed type 1 diabetes.
| Characteristics . | MZ proband (N = 80) . | DZ proband (N = 88) . | MZ cotwin (N = 80) . | DZ cotwin (N = 88) . | P value . |
|---|---|---|---|---|---|
| Sex, female | 46 (57.5%) | 40 (45.5%) | 46 (57.5%) | 38 (43.2%) | 0.116 |
| Race/ethnicity: | 66 (82.5%) | 77 (87.5%) | 67 (83.8%) | 77 (87.5%) | 0.967 |
| Non-Hispanic White | 4 (5%) | 3 (3.4%) | 4 (5%) | 3 (3.4%) | |
| Hispanic Other/mixed/unknown | 10 (12.5%) | 8 (9.1%) | 9 (11.3%) | 8 (9.1%) | |
| HLA DR3 haplotype presenta | 38 (47.5%) | 35 (40.2%) | 38 (48.1%) | 34 (39.1%) | 0.518 |
| HLA DR4 haplotype presenta | 45 (57.0%) | 60 (69.8%) | 44 (56.4%) | 42 (48.3%) | 0.039 |
| HLA DR 3/4 genotype presenta | 21 (26.3%) | 22 (25.3%) | 21 (26.6%) | 12 (13.8%) | 0.139 |
| Type 1 diabetes diagnosed | 80 (100%) | 88 (100%) | 12 (15.0%) | 5 (5.7%) | < 0.001 |
| Age at type 1 diabetes diagnosis (median yrs, interquartile range) | 9.3 (5.5, 13.9) | 6.7 (3.8, 10.7) | 15.2 (10.7, 19.8) | 13.1 (9.4, 16.9) | < 0.001 |
| Characteristics . | MZ proband (N = 80) . | DZ proband (N = 88) . | MZ cotwin (N = 80) . | DZ cotwin (N = 88) . | P value . |
|---|---|---|---|---|---|
| Sex, female | 46 (57.5%) | 40 (45.5%) | 46 (57.5%) | 38 (43.2%) | 0.116 |
| Race/ethnicity: | 66 (82.5%) | 77 (87.5%) | 67 (83.8%) | 77 (87.5%) | 0.967 |
| Non-Hispanic White | 4 (5%) | 3 (3.4%) | 4 (5%) | 3 (3.4%) | |
| Hispanic Other/mixed/unknown | 10 (12.5%) | 8 (9.1%) | 9 (11.3%) | 8 (9.1%) | |
| HLA DR3 haplotype presenta | 38 (47.5%) | 35 (40.2%) | 38 (48.1%) | 34 (39.1%) | 0.518 |
| HLA DR4 haplotype presenta | 45 (57.0%) | 60 (69.8%) | 44 (56.4%) | 42 (48.3%) | 0.039 |
| HLA DR 3/4 genotype presenta | 21 (26.3%) | 22 (25.3%) | 21 (26.6%) | 12 (13.8%) | 0.139 |
| Type 1 diabetes diagnosed | 80 (100%) | 88 (100%) | 12 (15.0%) | 5 (5.7%) | < 0.001 |
| Age at type 1 diabetes diagnosis (median yrs, interquartile range) | 9.3 (5.5, 13.9) | 6.7 (3.8, 10.7) | 15.2 (10.7, 19.8) | 13.1 (9.4, 16.9) | < 0.001 |
Abbreviations: DZ, dizygotic MZ, monozygotic.
aHLA class II genotyping was not available in 3 subjects.
| Characteristics . | MZ proband (N = 80) . | DZ proband (N = 88) . | MZ cotwin (N = 80) . | DZ cotwin (N = 88) . | P value . |
|---|---|---|---|---|---|
| Sex, female | 46 (57.5%) | 40 (45.5%) | 46 (57.5%) | 38 (43.2%) | 0.116 |
| Race/ethnicity: | 66 (82.5%) | 77 (87.5%) | 67 (83.8%) | 77 (87.5%) | 0.967 |
| Non-Hispanic White | 4 (5%) | 3 (3.4%) | 4 (5%) | 3 (3.4%) | |
| Hispanic Other/mixed/unknown | 10 (12.5%) | 8 (9.1%) | 9 (11.3%) | 8 (9.1%) | |
| HLA DR3 haplotype presenta | 38 (47.5%) | 35 (40.2%) | 38 (48.1%) | 34 (39.1%) | 0.518 |
| HLA DR4 haplotype presenta | 45 (57.0%) | 60 (69.8%) | 44 (56.4%) | 42 (48.3%) | 0.039 |
| HLA DR 3/4 genotype presenta | 21 (26.3%) | 22 (25.3%) | 21 (26.6%) | 12 (13.8%) | 0.139 |
| Type 1 diabetes diagnosed | 80 (100%) | 88 (100%) | 12 (15.0%) | 5 (5.7%) | < 0.001 |
| Age at type 1 diabetes diagnosis (median yrs, interquartile range) | 9.3 (5.5, 13.9) | 6.7 (3.8, 10.7) | 15.2 (10.7, 19.8) | 13.1 (9.4, 16.9) | < 0.001 |
| Characteristics . | MZ proband (N = 80) . | DZ proband (N = 88) . | MZ cotwin (N = 80) . | DZ cotwin (N = 88) . | P value . |
|---|---|---|---|---|---|
| Sex, female | 46 (57.5%) | 40 (45.5%) | 46 (57.5%) | 38 (43.2%) | 0.116 |
| Race/ethnicity: | 66 (82.5%) | 77 (87.5%) | 67 (83.8%) | 77 (87.5%) | 0.967 |
| Non-Hispanic White | 4 (5%) | 3 (3.4%) | 4 (5%) | 3 (3.4%) | |
| Hispanic Other/mixed/unknown | 10 (12.5%) | 8 (9.1%) | 9 (11.3%) | 8 (9.1%) | |
| HLA DR3 haplotype presenta | 38 (47.5%) | 35 (40.2%) | 38 (48.1%) | 34 (39.1%) | 0.518 |
| HLA DR4 haplotype presenta | 45 (57.0%) | 60 (69.8%) | 44 (56.4%) | 42 (48.3%) | 0.039 |
| HLA DR 3/4 genotype presenta | 21 (26.3%) | 22 (25.3%) | 21 (26.6%) | 12 (13.8%) | 0.139 |
| Type 1 diabetes diagnosed | 80 (100%) | 88 (100%) | 12 (15.0%) | 5 (5.7%) | < 0.001 |
| Age at type 1 diabetes diagnosis (median yrs, interquartile range) | 9.3 (5.5, 13.9) | 6.7 (3.8, 10.7) | 15.2 (10.7, 19.8) | 13.1 (9.4, 16.9) | < 0.001 |
Abbreviations: DZ, dizygotic MZ, monozygotic.
aHLA class II genotyping was not available in 3 subjects.
By age 20 years, the cumulative incidence of IA was 14.3% in monozygotic (Fig. 1A) and 9.2% in dizygotic (Fig. 1B) twins. The cumulative incidence of CDA by age 20 years was 10.0% in monozygotic twins and 12.3% in dizygotic twins. Risk for CDA plateaus at 15 years of age. Although the numbers of subjects are small, the cumulative incidence of IA by 30 years of age was 26% in monozygotic twins and 39% in dizygotic twins. The overall cumulative incidence of IA was not different between monozygotic and dizygotic twins (P = 0.63). Similarly, the overall cumulative incidence of CDA was not different between groups (P = 0.83).
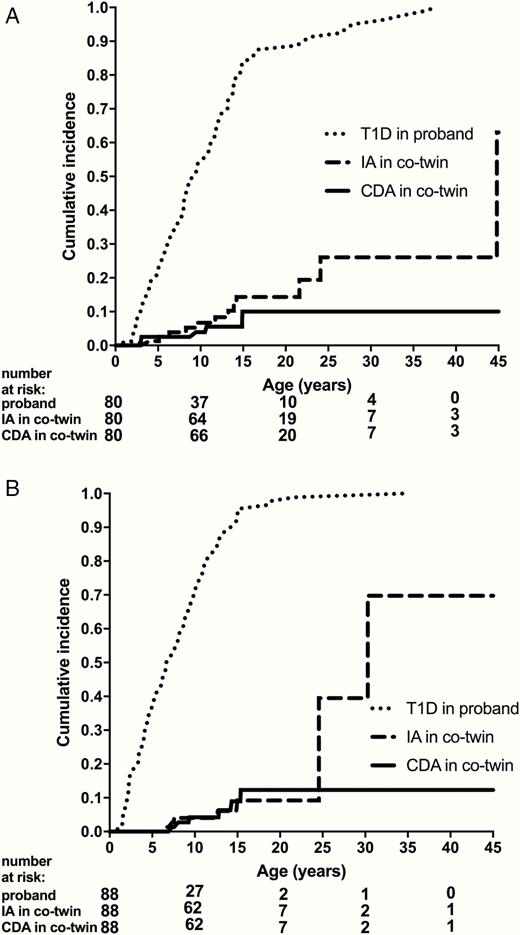
(A) Time to type 1 diabetes in monozygotic probands and time to islet autoimmunity and celiac autoimmunity in cotwins. (B) Time to type 1 diabetes in dizygotic probands and time to islet autoimmunity and celiac autoimmunity in cotwins.
Cox proportional hazards models were used to test whether the proband’s age at diagnosis, HLA status, or sex was associated with time to development of IA or CDA in the cotwins (Table 2). In monozygotic and dizygotic cotwins, a younger age at diagnosis of type 1 diabetes in the proband was associated with an increased risk for IA in the cotwin, while high risk HLA haplotypes or sex were not. Only younger age of the monozygotic proband was associated with increased risk of CDA in cotwins. There was no association of the dizygotic proband’s age at diagnosis, sex, or presence of the high-risk HLA haplotypes with time to CDA in the cotwin.
Cox proportional hazards models for time to islet autoimmunity and celiac autoimmunity in cotwins
| Covariate . | Hazard Ratio (95% Confidence Interval) . | P value . |
|---|---|---|
| Time to IA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.88 (0.78-1.00) | 0.047 |
| Proband’s sex | 0.37 (0.08-1.76) | 0.210 |
| DR3 present in proband | 0.95 (0.20-4.42) | 0.945 |
| DR4 present in proband | 0.79 (0.15-4.05) | 0.773 |
| Time to IA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.91 (0.84-0.98) | 0.012 |
| Proband’s sex | 0.97 (0.29-3.28) | 0.962 |
| 0.81 (0.26-2.51) | 0.716 | |
| DR4 present in proband | 1.05 (0.32-3.47) | 0.938 |
| Time to CDA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.85 (0.66-1.08) | 0.187 |
| Proband’s sex | 0.21 (0.02-1.91) | 0.164 |
| DR3 present in proband | 0.43 (0.09-1.93) | 0.268 |
| DR4 present in proband | 0.47 (0.05-4.12) | 0.495 |
| Time to CDA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.79 (0.68-0.91) | 0.001 |
| 0.44 (0.06-3.35) | 0.426 | |
| DR3 present in proband | 0.17 (0.02-1.46) | 0.106 |
| DR4 present in proband | 5.01 (0.38-65.8) | 0.220 |
| Covariate . | Hazard Ratio (95% Confidence Interval) . | P value . |
|---|---|---|
| Time to IA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.88 (0.78-1.00) | 0.047 |
| Proband’s sex | 0.37 (0.08-1.76) | 0.210 |
| DR3 present in proband | 0.95 (0.20-4.42) | 0.945 |
| DR4 present in proband | 0.79 (0.15-4.05) | 0.773 |
| Time to IA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.91 (0.84-0.98) | 0.012 |
| Proband’s sex | 0.97 (0.29-3.28) | 0.962 |
| 0.81 (0.26-2.51) | 0.716 | |
| DR4 present in proband | 1.05 (0.32-3.47) | 0.938 |
| Time to CDA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.85 (0.66-1.08) | 0.187 |
| Proband’s sex | 0.21 (0.02-1.91) | 0.164 |
| DR3 present in proband | 0.43 (0.09-1.93) | 0.268 |
| DR4 present in proband | 0.47 (0.05-4.12) | 0.495 |
| Time to CDA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.79 (0.68-0.91) | 0.001 |
| 0.44 (0.06-3.35) | 0.426 | |
| DR3 present in proband | 0.17 (0.02-1.46) | 0.106 |
| DR4 present in proband | 5.01 (0.38-65.8) | 0.220 |
Abbreviations: IA, islet autoimmunity; CDA, celiac autoimmunity.
Cox proportional hazards models for time to islet autoimmunity and celiac autoimmunity in cotwins
| Covariate . | Hazard Ratio (95% Confidence Interval) . | P value . |
|---|---|---|
| Time to IA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.88 (0.78-1.00) | 0.047 |
| Proband’s sex | 0.37 (0.08-1.76) | 0.210 |
| DR3 present in proband | 0.95 (0.20-4.42) | 0.945 |
| DR4 present in proband | 0.79 (0.15-4.05) | 0.773 |
| Time to IA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.91 (0.84-0.98) | 0.012 |
| Proband’s sex | 0.97 (0.29-3.28) | 0.962 |
| 0.81 (0.26-2.51) | 0.716 | |
| DR4 present in proband | 1.05 (0.32-3.47) | 0.938 |
| Time to CDA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.85 (0.66-1.08) | 0.187 |
| Proband’s sex | 0.21 (0.02-1.91) | 0.164 |
| DR3 present in proband | 0.43 (0.09-1.93) | 0.268 |
| DR4 present in proband | 0.47 (0.05-4.12) | 0.495 |
| Time to CDA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.79 (0.68-0.91) | 0.001 |
| 0.44 (0.06-3.35) | 0.426 | |
| DR3 present in proband | 0.17 (0.02-1.46) | 0.106 |
| DR4 present in proband | 5.01 (0.38-65.8) | 0.220 |
| Covariate . | Hazard Ratio (95% Confidence Interval) . | P value . |
|---|---|---|
| Time to IA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.88 (0.78-1.00) | 0.047 |
| Proband’s sex | 0.37 (0.08-1.76) | 0.210 |
| DR3 present in proband | 0.95 (0.20-4.42) | 0.945 |
| DR4 present in proband | 0.79 (0.15-4.05) | 0.773 |
| Time to IA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.91 (0.84-0.98) | 0.012 |
| Proband’s sex | 0.97 (0.29-3.28) | 0.962 |
| 0.81 (0.26-2.51) | 0.716 | |
| DR4 present in proband | 1.05 (0.32-3.47) | 0.938 |
| Time to CDA in Dizygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.85 (0.66-1.08) | 0.187 |
| Proband’s sex | 0.21 (0.02-1.91) | 0.164 |
| DR3 present in proband | 0.43 (0.09-1.93) | 0.268 |
| DR4 present in proband | 0.47 (0.05-4.12) | 0.495 |
| Time to CDA in Monozygotic Cotwins | ||
| Proband’s age at type 1 diabetes diagnosis | 0.79 (0.68-0.91) | 0.001 |
| 0.44 (0.06-3.35) | 0.426 | |
| DR3 present in proband | 0.17 (0.02-1.46) | 0.106 |
| DR4 present in proband | 5.01 (0.38-65.8) | 0.220 |
Abbreviations: IA, islet autoimmunity; CDA, celiac autoimmunity.
Fig. 2 shows the correlation of age of diagnosis of type 1 diabetes in monozygotic twins. Age of onset of type 1 diabetes in the proband was closely associated to that of the cotwin (r = 0.90, P < 0.001).
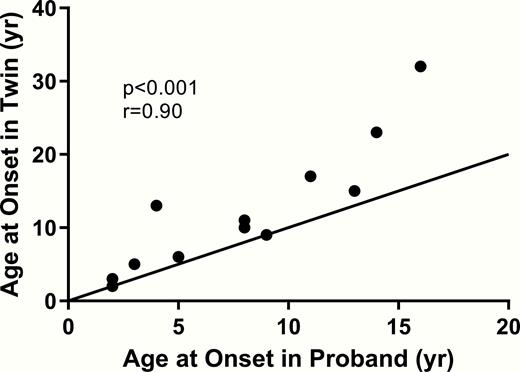
Correlation of age at diagnosis of type 1 diabetes in monozygotic probands and age at diagnosis of type 1 diabetes in their cotwins.
Figure 1. Time to type 1 diabetes in monozygotic (A) and dizygotic (B) probands and time to islet autoimmunity and celiac autoimmunity in cotwins. Time in years since birth are shown for the age of development of the proband with type 1 diabetes and the cotwin’s development of islet autoimmunity and celiac autoimmunity. Abbreviations: CDA, celiac autoimmunity; IA, islet autoimmunity; TID, type 1 diabetes.
Figure 2. Correlation of age at diagnosis of type 1 diabetes in monozygotic probands and age at diagnosis of type 1 diabetes in their cotwins. Age of onset of type 1 diabetes in the proband (x axis) compared to the age of onset of type 1 diabetes in the cotwin. Age of onset of the cotwin is closely correlated to age of onset of the proband (P < 0.01, r = 0.9). Abbreviation: yr, years.
3. Discussion
This large study of over 160 twin pairs with confirmed zygosity testing shows that the risk for development of IA in monozygotic and dizygotic cotwins is high and continues into adulthood. By age 20, the cumulative incidence of IA in both monozygotic and dizygotic cotwins was 14% and 9.2%, respectively. In twins followed to age 30, the risk for developing IA was high for both monozygotic and dizygotic twins (26% and 39%, respectively), although these risk estimates should be interpreted with caution as the number of subjects followed at that time was small. In monozygotic and dizygotic twins, younger age of the proband was associated with an increased risk of developing IA in the cotwin, while the proband’s sex and the presence of the high-risk HLA haplotypes were not significant factors. In addition, this is the first study to show risk of progression to CDA in cotwins of probands diagnosed with type 1 diabetes.
Individuals with type 1 diabetes are at an increased risk for additional autoimmune diseases, and little is known about the risk of CDA in cotwins of probands diagnosed with type 1 diabetes. Previous twin studies have demonstrated the development of celiac disease is known to have a strong genetic component [17]. The development of CDA by age 15 was about 10% for both monozygotic and dizygotic cotwins with no further increase in risk after age 15. In monozygotic twins, the proband’s younger age at type 1 diabetes diagnosis was associated with increased risk of development of CDA in the cotwin, while this association was not seen in dizygotic twins. A prospective birth cohort study following infants at increased genetic risk for type 1 diabetes, the Environmental Determinants of Diabetes in the Young (TEDDY) study, found that IA usually precedes CDA in early childhood and that IA significantly increases the risk of subsequent CDA [24]. On the other hand, the HLA class II haplotypes account for about 50% of the genetic risk for both type 1 diabetes [25] and celiac disease [26]; the 2 common haplotypes conferring increased risk in both diseases are DR4 and DR3 with the DR3 genotype especially known to increase risk for celiac disease [27]. In this study, however, we did not find any association between these high-risk HLA DR3 and DR4 haplotypes and time to development of CDA in the cotwins, possibly because of relatively small number of cases.
Previous studies have shown that monozygotic cotwins have a high concordance (>50%) of development of type 1 diabetes after the first twin is diagnosed [21], especially when the proband twin is diagnosed at a younger age, although the cut-off for young age has varied between less than 25 to less than 5 years of age [18,20,21,28-30]. Similar to prior studies [31], we found high concordance rates for monozygotic twins. Concordance rates for dizygotic twins has typically thought to be lower (5%-15%) [18,29,30]. More recent data from the TrialNet Pathway to Prevention, a multicenter study that screens and follows relatives of patients with type 1 diabetes for development of IA and type 1 diabetes showed that if nonidentical twins have multiple positive IA at screening, their risk of developing type 1 diabetes is similar to that of single and multiple IA positive identical twins [19]. This study suggests that, with long-term follow-up, cumulative incidence for IA is high in dizygotic twins, similar to monozygotic twins, indicating a role of possible early environmental factors shared by diabetes discordant cotwins. Given their presumed shared timing of exposures, it is possible that early environmental exposures such as in-utero factors, medication, early infant dietary factors, or infections (viruses, bacteria) could influence development of IA in twins [32].
Limitations of this study include that the cotwins were only screened and followed in the Twin Family Study after the twin proband was diagnosed with type 1 diabetes. Therefore, it is possible that that the cotwin may already have been IA or CDA positive before the diagnosis of the twin proband. Additionally, the median follow-up is 14 years for both monozygotic and dizygotic cotwins and the number of twins followed into adulthood at this point is small. The risk estimates after age 20 should be interpreted with caution. While the risk of IA for monozygotic cotwins is known to be high with long-term follow-up [19,21], it is possible that the high risk of development of IA in dizygotic cotwins reported in this study are influenced by the small numbers of subjects followed after age 20. Additional follow-up of this cohort over time will be beneficial to determine if the risk of development of IA in dizygotic cotwins remains similar to monozygotic cotwins. The risk of celiac disease is known to be near 10% for both patients with type 1 diabetes and first-degree relatives of patients with celiac disease [24,33]. In the general population, confirmed risk of CDA has previously been shown to be 5% by 15 years of age [34]. It is possible that the risk of CDA in this study was overestimated as the followed cotwins may have included subjects who were only transiently celiac autoantibody positive. As the main outcome of this study is to ascertain risk of IA and type 1 diabetes in cotwins, our data for CDA and development of celiac disease are limited in these subjects.
4. Conclusions
In conclusion, this study shows that the development of CDA is about 10% for both monozygotic and dizygotic cotwins by age 15. With long-term follow-up, cumulative incidence for IA is high in dizygotic cotwins and similar to monozygotic twins, suggesting a role of possible early environmental factors.
Acknowledgments
We would like to thank the subjects and their families.
Financial Support: TMT is funded by the National Institute of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K12 Grant (K12DK094712) and AKS is funded by the American Diabetes Association (ADA) Grant 1-14-CD-17.
Author Contributions: TMT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. TMT researched data and wrote the manuscript. SS researched data and reviewed and edited the manuscript. LP researched data and reviewed and edited the manuscript. LY researched data and reviewed and edited the manuscript. PAG contributed to discussion and reviewed and edited the manuscript. AKS designed the study, contributed to discussion, and reviewed and edited the manuscript.
Additional Information
Disclosure Summary: The authors have no conflicts of interest to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request



